Abstract
The startling capacity of the amphibian Spemann organizer to induce naïve cells to form a Siamese twin embryo with a second set of body axes is one of the hallmarks of developmental biology. However, the axis-inducing potential of the blastopore-associated tissue is commonly regarded as a chordate feature. Here we show that the blastopore lip of a non-bilaterian metazoan, the anthozoan cnidarian Nematostella vectensis, possesses the same capacity and uses the same molecular mechanism for inducing extra axes as chordates: Wnt/β-catenin signaling. We also demonstrate that the establishment of the secondary, directive axis in Nematostella by BMP signaling is sensitive to an initial Wnt signal, but once established the directive axis becomes Wnt-independent. By combining molecular analysis with experimental embryology, we provide evidence that the emergence of the Wnt/β-catenin driven blastopore-associated axial organizer predated the cnidarian-bilaterian split over 600 million years ago.
 In vertebrate embryos, Wnt/β-catenin signaling induces an organizer area guiding the formation of body axes and inducing extra axes upon transplantation. Here, Kraus et al. show that Wnt ligands also induce an organizer in a sea anemone, indicating that the organizer dates back over 600 million years.
In vertebrate embryos, Wnt/β-catenin signaling induces an organizer area guiding the formation of body axes and inducing extra axes upon transplantation. Here, Kraus et al. show that Wnt ligands also induce an organizer in a sea anemone, indicating that the organizer dates back over 600 million years.
The ability of the amphibian dorsal blastopore lip to induce ectopic body axes upon transplantation has fascinated biologists for nearly a century1. Although axis-forming blastoporal organizers were long thought to be a chordate-specific feature, transplantation of a fragment of the mid-gastrula blastopore lip in the sea anemone Nematostella vectensis, a member of the early branching non-bilaterian phylum Cnidaria (which, in addition to sea anemones, includes corals, hydroids and jellyfish), also resulted in the formation of an ectopic body axis2, just like the Mangold-Spemann organizer in amphibians. Whether this similarity reflects the homologous or convergent origin of the cnidarian and vertebrate blastoporal axial organizers remained unclear, since the molecular nature of the signal conveying axial organizer properties to an embryonic tissue has not been determined outside deuterostomes.
In the frog Xenopus, the Spemann organizer expresses Wnt and BMP antagonists3, whose microinjection in single vegetal blastomeres can induce ectopic rostral or dorsal structures correspondingly4,5. However, complete ectopic body axes, like the ones seen during transplantation of the dorsal blastopore lip1 or the Nieuwkoop center cells6, nearly never form. In contrast, complete ectopic body axes can be induced by single blastomere injection of Wnt-1, Xwnt-8, dishevelled, β-catenin and dominant negative GSK3β mRNA7,8,9,10, showing that Wnt/β-catenin signaling is crucial for setting up the organizer. Pharmacological activation of Wnt/β-catenin signaling in Nematostella larvae affects the oral-aboral patterning, as detected by changes in the expression of Wnt2, Wnt4, brachyury (Bra) and several other genes11,12,13. Moreover, it also results in the formation of double-headed polyps14, making canonical Wnt signaling the prime candidate for the role of the early axial inducer in Nematostella, similar to the situation in frog. We set out to test whether Wnt signaling is conveying inductive capacity to the blastoporal organizer of Nematostella.
In this paper, using a combination of experimental embryology and molecular analyses, we show that two Nematostella Wnt ligands are capable of conveying axial organizing capacity to any region of the gastrula ectoderm, where they are co-expressed. Our data suggest an ancient origin of the Wnt/β-catenin dependent blastopore-associated axial organizer, predating the cnidarian-bilaterian split.
Results
Wnt1 and Wnt3 convey axial organizer capacity
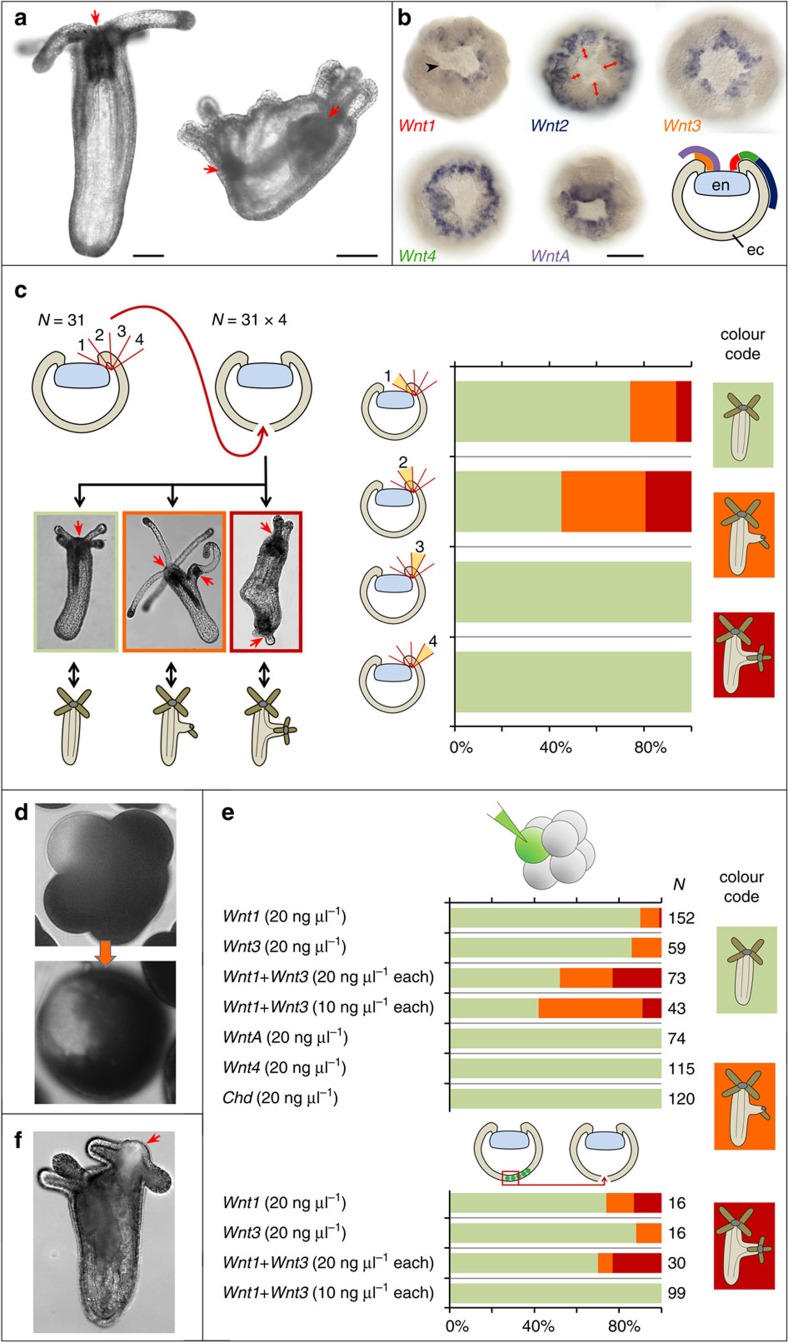
We addressed the role of Wnt signaling in axis formation in Nematostella by performing CRISPR/Cas9-mediated knockout of the putative intracellular Wnt/β-catenin signaling inhibitor APC. Primary polyps mosaic for the APC mutation formed multiple ectopic tentacles and oral openings in F0 (Fig. 1a, Supplementary Fig. 1) suggesting that ectopic activation of Wnt/β-catenin signaling in discrete cell patches is sufficient for the induction of head structures. The Nematostella genome harbors 13 Wnt genes, which are expressed in staggered domains along the oral-aboral axis of the embryo and larva15,16. Since only the blastopore lip is capable of inducing a second axis upon transplantation2, our search for a candidate Wnt was restricted to Wnt genes, which are expressed in