Abstract
Acute inflammatory reactions represent the major cause of irreversible neuropathy in leprosy. These tissue-destroying episodes have considerable overlap with acute immunological complications (flares) in several chronic (autoimmune) diseases that similarly warrant early detection. However, the lack of diagnostic tests impedes early diagnosis of these reactions. Here, we evaluated a user-friendly multiplex lateral flow assay for the simultaneous detection of IP-10 and anti-phenolic glycolipid I antibodies for longitudinally monitoring early onset and treatment of leprosy reactions.
TEXT
Leprosy, an infectious disease caused by Mycobacterium leprae, still poses a major health threat in developing countries. Additionally, leprosy represents an intriguing model of human immunoregulatory disease, since its interindividual variability in clinical manifestations closely parallels the ability of the host to establish effective immunity to M. leprae (1, 2). This has resulted in leprosy being the first disease for which researchers identified HLA-disease association (3), human regulatory cells (4), and the Th1/Th2 concept for human T cells (5).
Although leprosy can be treated effectively with multidrug therapy (MDT), it can be complicated by acute inflammatory episodes, called leprosy reactions, that may occur before, during, and after the completion of MDT (6). Two distinct types of reactions are distinguished: reversal reactions (RRs) and erythema nodosum leprosum (ENL). These immunological complications occur in up to 50% of leprosy patients and represent the major cause of irreversible neurological damage and consequent anatomical deformities. Prompt diagnosis and treatment aid recovery from inflammatory nerve damage and reduce the risk of permanent disability considerably (7). However, if diagnosis and treatment are delayed for more than 6 months after symptom initiation, neuropathy is likely to be permanent (8). Tests for the early detection of leprosy reactions may make significant differences in clinical outcomes, especially when the tests are user-friendly and robust.
Previous work has shown that gamma-interferon (IFN-γ)-inducible protein 10 (IP-10) is a useful biomarker for the detection of M. tuberculosis infection (9) or to indicate M. leprae exposure (10, 11). Moreover, increased IP-10 serum levels are part of the biomarker profile characterizing the early onset of RRs (12, 13). Levels of IP-10 decline again during antireactional therapy (13), similar to what has been described during tuberculosis treatment (14). With respect to the humoral immune response, IgM directed against the M. leprae-specific phenolic glycolipid I (PGL-I), although not informative for the identification of RR onset (13), represents a useful biomarker for monitoring the efficacy of treatment of leprosy (reactions), since IgM levels drop when reactions are effectively subdued (13). More importantly, since a significant percentage of new patients in many areas where leprosy is endemic initially seek care because of reactions and only then are diagnosed with leprosy, the detection of anti-PGL-I IgM serves as a confirmation of leprosy diagnosis.
Areas where leprosy is endemic are often short of sophisticated laboratories, which makes it imperative to develop diagnostic tests that are suitable for use in field settings. The combination of the user-friendly rapid lateral flow assay (LFA) format with the fluorescent quantitative up-converting phosphor (UCP) reporter technology has previously demonstrated usefulness for detecting and monitoring a variety of analytes (15–17). Additionally, besides having high sensitivity due to the complete absence of background fluorescence, UCP-LF test strips can be stored as permanent records, allowing reanalysis in a reference laboratory. To develop diagnostic assays for application in resource-limited areas, we previously developed UCP-LFAs for single detection of cytokines (IFN-γ, IP-10, interleukin 10 [IL-10], CCL4) as well as antibodies against M. leprae PGL-I for the diagnosis of nonreactional leprosy and tuberculosis (18–20). Generally, the performance of one biomarker can be significantly enhanced by using a custom-made grouping of independent biomarkers called a biomarker profile or signature.
