Abstract
Carcinoid tumors are rare and slow growing malignancies derived from enterochromaffin cells. Two-thirds of carcinoid tumors arise in the gastrointestinal tract, and in 3% of these cases the primary site cannot be determined. Presenting symptoms depend on the location of the primary tumor but may be nonspecific, and in 13% of patients distant metastases are discovered on diagnosis. The classic carcinoid syndrome occurs in less than 10% of cases and only after metastasis to the liver. We present a case of a young woman with a gastrointestinal carcinoid tumor of unknown site that had metastasized to the liver. We also provide a review of the current diagnostic modalities. Familiarity with the signs and symptoms of carcinoid tumors and the diagnostic techniques thereof may facilitate early detection and improved outcome.
Abbreviations: CT, computed tomography
Introduction
Carcinoid tumors are rare and slow growing malignancies that derive from neuroendocrine enterochromaffin cells, which are found in various regions of the body. Data from 13,715 patients compiled by the National Cancer Institute (NCI) over three series from 1950-1999 indicate that that 67% of carcinoid tumors arise from the gastrointestinal (GI) tract while another 25% are found in the bronchopulmonary system [1]. Although the majority of carcinoids derive from the gastrointestinal tract, they comprise only 2% of all GI maligancies [2]. Within the gastrointestinal tract, the three most common sites of carcinoid tumor occurrence are the ileum (21%), rectum (20%), and appendix (18%). In 3% of cases, the primary site of a digestive tract carcinoid tumor is unknown. In the most recent NCI series, distant metastases were found on diagnosis in 13% of all patients and 16% of patients with a primary lesion in the gastrointestinal tract. The overall 5 year survival rate for all carcinoid tumors is 67%. The prognosis is expectedly worse for patients with distant metastases, with the 5 year survival rate dropping to 39%. The liver is the most common site of distant metastasis. The average age at diagnosis for all carcinoid tumors is 61 years by the latest data, and there are notable differences depending on the primary site. Small bowel tumors have the latest age of diagnosis at 65 years, while the youngest diagnosed patients are those with appendiceal tumors, who have an average age of 49 years [1]. The classic “carcinoid syndrome,” in which patients present with flushing, diarrhea, and wheezing, occurs in less than 10% of cases [3]. Therefore the correct diagnosis relies on a combination of clinical signs, biochemical markers, and radiological studies. Radiological studies are used for both primary tumor localization and detection of metastases, which in turn determines the feasibility of various treatment modalities. We present a case of carcinoid tumor with an unknown primary site and liver metastasis in a young female.
Case Report
A 21-year-old woman presented to the emergency room with severe epigastric and right upper quadrant pain, nausea, and vomiting for one week. Prior to the onset of abdominal pain, she developed a diffuse erythematous petechial rash on her cheeks, arms, and abdomen as well as facial edema. The patient reported having chronic abdominal pain as a child, but the episodes never reached such intensity and were never accompanied by rash and edema. The patient also experienced a recent episode of night sweats and an intentional five-pound weight loss over the past few months. She was diagnosed with Type 1 diabetes mellitus at age five and reported poor glycemic control in the past week due to decreased appetite. On physical exam, the patient's vital signs were stable and within normal limits. She was diffusely tender to palpation over the abdomen, especially in the right upper quadrant. Laboratory studies revealed a borderline elevated white blood cell count and a normal hematocrit and platelet count. The complete metabolic panel was within normal limits except for a low serum glucose. Liver enzymes, amylase, lipase, and coagulation times were all normal. Urinalysis showed glucosuria.
In the emergency room, CT imaging of the abdomen and pelvis was performed with oral and IV contrast. Numerous heterogeneous hypodensities, many with central enhancement, were discovered throughout the liver. The lesions had poorly demarcated borders. Most of the lesions measured 1 cm or less in diameter, but several large masses measuring greater than 4 cm in diameter were present in both lobes (Figure 1A, Figure 1B). Both retroperitoneal and mesenteric lymphadenopathy was also present. The left adnexa also appeared mildly enlarged. The pancreas, spleen, kidneys, adrenal glands, gall bladder, small bowel, appendix, and colon all appeared normal. The patient was admitted for further investigation of the liver lesions and glycemic control.