the blastopore lip of the Nematostella mid-gastrula. Five Wnt genes are expressed in concentric, partially overlapping ectodermal rings around the blastopore at mid-gastrula: Wnt1, Wnt2, Wnt3, Wnt4 and WntA (Fig. 1b). To further narrow down the list of candidates, we subdivided the donor blastopore lips into four minute fragments along the oral-aboral axis and tested whether a certain part of the lip is particularly efficient in inducing secondary axes. We found that fragments immediately next to the bend of the lip were the most potent inducers (Fig. 1c). These fragments express Wnt1, Wnt3, WntA and, possibly, Wnt4, but not Wnt2, making the first four molecules the candidates for the role of head inducers. We then injected plasmids, where each of the four candidate Wnt genes was placed downstream of the EF1α promoter, into single blastomeres of the embryos at 8- or 16-cell stage. Dextran-Alexa488 fluorescence shows (Fig. 1d) that such injections result in the formation of a randomly located coherent patch of Wnt-expressing cells. While injections of 20 ng μl−1 solution of Wnt4 and WntA plasmids were unable to induce the formation of the secondary axis, Wnt1 and Wnt3 plasmid injections resulted in the formation of incomplete secondary axes in 9–14%, respectively (Fig. 1e, Supplementary Fig. 2). An incomplete secondary axis represents a lateral outgrowth with tentacles and, sometimes, pharynx, but not connected with the mesentery system of the polyp. Interestingly, co-injection of Wnt1 and Wnt3 (both, at 20 and 10 ng μl−1 each) led to a much stronger induction, increasing the rate of ectopic axis formation to about 50%, including a large number of complete ectopic axes with tentacles, pharynx and contractile mesenteries connected with the mesenterial system of the host polyp (Fig. 1e, Supplementary Fig. 2). Moreover, transplantation of fluorescent fragments of the aboral tissue ectopically expressing Wnt1 and/or Wnt3 was able to induce formation of the secondary axes (in 30% of the cases), while this part of the embryo is absolutely devoid of inductive capacity in the uninjected control embryos (Fig. 1e). Similar to the frog embryo, the blastopore lip of the Nematostella early and mid-gastrula also expresses BMP antagonist chordin (Chd)17. However, ectopic overexpression of Chd by single blastomere injection does not induce ectopic axes (n=0/120) (Fig. 1e). We conclude that Wnt/β-catenin signaling acts as head inducer in Nematostella, and Wnt1 and Wnt3 appear to act synergistically in conveying the inductive capacity to the blastoporal organizer tissue. Unfortunately, fluorescent dextran used as injection tracer is not detectable in the primary polyps. In order to test whether the progeny of the EF1α::Wnt1/EF1α::Wnt3 injected blastomere becomes part of the induced ectopic axis, we added the EF1α::mOrange2 plasmid to the injection mix and showed that the mOrange2-expressing tissue contributes to the pharynx and the hypostome of the induced secondary head (Fig. 1f).
Figure 1. Ectopic expression of Wnt1 and Wnt3 induces ectopic body axes.
(a) CRISPR-Cas9 knockout of APC results in the formation of ectopic tentacles and pharynges in F0. Left: wild type polyp; right: mosaic mutant polyp. Red arrows—pharynges. (b) Five ectodermal Wnt genes are expressed at mid-gastrula, however, Wnt2 is expressed at a distance to the bend of the blastopore lip (red double-headed arrows). Gene names are colour-coded as on the scheme showing a lateral view on a mid-gastrula with ectodermal Wnt expression domains depicted as coloured lines. en—invaginating endoderm, ec—ectoderm. Black arrowhead points at the bend of the blastopore lip. (c) Results of transplantation of four sequential blastopore lip fragments from donor gastrulae (N=31) to four different recipient gastrulae (N=31 × 4). Possible developmental outcomes: no axis duplication (green bars on graph), incomplete axis duplication (an outgrowth with tentacles and, sometimes, pharynx but without mesenteries; orange bars on graph), complete axis duplication (two contractile axes with head structures; red bars on graph). Red arrows—pharynges. Fragment 1 and fragment 2, closest to the bend of the blastopore lip, are inductive. (d) Co-injection of a plasmid with EF1α promoter driving the expression of a gene of interest and Dextran-Alexa488 into single cells in 8–16 cell stage embryos results in formation of a coherent patch of fluorescent cells. (e) Some embryos injected into single blastomeres at 8–16 cell stage with Wnt1 or Wnt3 or with both these Wnt's develop ectopic axes. WntA, Wnt4 and Chd never induce a second axis. Fluorescent aboral ectoderm from Wnt1 and Wnt3 injected embryos also acquires inductive capacity. The colour code of the bars is the same as on (c). (f) If EF1a::mOrange2 is co-injected with the Wnt plasmids, mOrange2 fluorescence is observed in the induced secondary head (red arrow). Scale bars: 100 μm.