In this study, we combined previous findings to evaluate the application of a multiplex UCP-LFA format for monitoring RR onset and treatment in leprosy patients. For this purpose, a UCP-LFA measuring IP-10 and anti-PGL-I IgM simultaneously was used to analyze serum samples from patients with borderline lepromatous leprosy collected prospectively in Bangladesh (n = 4 [13]), Brazil (n = 3 [13]), Nepal (n = 2 [13, 21]), and The Netherlands (n = 1 [22]). Newly diagnosed leprosy patients without reactions at recruitment were entered into the study after informed consent was obtained. Ethical approval of the study protocol was obtained through appropriate ethics committees: the Ethical Review Committee of ICDDR,B (no. PR-10032 and no. PR-2007-069), the Brazilian National Council of Ethics in Research (CONEP) and UFU Research Ethics Committee (no. 499/2008), and the Nepal Health Research Council (NHR no. 751). Leprosy was diagnosed based on clinical, bacteriological, and histological observations and classified by skin biopsy specimens according to Ridley and Jopling (23). For analysis by the UCP-LFA, leprosy patients who developed reactions during MDT were tested using samples obtained at three different time points: (i) without clinical signs of reactions ≥3 months before RR, (ii) at RR diagnosis, before steroid treatment, and (iii) after RR, ≥1 month after ending steroid treatment. Concentrations of antibodies against PGL-I and IP-10 were measured in all sera using a single UCP-LFA for either IP-10 or anti-PGL-I IgM and a multiplex UCP-LFA for both markers. Simultaneous detection of IP-10 and anti-PGL-I IgM was performed following a two-phase protocol described for single analyte detection (18, 24, 25). The protocol included a preflow incubation (60 min, 37°C, 900 rpm) of 10 μl 100-fold-diluted sample with 90 μl LF assay buffer containing 100 ng of the UCPαIP-10 conjugate and 100 ng of the UCPαIgM conjugate (18).
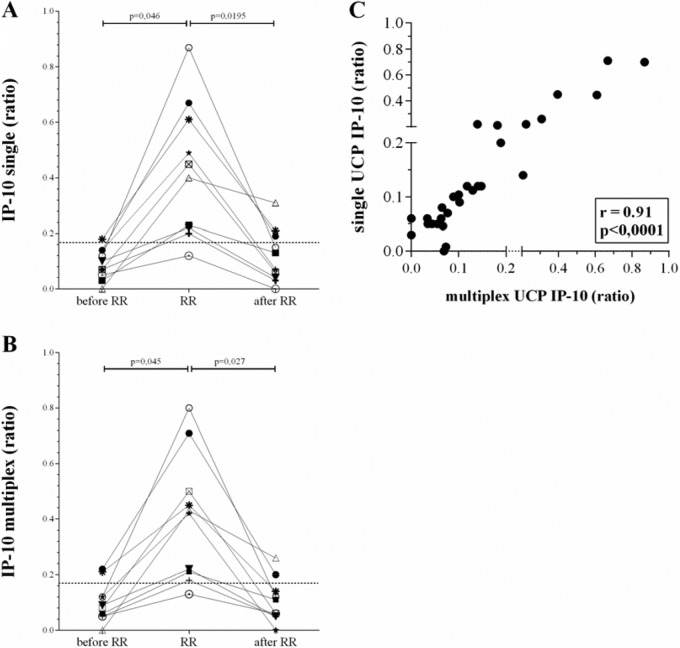
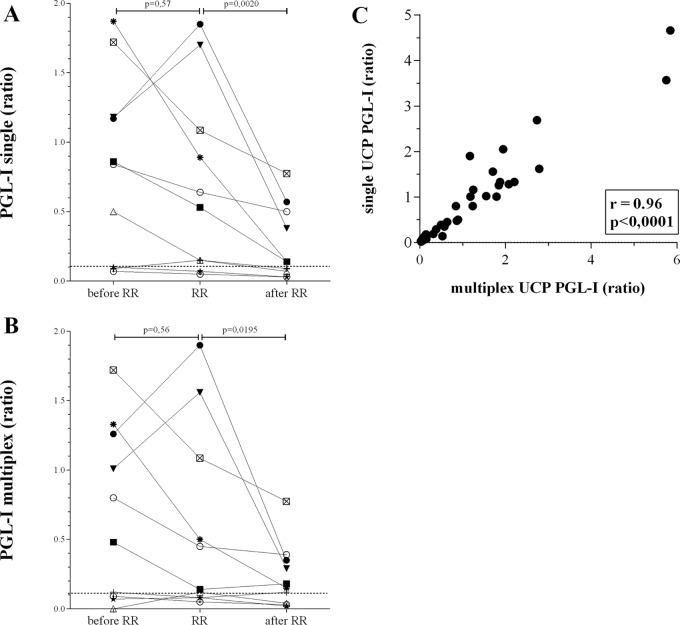
The serum levels of IP-10 measured with the multiplex UCP-LFA at RR onset differed between the patients but were all significantly higher than those in the absence of reactions (P = 0.045 [Fig. 1]). Similarly, IP-10 concentrations were significantly reduced after treatment (P = 0.027). On the other hand, the anti-PGL-I IgM levels detected with the multiplex UCP-LFA did not identify the onset of RRs, which is in agreement with our previous findings (13). Seven patients were positive for anti-PGL-I IgM (range, 0.5 to 1.87) at diagnosis, but at RR onset, only two patients, males from Bangladesh (aged 32 years; bacterial index [BI], ≥2) and Brazil (aged 25 years; BI, ≥3.2), showed increased antibody levels. These patients were not different from the others in BI or age (Table 1). However, serology clearly allowed monitoring of treatment efficacy for patients who were seropositive at RR onset, since levels were significantly reduced after treatment (P = 0.0195). Our data show that single and multiplex UCP-LFAs correlated well for IP-10 (r = 0.91 [Fig. 1C]) and anti-PGL-I IgM (r = 0.96 [Fig. 2C]), demonstrating no relevant interference between the two biomarkers.