Figure 1A.
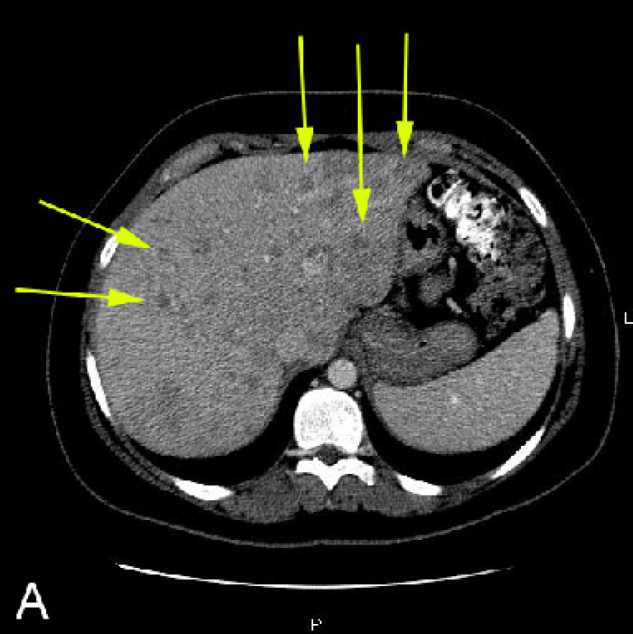
21-year-old woman with metastatic carcinoid tumor. A, Abdominal CT in liver window shows numerous small heterogeneous hypodense lesions in the liver, some with central enhancement (arrows). Most lesions have poorly demarcated borders.
Figure 1B.
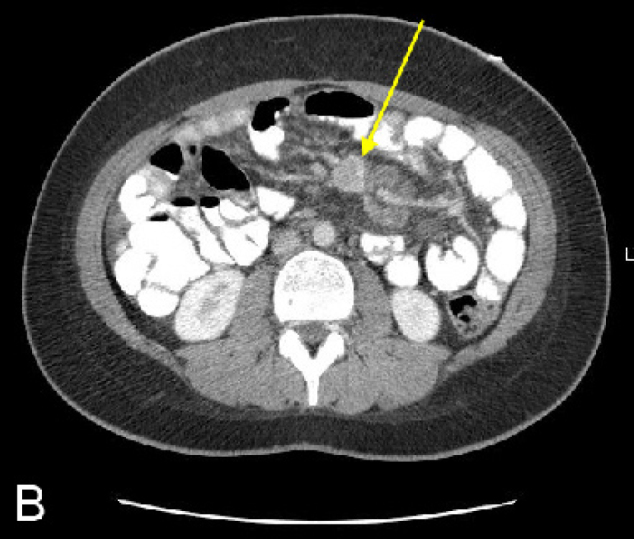
21-year-old woman with metastatic carcinoid tumor. B, Abdominal CT shows mesenteric (arrow) and retroperitoneal lymphadenopathy. There were no calcified mesenteric masses, radiating strands, or bowel wall thickening.