β-catenin signaling defines the location of the organizer
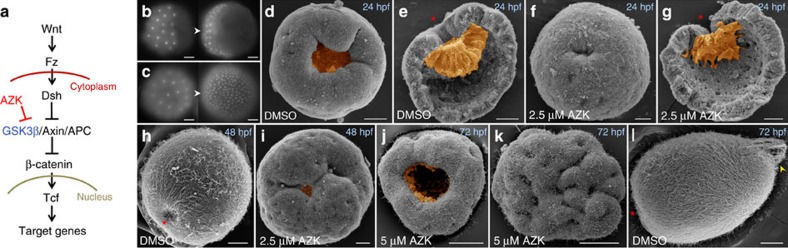
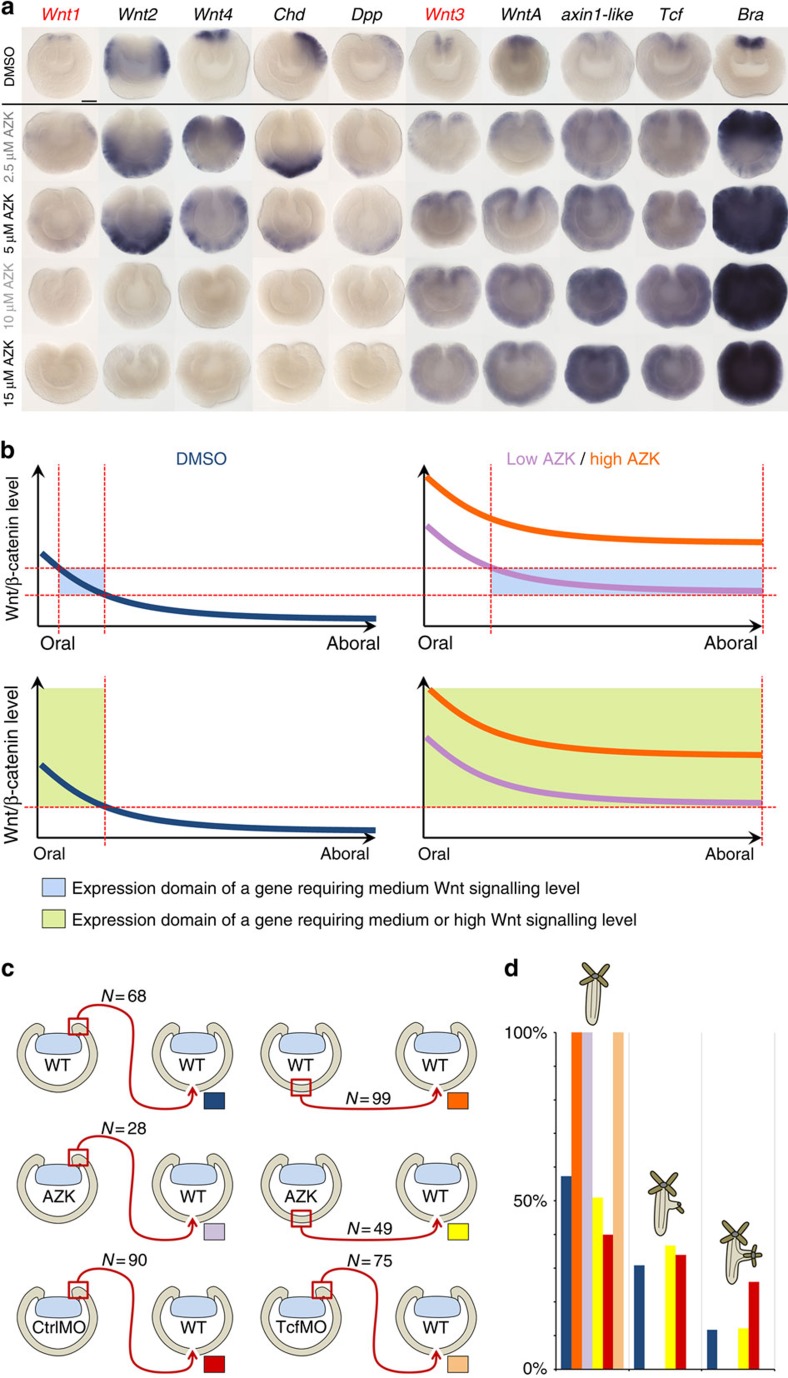
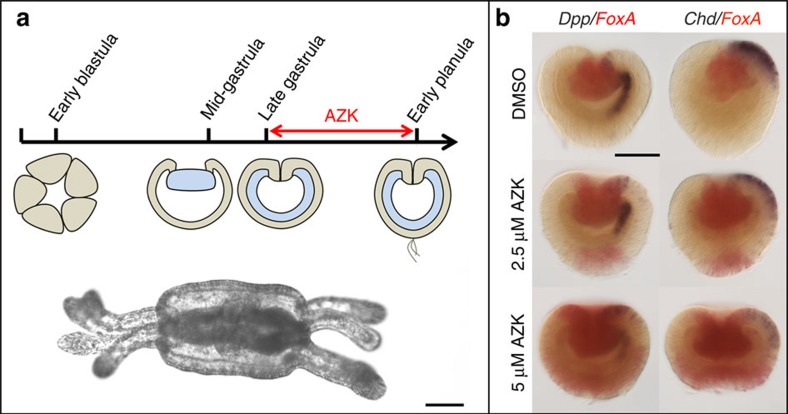
To get insight into the molecular response of the embryonic tissue and, particularly, the organizer to the elevated Wnt/β-catenin signaling in Nematostella, we modulated the Wnt/β-catenin signaling (Fig. 2a–c) by inhibiting GSK3β kinase with 1-Azakenpaullone (AZK) at different times during embryonic development. The treatment with 2.5 μM AZK from early cleavage to mid-gastrula (hereafter referred to as early treatment) resulted in the formation of gastrulae with smaller blastopores and pre-endodermal plates (Fig. 2d–g, Supplementary Fig. 3); however, the process of gastrulation was not perturbed. By day 2 (a stage corresponding to the early planula in controls), these AZK treated embryos flattened and their blastopore openings expanded (Fig. 2h,i). If higher concentrations of AZK were used (5 μM or more), additional openings appeared on their aboral sides (Fig. 2j–l, Supplementary Fig. 3). These severely oralized embryos did not develop further and died after several days. We then assayed the expression of the ectodermal Wnt genes and the putative Wnt signaling targets axin1-like, Tcf and Bra in the gastrulae subjected to early treatment with different concentrations of AZK. While 1 μM AZK treatments did not cause noticeable change in the expression of all the assayed genes, the treatments with 2.5, 5, 10 and 15 μM AZK showed a clear difference in the reaction of these genes to AZK treatment. Wnt3, WntA, axin1-like, Tcf and Bra were upregulated throughout the embryo (Fig. 3a, Supplementary Fig. 4, Supplementary Table 1), suggesting that these genes are activated by medium as well as high level of Wnt/β-catenin signaling (Fig. 3b). In contrast, in increasing AZK concentrations, Wnt1, Wnt2 and Wnt4 started to be upregulated in more and more aboral positions, while vacating the oral domain and finally disappearing from the embryo (Fig. 3a, Supplementary Fig. 4, Supplementary Table 1). This finding suggests that Wnt1, Wnt2 and Wnt4 are activated by medium levels but repressed, either directly or indirectly, by high levels of Wnt/β-catenin signaling (Fig. 3b). To control for the possibility that the observed changes in the expression were due to the described off-target effect of high concentrations of AZK on cyclin dependent kinase 1 (CDK1) rather than on GSK3β18, we treated the embryos with the CDK1/CDK2/CDK5 inhibitor Aminopurvalanol A (AMPV) in the same time window as the early AZK treatment, i.e., from early cleavage to mid-gastrula. As expected, higher concentrations of AMPV arrested the development, either immediately after addition of the drug (5 or 10 μM AMPV) or several cell cycles later (2 μM AMPV), and resulted in the death of the embryos by the time gastrulation had to start. We then used a sub-lethal concentration of AMPV (1 μM), in which the embryos were able to gastrulate, and performed α-phosphohistone H3 antibody staining with 1 μM AMPV treated embryos, 15 μM AZK treated embryos and DMSO control embryos at the gastrula stage. Metaphase plates were observed in all three treatments (Supplementary Fig. 5a), however no shifts in the expression domains of Wnt2, Wnt4 and Bra could be detected in the AMPV treated embryos (Supplementary Fig. 5b), suggesting that the observed effects of the AZK treatment on gene expression (Fig. 3a) were due to the inhibition of GSK3β.
Figure 2. Early AZK treatment results in formation of severely oralized embryos.