FIG 1.
Comparison between single and multiplex IP-10 UCP-LFAs. UCP-LFAs for detection of IP-10 were performed in a single (A) or multiplex (B) format as described previously (18) using sera from 10 leprosy patients at leprosy diagnosis before MDT in the absence of any clinical sign of reactions and at least 3 months before reaction (before RR), at diagnosis of reaction before steroids (RR), or after MDT and reactional treatment, at least 1 month after the end of steroid use (after RR) (13). Differences in cytokine concentrations between test groups were analyzed with the Wilcoxon matched-pairs signed-rank test for nonparametric distribution using GraphPad Prism version 5.01 for Windows (GraphPad Software, San Diego, CA, USA). The dotted line indicates the limit of detection (LOD) (0.17). The y axis indicates the ratio of the relative fluorescence units (RFUs) measured at the respective test and flow-control lines. The statistical significance level used was P ≤ 0.05. (C) For correlations, the Spearman r was calculated using GraphPad Prism version 5.01.
TABLE 1.
Clinical parameters of leprosy patients with RRa
| Country | Symbolb | BIc | Type | Sex | Age (yrs) | Before RRd |
At RR |
After RR |
|||
|---|---|---|---|---|---|---|---|---|---|---|---|
| PGL-I | IP-10 | PGL-I | IP-10 | PGL-I | IP-10 | ||||||
| Bangladesh | ■ | 2+ | BL | Male | 36 | 0.86 | 0.03 | 0.53 | 0.23 | 0.14 | 0.13 |
| Bangladesh | + | 2+ | BL | Female | 39 | 0.09 | 0.07 | 0.15 | 0.20 | 0.09 | 0.03 |
| Bangladesh | O | 0 | BL | Male | 36 | 0.84 | 0.12 | 0.64 | 0.87 | 0.50 | 0.15 |
| Bangladesh | ▼ | 2+ | BL | Male | 32 | 1.18 | 0.10 | 1.70 | 0.22 | 0.38 | 0.04 |
| Brazil | ⚫ | 3.2+ | BL | Male | 25 | 1.17 | 0.14 | 1.85 | 0.67 | 0.57 | 0.19 |
| Brazil | Δ | 2.42+ | BL | Male | 40 | 0.50 | 0.00 | 0.15 | 0.40 | 0.07 | 0.31 |
| Brazil | * | 4.28+ | BL | Male | 68 | 1.87 | 0.18 | 0.89 | 0.61 | 0.14 | 0.21 |
| Nepal | ⊙ | 0.25+ | BL | Female | 40 | 0.07 | 0.05 | 0.05 | 0.12 | 0.03 | 0.00 |
| Nepal | ★ | 0 | BL | Male | 33 | 0.10 | 0.14 | 0.07 | 0.49 | 0.03 | 0.07 |
| Netherlands | ☒ | 5+ | BL | Male | 17 | 1.72 | 0.07 | 1.09 | 0.45 | 0.78 | 0.06 |
Values for the ratio of the relative fluorescence units (RFUs) measured at the respective test and flow-control lines of single UCP-LFA for anti-PGL-I IgM and IP-10 are shown.
BI, bacterial index.
RR, reversal reaction.
FIG 2.