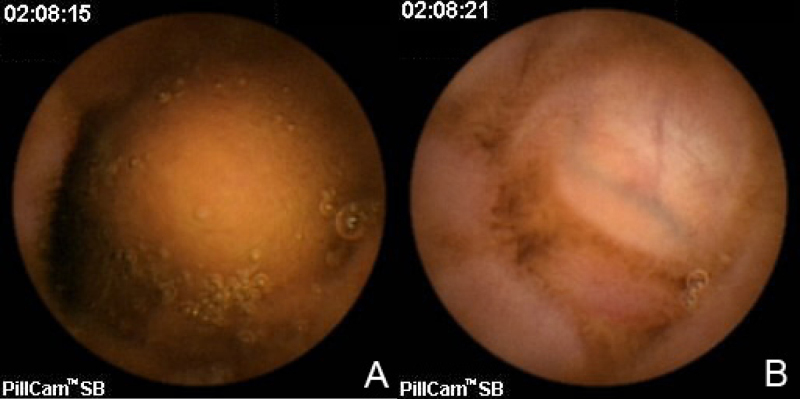
On hospital day 1, a chest CT and abdominal ultrasound were performed. The chest CT was normal and did not reveal hilar or mediastinal lymphadenopathy. The abdominal ultrasound revealed numerous hypoechoic nodules in the liver that were consistent with the lesions found on CT (Fig. 2). An ultrasound-guided core biopsy was taken from a large mass in the left hepatic lobe. The final pathologic diagnosis was metastatic carcinoid tumor. Staining patterns suggested that the primary tumor was located in the colon, and somatostatin receptor scintigraphy (OctreoScan) was performed for tumor localization. Increased uptake was detected throughout the liver and in the region of the aorta that most likely corresponded to the retroperitoneal lymphadenopathy at 4 hours and 24 hours post injection (Figure 3A, Figure 3B). At 24 hours, there was also increased activity in the colon. There was no activity in the lungs. A capsule endoscopy was performed to assess small bowel involvement. A small, non-ulcerated bulge was visualized in the mid-jejunum that may represent the primary tumor in the submucosa or a prominent intestinal fold (Fig. 4). Additional attempts to localize the primary lesion using upper endoscopy and colonoscopy were unsuccessful. The patient developed severe headaches during her hospital course, and brain metastases were ruled out after a head CT.
Figure 2.
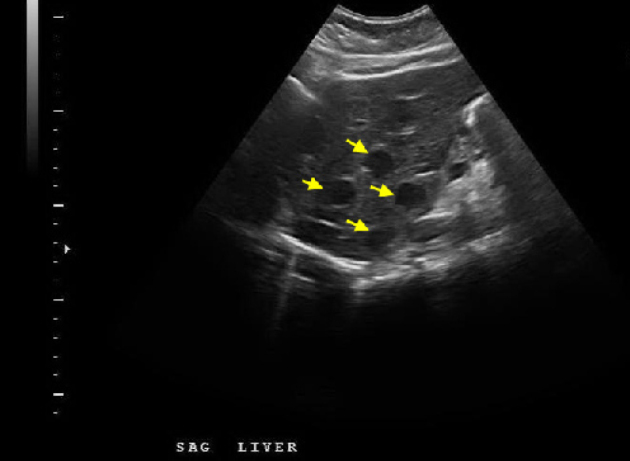
21-year-old woman with metastatic carcinoid tumor. Sagittal right upper quadrant sonogram shows numerous hypoechoic hepatic masses.
Figure 3A.
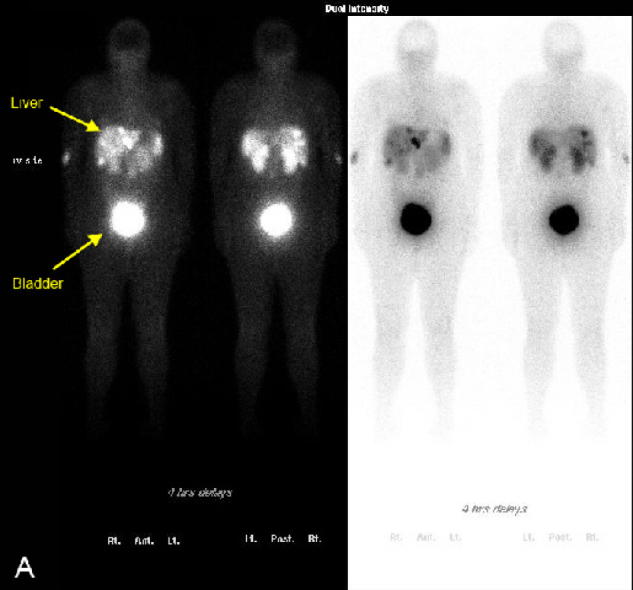
21-year-old woman with metastatic carcinoid tumor. Octreotide scintigraphy. A, At 4 hours after injection, there is increased uptake in the liver and retroperitoneal lymph nodes. There is no increased uptake in the lungs.