(a) AZK upregulates Wnt/β-catenin signaling by inhibiting GSK3β. (b) Nuclear translocation of β-catenin-venus fusion protein on one side in 6 and 9 h post fertilization (hpf) embryos injected with β-catenin-venus mRNA. (c) Nuclear β-catenin-venus in all cells at 6 and 9 hpf of AZK treated blastulae. (d,e) SEM of control 24 hpf mid-gastrulae; oral (d) and lateral (e) views. Note large blastopore and pre-endodermal plate. (f,g) Small blastopore and pre-endodermal plate on SEM of mid-gastrulae after early treatment with 2.5 μM AZK. Gastrulae on (e) and (g) were split into halves to make inner structures visible. (h,i) Control 48 hpf planula (h lateral view) and a planula subjected to the early 2.5 μM AZK treatment (i, oral view). In treated planula, blastopore starts to re-open and small pits appear throughout the surface. (j–l) oral (j) and aboral (k) views of the 72 hpf planula after early 5 μM AZK treatment and of the control 72 hpf planula (l). The oral surface of the treated embryo carries a re-opened blastopore (j), the aboral surface shows multiple folds and holes. The embryo fails to elongate and form aboral structures (e.g., apical tuft - yellow arrowhead on (l)). Scale bars: 30 μm. Endoderm highlighted orange. Red asterisks on lateral views denote blastopores.
Figure 3. Organizer capacity shifts to follow changes in Wnt1 and Wnt3 expression.
(a) Expression of Wnt1, Wnt2, Wnt4, Chd and Dpp expands aborally while clearing from the oral domain and disappears with the increasing AZK concentrations; expression of Wnt3, axin1-like, Tcf and Bra expands globally and reaches saturation. Blastopores point up; scale bar: 50 μm. (b) Model of expression of a gene activated at medium Wnt/β-catenin signaling levels (upper row; blue highlight represents the range of Wnt/β-catenin signaling levels when the gene can be expressed) versus a gene activated at medium and high Wnt/β-catenin signaling levels (lower row; green highlight represents the range of Wnt/β-catenin signaling levels when the gene can be expressed) in the AZK treatments. Blue curve: wild type level of Wnt/β-catenin signaling along the oral-aboral axis. Lilac curve: Wnt/β-catenin signaling level in a low concentration of AZK. Orange curve: Wnt/β-catenin signaling level in a high concentration of AZK. Red dashed lines mark the Wnt/β-catenin signaling thresholds and the corresponding positions on the oral-aboral axis where these thresholds are reached. (c,d) Scheme (c) and developmental outcomes (d) of transplantation of wild type (wt) and 2.5 μM AZK treated (AZK) blastopore lips and aboral ectoderm fragments as well as blastopore lips of the CtrlMO and TcfMO injected gastrulae into wild type recipient gastrulae. Coloured rectangles on (c) correspond to the colours of the bars on (d). Only untreated or CtrlMO blastopore lips and AZK treated aboral ectoderm fragments are inductive.
To test whether the change of expression domains of Wnt1 and Wnt3 in AZK treated embryos correlated with the changes in the inductive capacity of the gastrula tissue, we performed transplantations using donor embryos subjected to the early treatment with 2.5 μM AZK. Strikingly, and concomitant with the loss of Wnt1 expression in the blastopore lip in the AZK treated embryos, transplanted blastopore lips of the AZK treated embryos were completely unable to induce secondary axes in the recipients (Fig. 3c,d). However, the normally non-inductive aboral tissue, which in the AZK-treated embryos represents the tissue co-expressing Wnt1 and Wnt3 (Fig. 3a), could induce ectopic axis formation in the recipients just like the wild type blastopore lips (Fig. 3c,d), suggesting a shift of the organizer capacity from the blastopore lip to an aboral location. Further supporting the role of Wnt/β-catenin signaling in organizer formation, morpholino knockdown of Tcf, the conserved transcriptional co-factor of β-catenin, abolished the inductive capacity in the morphant blastopore lip (Fig. 3c,d).
Establishment of the inductive territory
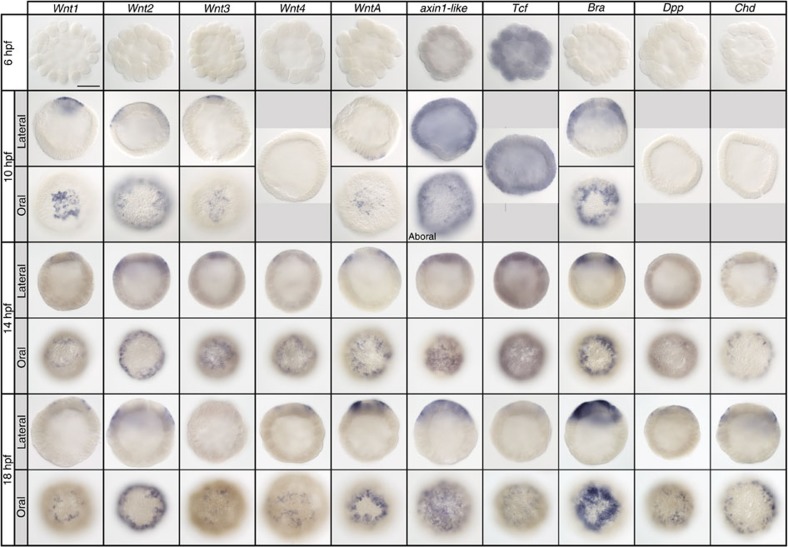
Our data clearly show that axial organizer formation in Nematostella is a Wnt/β-catenin signaling dependent process. It has been previously demonstrated that nuclear β-catenin starts to accumulate at the future oral side of the Nematostella embryo already during early cleavage19 and that Dishevelled protein is localized at the animal pole of the unfertilized egg20. To get further insight into the establishment of the future inductive territory during development, we analyzed the expression of Wnt1, Wnt2, Wnt3, Wnt4, WntA, axin1-like, Tcf and Bra from early blastula to pre-gastrula in the wild type embryos (Fig. 4). Tcf, is strongly expressed in the 6 h post fertilization (hpf) blastula (although we did not see an accumulation at the future oral pole as previously suggested16), and axin1-like, is weakly expressed at 6 hpf. In contrast, the ectodermal Wnt genes (except Wnt4) become detectable in 10 hpf blastulae, together with Bra (Fig. 4). Interestingly, Wnt1, Wnt3 and WntA are first expressed in a patch of cells and only later refine into a ring, while Wnt2 and Bra are detected as a ring from the start. The early expression patterns of Wnt1, Wnt3 and WntA suggest that they are either involved in the activation of the genetic programme defining the pre-endodermal plate or co-regulated by the same early β-catenin input that activates the endoderm determination programme throughout Eumetazoa19,20,21,22,23,24,25. Once the endodermal territory is determined, Wnt1, Wnt3 and WntA expression becomes displaced out of the prospective endodermal cells, likely by a yet unknown negative feedback mechanism, and becomes confined to the ectodermal tissue surrounding the endoderm. These ectodermal cells will become the axial organizer.
Figure 4. Establishment of the oral inductive territory in Nematostella.
At 10 hpf Wnt1, Wnt2, Wnt3 and WntA are the first Wnt genes to be expressed. Wnt2 expression starts as a ring excluding the presumptive pre-endodermal plate. Wnt1, Wnt3 and WntA expression starts as a patch in the presumptive pre-endodermal plate and by 14–18 hpf begins to form a ring around it. Brachyury expression is first detectable as a ring at 10 hpf. Wnt4, Dpp and Chd start to be expressed in a ring around the pre-endodermal plate by 14 hpf. On lateral views, animal/oral ends of the embryos point up. Scale bar: 100 μm.