Comparison between single and multiplex PGL-I UCP-LFAs. UCP-LFAs for detection of anti-PGL-I IgM antibodies were performed in a single (A) or multiplex (B) format as described previously (18) using sera from 10 leprosy patients (see Fig. 1). Differences in IgM levels between test groups were analyzed with the Wilcoxon matched-pairs signed-rank test for nonparametric distribution using GraphPad Prism version 5.01 for Windows (GraphPad Software). The dotted line indicates the LOD (0.11). The y axis indicates the ratio of the relative fluorescence units (RFUs) measured at test and flow-control lines. The statistical significance level used was P ≤ 0.05. (C) For correlations, the Spearman r was calculated using GraphPad Prism version 5.01.
Biomarkers as reliable correlates of disease complications and response to therapy represent essential tools for early diagnosis of disease states in chronic infections. In areas where leprosy is endemic, leprosy reactions are frequently misdiagnosed due to a lack of expertise. The evaluation of this multiplex UCP-LFA shows that this quantitative test, when used for intraindividual monitoring, can aid health care workers in the early diagnosis of reactional episodes, allowing timely treatment. In view of the frequent recurrence of these episodes, this test is particularly useful for monitoring treatment efficacy.
Since acute inflammatory (delayed hypersensitivity) reactions are a frequently occurring, tissue-destroying phenomenon in chronic (infectious) diseases as well as in autoimmune diseases, similar multiplex IP-10 UCP-LFAs adapted, e.g., for the detection of antibodies against tumor necrosis factor alpha (TNF-α) (25) could also be applied to diagnose similar episodes in other diseases, such as rheumatoid arthritis (26), psoriasis (27), and Crohn's disease, (28) for which conscientious, personalized treatment monitoring is also vital.
ACKNOWLEDGMENTS
We gratefully acknowledge the patients for their participation and Sayera Banu (IDDRB, Dhaka), Deanna Hagge (MLR, Kathmandu), Isabela Goulart (National Reference Center for Sanitary Dermatology and Leprosy Uberlandia), and Colette van Hees (EMC, Rotterdam) for organizing the recruitment of leprosy patients.
We declare no financial or commercial conflicts of interest.
REFERENCES
- 1.Scollard DM, Adams LB, Gillis TP, Krahenbuhl JL, Truman RW, Williams DL. 2006. The continuing challenges of leprosy. Clin Microbiol Rev 19:338–381. doi: 10.1128/CMR.19.2.338-381.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Geluk A. 2013. Biomarkers for leprosy: would you prefer T (cells)? Lepr Rev 84:3–12. [PubMed] [Google Scholar]
- 3.Ottenhoff TH, Gonzalez NM, de Vries RR, Convit J, van Rood JJ. 1984. Association of HLA specificity LB-E12 (MB1, DC1, MT1) with lepromatous leprosy in a Venezuelan population. Tissue Antigens 24:25–29. [DOI] [PubMed] [Google Scholar]
- 4.Ottenhoff TH, Elferink DG, Klatser PR, de Vries RR. 1986. Cloned suppressor T cells from a lepromatous leprosy patient suppress Mycobacterium leprae reactive helper T cells. Nature 322:462–464. doi: 10.1038/322462a0. [DOI] [PubMed] [Google Scholar]
- 5.Modlin RL. 1994. Th1-Th2 paradigm: insights from leprosy. J Investig Dermatol 102:828–832. doi: 10.1111/1523-1747.ep12381958. [DOI] [PubMed] [Google Scholar]