Figure 3B.
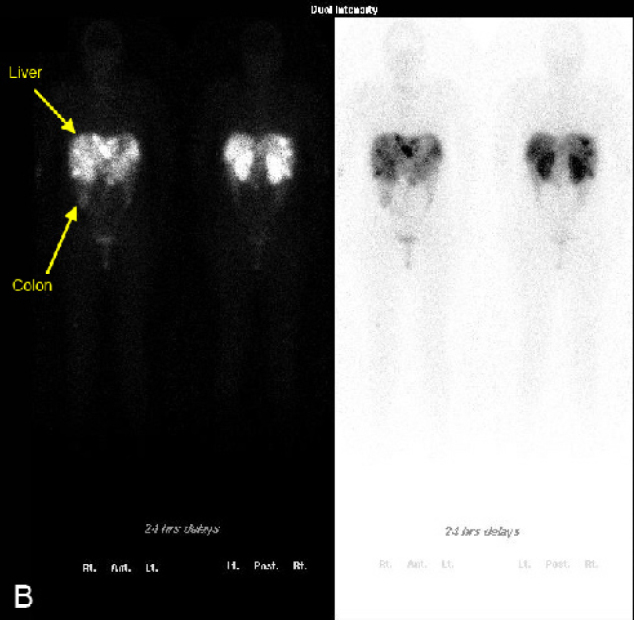
21-year-old woman with metastatic carcinoid tumor. Octreotide scintigraphy. B, At 24 hours after injection, there is increased uptake in the liver, retroperitoneal lymph nodes, and the colon. There is no increased uptake in the lungs.
Figure 4.
21-year-old woman with metastatic carcinoid tumor. A and B, Capsule endoscopy images taken 6 seconds apart show a bulge protruding into the gastrointestinal lumen at the level of the mid-jejunum. This may represent the primary tumor in the submucosa or a normal prominent intestinal fold.
Other notable laboratory values during the hospital course included the following: an elevated urine 5-HIAA (161 mg/24 hr), elevated CA 19-9 antigen (62 units/mL), low cortisol (0.6 mcg/dL), and elevated gastrin (142 pg/mL). Alpha fetoprotein, CEA, serum glucagon, and CA 125 antigen were within normal range. Serology studies showed reactivated EBV and was negative for CMV, HIV, and Toxoplasma.
The patient was not considered a surgical candidate due to her widespread metastatic disease, and further workup to definitively determine the primary tumor site was not conducted as it would not have altered the treatment plan. She was referred to a cancer center and has been enrolled in a clinical trial.
Discussion
Gastrointestinal carcinoids can present in various ways, depending on the site of the primary tumor and whether metastases are present. Carcinoids of the foregut can secrete substances such as 5-hydroxytryptophan (5-HTP), adrenocorticotropic hormone (ACTH), and histamine and cause an atypical carcinoid syndrome. This is less often associated with flushing than the classic carcinoid syndrome and typically presents with abdominal pain, diarrhea, and weight loss. Midgut carcinoids, which can secrete 5-hydroxytryptamine (5-HT), kinins, prostaglandins, and substance P, manifest with the classic carcinoid syndrome after metastasis to the liver. Symptoms include flushing, diarrhea, cardiac valvular disease, wheezing, and pellagra. Carcinoids originating from the hindgut usually do not secrete 5-HT and therefore rarely present with symptoms of the carcinoid syndrome. The presence of other clinical findings, such as recurrent abdominal pain and hepatosplenomegaly, should also increase the suspicion for carcinoid tumor.