Wnt/β-catenin signaling and the directive axis
In the vertebrate organizer, the initial β-catenin signal activates the expression of BMP antagonists such as chordin, thus suppressing ventral fates3. Anthozoan cnidarians, unlike their radially symmetric hydroid and jellyfish relatives, possess not only the oral-aboral body axis but also a second, directive axis, orthogonal to the oral-aboral axis. The key players for the establishment of the directive axis are the bmp4 homologue Dpp and the chordin homologue Chd26. Their initially radial expression starts at 14 hpf (Fig. 4) and shifts to the same side of the directive axis upon BMP signaling-dependent symmetry break in late gastrula17,26. It has been previously shown that Dpp and Chd is negatively affected by suppression of Wnt/β-catenin signaling by iCRT14 (ref. 11), and microarray analysis showed that both genes react to 10 μM AZK13. Interestingly, the expression of Dpp and Chd reacted to the early AZK treatment in a similar way as the expression of Wnt1, Wnt2 and Wnt4, displaying an aboral expansion at lower AZK concentrations and disappearing at higher AZK concentrations, indicative of the lack of directive axis (Fig. 3a, Supplementary Fig. 5). The disappearance of Chd expression was previously described11 for a different GSK3β inhibitor, alsterpaullone (ALP), although only one concentration of ALP was used. Surprisingly, we did not observe the reported upregulation11,13 and ubiquitous expansion11 of Dpp expression in early AZK treatment. The effect of the early AZK treatment is in stark contrast to the effects of the late AZK treatment (24 h incubation in 2.5 or 5 μM AZK starting from late gastrula), which we performed after the time of blastoporal organizer formation and directive axis establishment. Late AZK treatment did not prevent larva development and primary polyp formation, but resulted in the oral transformation of the aboral end of the embryo. Notably, such embryos never developed ectopic axes laterally, but rather re-specified the aboral half of their oral-aboral axis to acquire oral fates (Fig. 5a). Strikingly, late AZK treatment did not affect the asymmetric expression of Dpp and Chd (Fig. 5b, Supplementary Fig. 4, Supplementary Table 2), while the expression of the oral marker FoxA27 clearly demonstrated the transformation of the aboral domain into an oral domain (Fig. 5b). These observations suggest that the initial, radially symmetric, circumblastoporal expression of Dpp and Chd might rely on the correct intensity of β-catenin signaling, however, Dpp and Chd expression becomes self-regulating and does not require Wnt/β-catenin input, once the directive axis is established.
Figure 5. Late AZK treatment affects the oral-aboral but not the directive axis.
(a) Scheme of late AZK treatment and a resulting double-headed primary polyp. (b) An ectopic domain positive for the oral marker FoxA appears on the aboral end of the planula subjected to late AZK treatment, however, unilateral expression of Dpp and Chd in treated embryos is retained. Blastopores point up. Scale bars: 100 μm.
Discussion
In this paper, we showed that the key molecules conveying the inductive capacity to the Nematostella organizer are Wnt1 and Wnt3. Their co-expression in any region of the ectoderm of the gastrula following the single blastomere injection appears to be sufficient for the tissue to acquire axial organizer properties. If expression domains of Wnt/β-catenin signaling dependent genes in the mid-gastrula are modulated by AZK treatment, the inductive capacity is lost in the blastopore lip and becomes confined to the aboral ectoderm, correlated with the shifts in the Wnt1 and Wnt3 expression. Our analysis of the onset of Wnt1 and Wnt3 expression in the wild type Nematostella embryos showed that their early expression is first observed not in a ring, but in a patch of cells in the 10 hpf blastula, and only later it starts to refine into a ring surrounding the presumptive pre-endodermal plate. It seems reasonable to predict that transplantation of a patch of Wnt1 and Wnt3 expressing cells from a 10 hpf blastula (Fig. 4) into an aboral position of a recipient embryo would also lead to axis induction. Currently, due to the lack of morphological landmarks in the blastula, such experiment is not possible, however, once transgenic lines expressing a fluorescent reporter under control of Wnt1 or Wnt3 regulatory elements are generated, it will be feasible to test whether these early presumptive endodermal cells expressing Wnt1 and Wnt3 already have the inductive capacity, reminiscent of the Nieuwkoop center cells6, which are also located in the endodermal territory in the frog embryo.
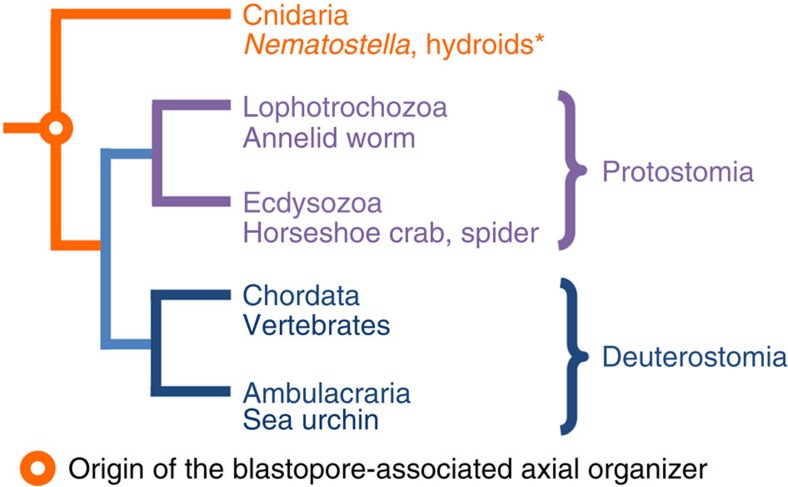
In summary, Wnt/β-catenin signaling dependent blastopore lip organizers are capable of inducing twin new body axes in the animals evolutionary as far apart as cnidarian Nematostella and vertebrates. Axial organizer capacity has been reported for other cnidarians as well. The swimming posterior pole of hydrozoan planulae, which corresponds to the blastoporal end of the sea anemone planula, is capable of inducing ectopic axes28,29. Also, the hypostome (oral cone) tissue of the solitary hydroid polyp Hydra, as well as of its colonial relative Clava, has axial organizer capacity28,30, and this adult organizer capacity in Hydra is Wnt/β-catenin dependent31,32. Maybe due to the difficulty of performing transplantation experiments or because inductive capacity was not expected in embryos, whose body axes are established maternally, or whose development is invariant, little is known about the axial organizing capacity of the blastopore-associated tissue in non-chordate Bilateria. However, there is evidence suggesting that blastopore-associated axial organizers also exist in the non-chordate deuterostomes as well as in the ecdysozoan and lophotrochozoan protostomes. Grafting of the micromeres from the 16-cell sea urchin embryo from the vegetal pole into the animal position induces the formation of the twin gut and a complete second set of larval axial structures33. The sea urchin micromere lineage is characterized by strong nuclear β-catenin from the fifth cleavage cycle on24. Grafting of the center cells from the vicinity of the blastopore in a horseshoe crab, which is an early-branching chelicerate arthropod, results in the formation of larvae with complete duplicated axes34. The same effect is seen in cumulus transplantations in a spider35. Moreover, even in the oligochaete annelid Tubifex—displaying a highly invariant, cell lineage based development—grafting of the 2d11 and 4d blastomeres into an ectopic position also results in the formation of ectopic axes36, although concerns have been raised whether the grafted cells actually induce the host cells to contribute to the formation of the ectopic axis or whether they form the second axis autonomously37. A more recent blastomere ablation study on a different annelid, the polychaete Capitella, showed that the 2d somatoblast emits a signal required for trunk formation and the D-V axis organization in the head37, although the authors did not perform transplantations of the 2d cell, and the molecular nature of the signal remains unknown37. In general, the D-quadrant and, particularly, the 4d cell and its descendants in Spiralia are the site of nuclear β-catenin stabilization21,22. In the invaginating gastrulae of the species with homoquadrant cleavage these cells are located at the dorsal margin of the blastopore38. However, the possible role of β-catenin in the formation of axial organizers in protostome Bilateria requires further thorough analysis. Taken together, our study strongly suggests an ancient origin of the blastopore-associated axial organizer (Fig. 6) based on Wnt/β-catenin signaling, which was maintained in the cnidarian, deuterostome and, possibly, in several protostome lineages.