- 6.Scollard DM, Martelli CM, Stefani MM, Maroja MF, Villahermosa L, Pardillo F, Tamang KB. 2015. Risk factors for leprosy reactions in three endemic countries. Am J Trop Med Hyg 92:108–114. doi: 10.4269/ajtmh.13-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lockwood DN, Saunderson P. 2012. Nerve damage in leprosy: a continuing challenge for scientists, clinicians and service providers. Int Health 4:77–85. doi: 10.1016/j.inhe.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 8.Ranque B, Nguyen VT, Vu HT, Nguyen TH, Nguyen NB, Pham XK, Schurr E, Abel L, Alcais A. 2007. Age is an important risk factor for onset and sequelae of reversal reactions in Vietnamese patients with leprosy. Clin Infect Dis 44:33–40. doi: 10.1086/509923. [DOI] [PubMed] [Google Scholar]
- 9.Ruhwald M, Dominguez J, Latorre I, Losi M, Richeldi L, Pasticci MB, Mazzolla R, Goletti D, Butera O, Bruchfeld J, Gaines H, Gerogianni I, Tuuminen T, Ferrara G, Eugen-Olsen J, Ravn P. 2011. A multicentre evaluation of the accuracy and performance of IP-10 for the diagnosis of infection with M. tuberculosis. Tuberculosis (Edinb) 91:260–267. doi: 10.1016/j.tube.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 10.Geluk A, Bobosha K, van der Ploeg-van Schip JJ, Spencer JS, Banu S, Martins SB, Cho SN, Franken KL, Kim HJ, Bekele Y, Uddin MK, Abdul HS, Aseffa A, Pessolani MC, Pereira GM, Dockrell HM, Ottenhoff TH. 2012. New biomarkers with relevance to leprosy diagnosis applicable in areas hyperendemic for leprosy. J Immunol 188:4782–4791. doi: 10.4049/jimmunol.1103452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bobosha K, Tang ST, van der Ploeg-van Schip JJ, Bekele Y, Martins MV, Lund O, Franken KL, Khadge S, Pontes MA, Goncalves HS, Hussien J, Thapa P, Kunwar CB, Hagge DA, Aseffa A, Pessolani MC, Pereira GM, Ottenhoff TH, Geluk A. 2012. Mycobacterium leprae virulence-associated peptides are indicators of exposure to M. leprae in Brazil, Ethiopia and Nepal. Mem Inst Oswaldo Cruz 107(Suppl 1):S112–S123. [DOI] [PubMed] [Google Scholar]
- 12.Scollard DM, Chaduvula MV, Martinez A, Fowlkes N, Nath I, Stryjewska BM, Kearney MT, Williams DL. 2011. Increased CXC ligand 10 levels and gene expression in type 1 leprosy reactions. Clin Vaccine Immunol 18:947–953. doi: 10.1128/CVI.00042-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khadge S, Banu S, Bobosha K, van der Ploeg-van Schip JJ, Goulart IM, Thapa P, Kunwar CB, van Meijgaarden KE, van den Eeden SJ, Wilson L, Kabir S, Dey H, Goulart LR, Lobato J, Carvalho W, Bekele Y, Franken KL, Aseffa A, Spencer JS, Oskam L, Otttenhoff TH, Hagge DA, Geluk A. 2015. Longitudinal immune profiles in type 1 leprosy reactions in Bangladesh, Brazil, Ethiopia and Nepal. BMC Infect Dis 15:477. doi: 10.1186/s12879-015-1128-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wergeland I, Pullar N, Assmus J, Ueland T, Tonby K, Feruglio S, Kvale D, Damas JK, Aukrust P, Mollnes TE, Dyrhol-Riise AM. 2015. IP-10 differentiates between active and latent tuberculosis irrespective of HIV status and declines during therapy. J Infect 70:381–391. doi: 10.1016/j.jinf.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 15.van Dam GJ, de Dood CJ, Lewis M, Deelder AM, van Lieshout L, Tanke HJ, van Rooyen LH, Corstjens PL. 2013. A robust dry reagent lateral flow assay for diagnosis of active schistosomiasis by detection of Schistosoma circulating anodic antigen. Exp Parasitol 135:274–282. doi: 10.1016/j.exppara.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zuiderwijk M, Tanke HJ, Sam NR, Corstjens PL. 2003. An amplification-free hybridization-based DNA assay to detect Streptococcus pneumoniae utilizing the up-converting phosphor technology. Clin Biochem 36:401–403. doi: 10.1016/S0009-9120(03)00057-2. [DOI] [PubMed] [Google Scholar]