A defining characteristic of enterochromaffin cells is their ability to secrete serotonin, and therefore the urinary measurement of the serotonin metabolite 5-HIAA is used in the laboratory diagnosis of carcinoids. The specificity of a 24 hour urinary 5-HIAA measurement for carcinoid tumors is approximately 88%. Serum chromogranin A is the most sensitive biochemical marker for carcinoids, but because it is constitutively secreted by most neuroendocrine tumors, it has a lower specificity than urinary 5-HIAA.
Nuclear medicine studies are the first option for primary tumor localization and detection of metastases. Somatostatin receptor scintigraphy uses radiolabelled somatostatin analogues to bind and detect two somatostatin receptor subtypes (2, 5) that are expressed by 70-90% of carcinoid tumors [4]. 111-Indium-DTPA-octreotide is the most commonly used radioligand and has a sensitivity of 80-90% irrespective of the primary tumor site [5, 6]. Positron emission tomography (PET) using 18-F-FDG has been shown to have a low sensitivity in carcinoid detection [7]. However, PET studies using 11-C-5HTP [8] and 68-Ga-octreotide [9] tracers have demonstrated higher detection rates with than with octreotide scintigraphy. Currently, PET remains an investigational technique for carcinoid detection. Metaiodobenzylguanidine (MIBG) labelled with iodine-123 or iodine-131 is the oldest of the nuclear medicine methods. It is highly specific for neuroendocrine tumors [10] but has a lower sensitivity than somatostatin receptor scintigraphy [11]. 111-In-DTPA-octreotide is recommended as the study of choice for imaging carcinoid tumors [12].
If nuclear medicine studies fail to localize the primary tumor, as was the case for our patient, direct visualization with endoscopy and endoscopic ultrasound may be attempted. Upper endoscopy and colonoscopy are both operator-dependent procedures that are limited by anatomic boundaries. Case reports suggest that capsule endoscopy has a role in the detection of small bowel lesions, which are poorly visualized by esophagogastroduodenoscopy (EGD) [13, 14]. No modality has been established as the “gold standard” diagnostic study for small bowel tumors. Push enteroscopy is also limited in reach and cannot extend beyond the proximal jejunum [15]. In a randomized control trial, capsule endoscopy was shown to have a higher detection rate but a lower specificity than push enteroscopy [16]. A prospective study comparing capsule endoscopy with octreotide scintigraphy concluded that the two modalities have similar diagnostic yields, but it is impossible to differentiate between intestinal and mesenteric localization on scintigraphy [17]. The main disadvantage of capsule endoscopy is the inability to control the angle or duration of visualization of any area of interest. Since findings may only appear in a single frame of the recorded video, it is often difficult to differentiate between a normal prominent plica and an abnormality. No data exists on the false-positive rate for capsule endoscopic diagnosis of small bowel tumors, but a colonic polyp study showed that false-positive findings based on expert interpretation of capsule endoscopy were present in 33% of cases when compared to conventional colonoscopy [18]. Endoscopic ultrasound is highly sensitive in detecting foregut caricinoids and can detect small lesions measuring 2-3 mm, but again its usefulness is limited by the mechanical difficulty of traversing the small bowel [19]. In our case, the capsule endoscopy captured a mid-jejunal bulge, but the notion of a small bowel primary lesion was not supported by either the octreotide scintigraphy or the liver specimen staining pattern, which pointed to the colon as the likely primary site. Push enteroscopy and endoscopic ultrasound were not performed, but they would have been unlikely to yield additional information given the length limitation of the endoscopes. The significance of the small bowel finding visualized on capsule endoscopy could not be determined in the absence of histological evidence. Radiological studies such as CT, MRI, ultrasonography, and angiography have lower detection rates than nuclear medicine studies and are considered ancillary tests in the diagnosis of carcinoid tumors. However, CT is often the initial radiological study used to evaluate patients who present with abdominal pain to the emergency room, and abdominal pain may be the only symptom of a gastrointestinal carcinoid tumor. Findings on CT that suggest the presence of a carcinoid tumor in the small bowel include the triad of calcified mesenteric masses, radiating strands, and adjacent bowel wall thickening [20]. None of these three findings were noted in our patient.