Figure 6. The origin of the blastopore-associated axial organizer.
In hydroids (marked by *), organizing capacity is detected in the oralmost tissue: the swimming posterior tips of the planula larvae and the hypostomes of polyps.
Methods
Animal culture and plasmid microinjection
Animal husbandry was performed as described39. Briefly, adult polyps were kept in Nematostella medium (16‰ artificial sea water) at 18 °C in the dark. Induction of spawning was performed by placing the animals into 25 °C illuminated incubator for 10 h. Developing embryos were kept at 21 °C. Plasmids carrying different untagged Nematostella Wnt's and mOrange2 driven by EF1α promoter were injected40 into individual blastomeres at 8–16 cell stage. Fluorescent Dextran-Alexa488 (Life Technologies) was co-injected as tracer.
CRISPR-Cas9 mediated mutagenesis
Single guide RNAs for CRISPR-Cas9 mediated mutagenesis of Nematostella APC (Genbank KT381584) were synthesized41 using the following oligos: 5′TAGGCACAGCTATGAGGGCCAC, 5′AAACGTGGCCCTCATAGCTGTG. nls-Cas9 protein was obtained from PNA bio (Thousand Oaks, CA, USA). 500 ng/μl single guide RNA and 1.5 μg/μl nls-Cas9 were injected into single cell embryos42. Control embryos were injected with 500 ng/μl single guide RNA alone. DNA was prepared from individual polyps and a 248 bp long fragment containing the recognition sequence of the guide RNA was amplified using the following oligos: 5′AGAATCCTGCAGAAGATGAACA, 5′ CCTGGCATACAAAGGTGACA. For the controls, 10 animals were pooled prior to DNA preparation. PCR products harboring the mutation site were directly sequenced by Microsynth (Balgach, Switzerland), and sequencing chromatograms were analyzed to assess the presence of the discrepancies in comparison to the wild type sequence.
Inhibitor treatments and morpholino experiments
1-Azakenpaullone (Sigma) and Aminopurvalanol A (abcam) treatments were performed in the time windows and with concentrations specified in the main text. Tcf knockdown was performed by injecting a previously published antisense translation blocking morpholino11,13 (TcfMO; 5′ CTGAGGCATACTACCGTCTCATGTG) into fertilized eggs at 500 μM concentration. A morpholino recognizing the splice acceptor in front of the exon 9 of Nvmef2, which does not produce any noticeable developmental phenotype43, was used as control (CtrlMO; 5′ GATGTGCCTAGGGTACAACAACAAT) at the same concentration. Transplantation of different fragments of the gastrulae was performed as previously described2. Briefly, the fragments of the donor gastrula tissue were excised with a feather microscalpel (Science Services, blade angle 30° or 45°) and implanted into small windows cut out in the aboral pole of the recipient gastrulae under a dissecting scope (Nikon SMZ745). For transplantation of the fluorescently labeled tissue, a fluorescent dissecting scope (Nikon SMZ18) was used. During and after transplantation, the embryos were kept in Nematostella medium.
In situ hybridization and morphological analysis
In situ hybridization was performed as described44. Briefly, embryos from cleavage stage till gastrula were fixed for 1 min in 2% glutaraldehyde/3.7% formaldehyde in Nematostella medium (NM) and then for an additional hour in 3.7% formaldehyde/NM. Older embryos and larvae were fixed for 1 min in 0.2% glutaraldehyde/3.7% formaldehyde in Nematostella medium and then for an additional hour in 3.7% formaldehyde/NM. After 5 PTw (1x PBS with 0.1% Tween 20) washes, the embryos were transferred into methanol, then rehydrated with PTw and treated for 3 min with 80 μg/ml proteinase K (Ambion). After stopping the digest with two 4 mg/ml Glycin/PTw washes, the embryos were washed four times in 1% triethanolamine/PTw for 5 min. The last two triethanolamine washes contained 3 μl/ml and 6 μl/ml of acetic anhydride. After two additional PTw washes, the samples were post-fixed for 1 h in 3.7% formaldehyde/PTw, washed 5x with PTw, prehybridized at 60 °C for 2 h in the Hybe buffer (50% formamide, 5 × SSC pH4.5, 1% SDS, 0.1% Tween 20, 50 μg/ml heparin, 5 mg/ml Torula yeast RNA) and then hybridized at 60 °C for 36–60 h in Hybe containing 0.5 ng/μl of the Digoxigenin or FITC-labeled antisense RNA probe against Wnt1 (nucleotides 44–1135 of Genbank AY530300), Wnt2 (full length Genbank AY725201), Wnt3 (nucleotides 11–1051 of Genbank DQ492689), Wnt4 (nucleotides 53–1114 of Genbank AY687348), WntA (nucleotides 35–1129 of Genbank AY534532), Bra (nucleotides 52–1222 of Genbank AF540387), axin1-like (nucleotides 1–1123 of Genbank JQ959548), Tcf (nucleotides 1–1191 of Genbank DQ497247), Dpp (nucleotides 134–1267 of Genbank AY363391), Chd (nucleotides 1768–2584 of Genbank DQ286294) or FoxA (nucleotides 233–1093 of Genbank AY457634). After gradually transferring the samples from Hybe into 2 × SSCT (2 × SSC with 0.1% Tween 20) in three 30 min steps at 60°C, the embryos were washed once for 30 min with 2 × SSCT and three times for 30 min with 0.05 × SSCT at 60 °C. Then the samples were briefly washed with PTw and blocked in 1% Blocking reagent (Roche) in MAB for 1 h at room temperature. Then the samples were incubated overnight at 4 °C with alkaline phosphatase conjugated Anti-Dig or Anti-FITC Fab fragments (Roche) diluted 1:2000 in the 1% Blocking reagent/MAB, washed 10 times for 15 min in PTw and twice for 5 min in the buffer for alkaline phosphatase and stained with NBT/BCIP (Roche) or Fast Red (Roche). Double in situ staining was performed as described45. Briefly, after staining overnight at 4 °C with anti-FITC Fab fragments conjugated with alkaline phosphatase, washing 10 times for 15 min in PTw and performing the first substrate reaction with Fast Red according to the protocol of the manufacturer, the samples were rinsed twice in PTw, and alkaline phosphatase was inactivated by a 10 min wash in 100 mM glycine-HCl (pH 2.2) at room temperature. Then, the samples were rinsed twice in PTw, re-blocked in 1% Blocking reagent/MAB, stained with the anti-Dig Fab fragments conjugated with alkaline phosphatase overnight at 4 °C, washed 10 times for 15 min in PTw and stained with NBT/BCIP. After staining, the samples were rinsed with water, washed with absolute ethanol (this step has to be done carefully if ethanol-sensitive Fast Red staining was performed), then rinsed with PTw and embedded in 86% glycerol. In situ patterns were imaged and quantified under a Nikon 80i compound microscope.