- 17.Corstjens PL, Chen Z, Zuiderwijk M, Bau HH, Abrams WR, Malamud D, Sam NR, Tanke HJ. 2007. Rapid assay format for multiplex detection of humoral immune responses to infectious disease pathogens (HIV, HCV, and TB). Ann N Y Acad Sci 1098:437–445. doi: 10.1196/annals.1384.016. [DOI] [PubMed] [Google Scholar]
- 18.Bobosha K, Tjon Kon Fat EM, van den Eeden SJ, Bekele Y, van der Ploeg-van Schip JJ, de Dood CJ, Dijkman K, Franken KL, Wilson L, Aseffa A, Spencer JS, Ottenhoff TH, Corstjens PL, Geluk A. 2014. Field-evaluation of a new lateral flow assay for detection of cellular and humoral immunity against Mycobacterium leprae. PLoS Negl Trop Dis 8:e2845. doi: 10.1371/journal.pntd.0002845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Corstjens PL, Zuiderwijk M, Tanke HJ, van der Ploeg-van Schip JJ, Ottenhoff TH, Geluk A. 2008. A user-friendly, highly sensitive assay to detect the IFN-gamma secretion by T cells. Clin Biochem 41:440–444. doi: 10.1016/j.clinbiochem.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corstjens PL, Tjon Kon Fat EM, de Dood CJ, van der Ploeg-van Schip JJ, Franken KL, Chegou NN, Sutherland JS, Howe R, Mihret A, Kassa D, van der Vyver M, Sheehama VJ, Simukonda F, Mayanja-Kizza H, Ottenhoff TH, Walzl G, Geluk A. 2015. Multi-center evaluation of a user-friendly lateral flow assay to determine IP-10 and CCL4 levels in blood of TB and non-TB cases in Africa. Clin Biochem 49:22–31. doi: 10.1016/j.clinbiochem.2015.08.013. [DOI] [PubMed] [Google Scholar]
- 21.Mayboroda OA, van Hooij A, Derks R, van den Eeden SJ, Dijkman K, Khadge S, Thapa P, Kunwar CB, Hagge DA, Geluk A. 2016. Exploratory urinary metabolomics of type 1 leprosy reactions. Int J Infect Dis 45:46–52. doi: 10.1016/j.ijid.2016.02.012. [DOI] [PubMed] [Google Scholar]
- 22.Geluk A, van Meijgaarden KE, Wilson L, Bobosha K, van der Ploeg-van Schip JJ, van den Eeden SJ, Quinten E, Dijkman K, Franken KL, Haisma EM, Haks MC, van Hees CL, Ottenhoff TH. 2014. Longitudinal immune responses and gene expression profiles in type 1 leprosy reactions. J Clin Immunol 34:245–255. doi: 10.1007/s10875-013-9979-x. [DOI] [PubMed] [Google Scholar]
- 23.Ridley DS, Jopling WH. 1966. Classification of leprosy according to immunity. A five-group system. Int J Lepr Other Mycobact Dis 34:255–273. [PubMed] [Google Scholar]
- 24.Corstjens PL, de Dood CJ, van der Ploeg-van Schip JJ, Wiesmeijer KC, Riuttamaki T, van Meijgaarden KE, Spencer JS, Tanke HJ, Ottenhoff TH, Geluk A. 2011. Lateral flow assay for simultaneous detection of cellular- and humoral immune responses. Clin Biochem 44:1241–1246. doi: 10.1016/j.clinbiochem.2011.06.983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Corstjens PL, Fidder HH, Wiesmeijer KC, de Dood CJ, Rispens T, Wolbink GJ, Hommes DW, Tanke HJ. 2013. A rapid assay for on-site monitoring of infliximab trough levels: a feasibility study. Anal Bioanal Chem 405:7367–7375. doi: 10.1007/s00216-013-7154-0. [DOI] [PubMed] [Google Scholar]
- 26.Hanaoka R, Kasama T, Muramatsu M, Yajima N, Shiozawa F, Miwa Y, Negishi M, Ide H, Miyaoka H, Uchida H, Adachi M. 2003. A novel mechanism for the regulation of IFN-gamma inducible protein-10 expression in rheumatoid arthritis. Arthritis Res Ther 5:R74–R81. doi: 10.1186/ar616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levis WR, Martiniuk F. 2013. Psoriasis and leprosy are teaching us T-cell plasticity. J Drugs Dermatol 12:1082. [PubMed] [Google Scholar]
- 28.Grant AV, Alter A, Huong NT, Orlova M, Thuc NV, Ba NN, Thai VH, Abel L, Schurr E, Alcais A. 2012. Crohn's disease susceptibility genes are associated with leprosy in the Vietnamese population. J Infect Dis 206:1763–1767. doi: 10.1093/infdis/jis588. [DOI] [PubMed] [Google Scholar]