Semithin sectioning and SEM was performed as specified46. Briefly, the embryos were fixed in 2.5% glutaraldehyde/0.1 M cacodylate buffer (pH 7.2) and stored in 1.25% glutaraldehyde/0.1 M cacodylate buffer. Then they were washed with 0.1 cacodylate buffer, post-fixed for 1 h in 1% OsO4/0.1 M cacodylate buffer, washed, dehydrated in ethanol and acetone and either dried by critical point technique and sputter coated for SEM or embedded into a standard mixture of Araldite and Epon (Electron Microscopy Sciences) for semithin sectioning. 1 μm semithin sections were stained with 1% toluidine blue and imaged under the Nikon 80i compound microscope. SEM was performed with the JEOL JSM-6380LA or CamScan S2 microscopes.
Antibody staining
Rabbit anti-phospho-Histone H3 (Ser10) antibody (Merck Millipore) was used in 1:400 dilution using the antibody staining protocol from47. Briefly, the embryos were fixed in 4% paraformaldehyde/PBS-TT (1xPBS, 0.2% Triton X100, 0.2% Tween 20), washed five times in PBS-TT, blocked in 20% heat inactivated sheep serum (Sigma)/1% BSA in PBS-TT, and incubated with the primary antibody overnight at 4 °C. After eight 15 min PBS-TT washes, the embryos were blocked again and incubated with the goat anti-rabbit Alexa568 (Life Technologies) at 1:1000 dilution overnight. DAPI was added at the final concentration of 300 nM together with the secondary antibody to counterstain chromatin. The samples were then washed again eight times 15 min with PBS-TT, embedded in Vectashield (Vectorlabs) and imaged under a Leica SP5X CLSM.
Data availability
The data supporting the findings of this study are available within the article and its Supplementary Information. Any further relevant data concerning the techniques used in the paper are available on request.
Additional information
How to cite this article: Kraus, Y. et al. Pre-bilaterian origin of the blastoporal axial organizer. Nat. Commun. 7:11694 doi: 10.1038/ncomms11694 (2016).
Supplementary Material
Supplementary Figures 1-5 and Supplementary Tables 1-2
Acknowledgments
This work was funded by Austrian Science Foundation (FWF) grants P22717 (to U.T.) and P26962 (to G.G.). Patrick Steinmetz cloned and tested the EF1α promoter. Y.K. was supported by the Lise Meitner FWF fellowship (M1140-B17). A.A. was supported by an HFSP postdoctoral fellowship (LT000809/2012-L). We are grateful to the Electron Microscopy Laboratory of the Shared Facilities Center of the Lomonosov Moscow State University for the possibility to perform SEM and to the Core Facility for Cell Imaging and Ultrastructure Research of the University of Vienna for the access to the confocal microscope. We thank Claude Desplan, Ira Blitz and Alexandra Schauer for valuable comments on the manuscript.
Footnotes
Author contributions G.G., Y.K. and U.T. designed the experiments, G.G., Y.K. and A.A. performed the experiments. G.G. and Y.K. analyzed data. G.G. wrote the manuscript. All authors edited the manuscript.
References
- Spemann H. & Mangold H. Über Induktion von Embryonalanlagen durch Implantation artfremder Organisatoren. W. Roux's Arch. Entwicklungsmech. Organ 100, 599–638 (1924). [Google Scholar]
- Kraus Y., Fritzenwanker J. H., Genikhovich G. & Technau U. The blastoporal organiser of a sea anemone. Curr. Biol. 17, R874–R876 (2007). [DOI] [PubMed] [Google Scholar]
- De Robertis E. M. & Kuroda H. Dorsal-ventral patterning and neural induction in Xenopus embryos. Ann. Rev. Cell & Dev. Biol. 20, 285–308 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouwmeester T., Kim S., Sasai Y., Lu B. & De Robertis E. M. Cerberus is a head-inducing secreted factor expressed in the anterior endoderm of Spemann's organizer. Nature 382, 595–601 (1996). [DOI] [PubMed] [Google Scholar]
- Sasai Y. et al. Xenopus chordin: a novel dorsalizing factor activated by organizer-specific homeobox genes. Cell 79, 779–790 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimlich R. L. Cytoplasmic localization and chordamesoderm induction in the frog embryo. J. Embryol. Exp. Morphol 89, Suppl 89–111 (1985). [PubMed] [Google Scholar]
- Dominguez I., Itoh K. & Sokol S. Y. Role of glycogen synthase kinase 3 beta as a negative regulator of dorsoventral axis formation in Xenopus embryos. Proc. Natl. Acad. Sci. U.S.A. 92, 8498–8502 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funayama N., Fagotto F., McCrea P. & Gumbiner B. M. Embryonic axis induction by the armadillo repeat domain of beta-catenin: evidence for intracellular signaling. J. Cell. Biol. 128, 959–968 (1995). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sokol S., Christian J. L., Moon R. T. & Melton D. A. Injected Wnt RNA induces a complete body axis in Xenopus embryos. Cell 67, 741–752 (1991). [DOI] [PubMed] [Google Scholar]
- Sokol S. Y., Klingensmith J., Perrimon N. & Itoh K. Dorsalizing and neuralizing properties of Xdsh, a maternally expressed Xenopus homolog of dishevelled. Development 121, 1637–1647 (1995). [DOI] [PubMed] [Google Scholar]
- Watanabe H. et al. Sequential actions of beta-catenin and Bmp pattern the oral nerve net in Nematostella vectensis. Nat. Commun 5, 5536 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlow H., Matus D. Q. & Martindale M. Q. Ectopic activation of the canonical wnt signaling pathway affects ectodermal patterning along the primary axis during larval development in the anthozoan Nematostella vectensis. Dev. Biol. 380, 324–334 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Röttinger E., Dahlin P. & Martindale M. Q. A framework for the establishment of a cnidarian gene regulatory network for ‘endomesoderm' specification: the inputs of β-catenin/TCF Signaling. PLoS Genet. 8, e1003164 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guder C. et al. The Wnt code: cnidarians signal the way. Oncogene 25, 7450–7460 (2006). [DOI] [PubMed] [Google Scholar]
- Kusserow A. et al. Unexpected complexity of the Wnt gene family in a sea anemone. Nature 433, 156–160 (2005). [DOI] [PubMed] [Google Scholar]
- Lee P. N., Pang K., Matus D. Q. & Martindale M. Q. A WNT of things to come: evolution of Wnt signaling and polarity in cnidarians. Sem. Cell & Dev. Biol. 17, 157–167 (2006). [DOI] [PubMed] [Google Scholar]
- Rentzsch F. et al. Asymmetric expression of the BMP antagonists chordin and gremlin in the sea anemone Nematostella vectensis: implications for the evolution of axial patterning. Dev. Biol. 296, 375–387 (2006). [DOI] [PubMed] [Google Scholar]
- Kunick C., Lauenroth K., Leost M., Meijer L. & Lemcke T. 1-Azakenpaullone is a selective inhibitor of glycogen synthase kinase-3 beta. Bioorg. Med. Chem. Lett. 14, 413–416 (2004). [DOI] [PubMed] [Google Scholar]
- Wikramanayake A. H. et al. An ancient role for nuclear beta-catenin in the evolution of axial polarity and germ layer segregation. Nature 426, 446–450 (2003). [DOI] [PubMed] [Google Scholar]
- Lee P. N., Kumburegama S., Marlow H. Q., Martindale M. Q. & Wikramanayake A. H. Asymmetric developmental potential along the animal-vegetal axis in the anthozoan cnidarian, Nematostella vectensis, is mediated by Dishevelled. Dev. Biol. 310, 169–186 (2007). [DOI] [PubMed] [Google Scholar]
- Henry J. Q., Perry K. J. & Martindale M. Q. beta-catenin and early development in the gastropod, Crepidula fornicata. Integr. Comp. Biol. 50, 707–719 (2010). [DOI] [PubMed] [Google Scholar]
- Henry J. Q., Perry K. J., Wever J., Seaver E. & Martindale M. Q. Beta-catenin is required for the establishment of vegetal embryonic fates in the nemertean, Cerebratulus lacteus. Dev. Biol. 317, 368–379 (2008). [DOI] [PubMed] [Google Scholar]
- Lhomond G., McClay D. R., Gache C. & Croce J. C. Frizzled1/2/7 signaling directs beta-catenin nuclearisation and initiates endoderm specification in macromeres during sea urchin embryogenesis. Development 139, 816–825 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Logan C. Y., Miller J. R., Ferkowicz M. J. & McClay D. R. Nuclear beta-catenin is required to specify vegetal cell fates in the sea urchin embryo. Development 126, 345–357 (1999). [DOI] [PubMed] [Google Scholar]
- Imai K., Takada N., Satoh N. & Satou Y. (beta)-catenin mediates the specification of endoderm cells in ascidian embryos. Development 127, 3009–3020 (2000). [DOI] [PubMed] [Google Scholar]
- Saina M., Genikhovich G., Renfer E. & Technau U. BMPs and chordin regulate patterning of the directive axis in a sea anemone. Proc. Natl. Acad. Sci. U.S.A. 106, 18592–18597 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fritzenwanker J. H., Saina M. & Technau U. Analysis of forkhead and snail expression reveals epithelial-mesenchymal transitions during embryonic and larval development of Nematostella vectensis. Dev. Biol. 275, 389–402 (2004). [DOI] [PubMed] [Google Scholar]
- Mayorova T., Kosevich I., Dulin N., Savina E. & Kraus Y. Organizer regions in marine colonial hydrozoans. Zoology 118, 89–101 (2015). [DOI] [PubMed] [Google Scholar]
- Stumpf M. et al. An organizing region in metamorphosing hydrozoan planula larvae - stimulation of axis formation in both larval and in adult tissue. Int. J. Dev.Biol. 54, 795–802 (2010). [DOI] [PubMed] [Google Scholar]
- Browne E. The production of new hydrants by the insertion of small grafts. J. Exp. Zool. 7, 1–37 (1909). [Google Scholar]
- Gee L. et al. beta-catenin plays a central role in setting up the head organizer in hydra. Dev. Biol. 340, 116–124 (2010). [DOI] [PubMed] [Google Scholar]
- Lengfeld T. et al. Multiple Wnts are involved in Hydra organizer formation and regeneration. Dev. Biol. 330, 186–199 (2009). [DOI] [PubMed] [Google Scholar]
- Ransick A. & Davidson E. H. A complete second gut induced by transplanted micromeres in the sea urchin embryo. Science 259, 1134–1138 (1993). [DOI] [PubMed] [Google Scholar]
- Itow T., Kenmochi S. & Mochizuki T. Induction of secondary embryos by intra- and interspecific grafts of center cells under the blastopore in horseshoe crabs. Dev. Growth & Differ 33, 251–258 (1991). [DOI] [PubMed] [Google Scholar]
- Holm Å. Experimentelle Untersuchungen über die Entwicklung und Entwicklungsphysiologie des Spinnenembryos. Zool. BiDr Uppsala 29, 293–424 (1952). [Google Scholar]
- Nakamoto A., Nagy L. M. & Shimizu T. Secondary embryonic axis formation by transplantation of D quadrant micromeres in an oligochaete annelid. Development 138, 283–290 (2011). [DOI] [PubMed] [Google Scholar]
- Amiel A. R., Henry J. Q. & Seaver E. C. An organizing activity is required for head patterning and cell fate specification in the polychaete annelid Capitella teleta: new insights into cell-cell signaling in Lophotrochozoa. Dev. Biol. 379, 107–122 (2013). [DOI] [PubMed] [Google Scholar]
- Woltereck R. Trochophorastudien. I. Über die Entwicklung des Annelides bei den Polygordiusarten in der Nordsee. Zoologica 34, 1–71 (1902). [Google Scholar]
- Genikhovich G. & Technau U. Induction of spawning in the starlet sea anemone Nematostella vectensis, in vitro fertilization of gametes, and dejellying of zygotes. CSH protocols 2009, pdb prot5281 (2009). [DOI] [PubMed] [Google Scholar]
- Renfer E., Amon-Hassenzahl A., Steinmetz P. R. & Technau U. A muscle-specific transgenic reporter line of the sea anemone, Nematostella vectensis. Proc. Natl. Acad. Sci. U.S.A. 107, 104–108 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hwang W. Y. et al. Efficient genome editing in zebrafish using a CRISPR-Cas system. Nat. Biotechnol. 31, 227–229 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikmi A., McKinney S. A., Delventhal K. M. & Gibson M. C. TALEN and CRISPR/Cas9-mediated genome editing in the early-branching metazoan Nematostella vectensis. Nat. Commun 5, 5486 (2014). [DOI] [PubMed] [Google Scholar]
- Genikhovich G. & Technau U. Complex functions of Mef2 splice variants in the differentiation of endoderm and of a neuronal cell type in a sea anemone. Development 138, 4911–4919 (2011). [DOI] [PubMed] [Google Scholar]
- Genikhovich G. & Technau U. In situ hybridization of starlet sea anemone (Nematostella vectensis) embryos, larvae, and polyps. CSH protocols 2009, pdb prot5282 (2009). [DOI] [PubMed] [Google Scholar]
- Denker E., Manuel M., Leclère L., Le Guyader H. & Rabet N. Ordered progression of nematogenesis from stem cells through differentiation stages in the tentacle bulb of Clytia hemisphaerica (Hydrozoa, Cnidaria). Dev. Biol. 315, 99–113 (2008). [DOI] [PubMed] [Google Scholar]
- Fritzenwanker J. H., Genikhovich G., Kraus Y. & Technau U. Early development and axis specification in the sea anemone Nematostella vectensis. Dev. Biol. 310, 264–279 (2007). [DOI] [PubMed] [Google Scholar]
- Genikhovich G. et al. Axis patterning by BMPs: cnidarian network reveals evolutionary constraints. Cell Rep 10, 1646–1654 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figures 1-5 and Supplementary Tables 1-2
Data Availability Statement
The data supporting the findings of this study are available within the article and its Supplementary Information. Any further relevant data concerning the techniques used in the paper are available on request.