Several aspects of this case are unusual and noteworthy. First, despite a thorough localization workup with 111-In-labelled octreotide scintigraphy, upper endoscopy, colonoscopy, and capsule endoscopy, the primary tumor site was not determined. Furthermore, the pathologic and capsule endoscopic evidence point to different regions as the likely site of origin. Whereas the tissue staining pattern suggests a colonic origin, the capsule endoscopy suggests a small bowel origin for the primary tumor. The primary lesion remains unknown in only 3% of gastrointestinal carcinoid cases. A second noteworthy aspect of the case is the patient's age, which at 21 years is far less than the average age of diagnosis of 61 years. The youngest patient in the literature with a diagnosis of carcinoid tumor is a 3 year old with an appendiceal carcinoid, which is a well known condition in the pediatric population [21]. Our review of the literature finds that the youngest patient with a non-appendiceal GI carcinoid is 12 years of age [22]. Our patient is likely among the youngest group of patients with metastatic carcinoid tumor, although the lack of analysis with regard to age of incidence in the literature makes this difficult to substantiate.
Carcinoids are rare and slow-growing tumors which do not usually present with the classically described carcinoid syndrome. In order to improve survival, early diagnosis before metastatic spread of disease is necessary and the clinician should consider carcinoid tumors in the differential diagnosis of nonspecific symptoms such as recurrent abdominal pain and hepatosplenomegaly. Somatostatin receptor scintigraphy with 111-In-DTPA-octreotide is currently the diagnostic study of choice for carcinoid tumors, and new PET techniques hold promise for increased rates of detection in the future.
Footnotes
Published: August 13, 2007
Contributor Information
Peter S. Liang, Email: peter_liang@hms.harvard.edu.
Kitt Shaffer, Email: kshaffer@challiance.org.
References
- 1.Modlin IM, Lye KD, Kidd M. A 5-decade analysis of 13,715 carcinoid tumors. Cancer. 2003;97(4):934–959. doi: 10.1002/cncr.11105. [PubMed] [DOI] [PubMed] [Google Scholar]
- 2.Oberg K, Eriksson B. Nuclear medicine in the detection, staging and treatment of gastrointestinal carcinoid tumours. Best Pract Res Clin Endocrinol Metab. 2005;19(2):265–276. doi: 10.1016/j.beem.2004.11.016. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.van der Lely AJ, de Herder WW. Carcinoid syndrome: diagnosis and medical management. Arq Bras Endocrinol Metabol. 2005;49(5):850–860. doi: 10.1590/s0004-27302005000500028. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Reubi JC, Kvols L, Krenning E, Lamberts SW. Distribution of somatostatin receptors in normal and tumor tissue. Metabolism. 1990;39:78–81. doi: 10.1016/0026-0495(90)90217-z. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Krenning EP, Kwekkeboom DJ, Oei HY. Somatostatin receptor scintigraphy in carcinoids, gastrinomas and Cushing's syndrome. Digestion. 1994;55(suppl. 3):54–59. doi: 10.1159/000201202. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Kwekkeboom DJ, Krenning EP. Somatostatin receptor scintigraphy in patients with carcinoid tumors. World J Surg. 1996;20(2):157–161. doi: 10.1007/s002689900024. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Adams S, Baum R, Rink T. Limited value of fluorine-18 fluorodeoxyglucose positron emission tomography for the imaging of neuroendocrine tumours. Eur J Nucl Med. 1998;25:79–83. doi: 10.1007/s002590050197. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Eriksson B, Orlefors H, Oberg K, Sundin A, Bergstrom M, Langstrom B. Developments in PET for the detection of endocrine tumours. Best Pract Res Clin Endocrinol Metab. 2005;19(2):311–324. doi: 10.1016/j.beem.2004.11.001. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Hofmann M, Maecke H, Borner A. Biokinetics and imaging with the somatostatin receptor PET radioligand (68) Ga-DOTATOC: preliminary data. EurJ Nucl Med. 2001;28:1751–1757. doi: 10.1007/s002590100639. [PubMed] [DOI] [PubMed] [Google Scholar]
- 10.Hanson MW, Feldman JM, Blinder RA, Moore JO, Coleman RE. Carcinoid tumors: iodine-131 MIBG scintigraphy. Radiology. 1989;172(3):699–703. doi: 10.1148/radiology.172.3.2772175. [PubMed] [DOI] [PubMed] [Google Scholar]
- 11.Kaltsas G, Korbonits M, Heintz E. Comparison of somatostatin analog and meta-iodobenzylguanidine radionuclides in the diagnosis and localization of advanced neuroendocrine tumors. J Clin Endocrinol Metab. 2001;86:895–902. doi: 10.1210/jcem.86.2.7194. [PubMed] [DOI] [PubMed] [Google Scholar]
- 12.Plockinger U, Rindi G, Arnold R. Guidelines for the diagnosis and treatment of neuroendocrine gastrointestinal tumours. Neuroendocrinology. 2005;80(6):394–424. doi: 10.1159/000085237. [PubMed] [DOI] [PubMed] [Google Scholar]
- 13.Forner A, Mata A, Puig M. Ileal carcinoid tumor as a cause of massive lower-GI bleeding: the role of capsule endoscopy. Gastrointest Endosc. 2004;60(3):483–485. doi: 10.1016/s0016-5107(04)01814-0. [PubMed] [DOI] [PubMed] [Google Scholar]
- 14.Coates SW, Jr, DeMarco DC. Metastatic carcinoid tumor discovered by capsule endoscopy and not detected by esophagogastroduodenoscopy. Dig Dis Sci. 2004;49(4):639–641. doi: 10.1023/b:ddas.0000026311.62364.0b. [PubMed] [DOI] [PubMed] [Google Scholar]
- 15.Gill SS, Heuman DM, Mihas AA. Small intestinal neoplasms. J Clin Gastroenterol. 2001;33(4):267–282. doi: 10.1097/00004836-200110000-00004. [PubMed] [DOI] [PubMed] [Google Scholar]
- 16.Appleyard M, Fireman Z, Glukhovsky A. A randomized trial comparing wireless capsule endoscopy with push enteroscopy for the detection of small-bowel lesions. Gastroenterology. 2000;119(6):1431–1438. doi: 10.1053/gast.2000.20844. [PubMed] [DOI] [PubMed] [Google Scholar]
- 17.van Tuyl SAC, van Noorden JT, Timmer R. Detection of small-bowel neuroendocrine tumors by video capsule endoscopy. Gastrointest Endosc. 2006;64(1):66–72. doi: 10.1016/j.gie.2006.01.054. [PubMed] [DOI] [PubMed] [Google Scholar]
- 18.Eliakim R, Fireman Z, Gralnek IM. Evaluation of the PillCam Colon capsule in the detection of colonic pathology: results of the first multicenter, prospective, comparative study. Endoscopy. 2006;38(10):963–970. doi: 10.1055/s-2006-944832. [PubMed] [DOI] [PubMed] [Google Scholar]
- 19.Gibril F, Jensen RT. Comparative analysis of diagnostic techniques for localization of gastrointestinal neuroendocrine tumors. Yale J Biol Med. 1997;70(5-6):509–522. [PubMed] [PMC free article] [PubMed] [Google Scholar]
- 20.Pantongrag-Brown L, Buetow PC, Carr NJ. Calcification and fibrosis in mesenteric carcinoid tumor: CT findings and pathologic correlation. AJR Am J Roentgenol. 1995;164(2):387–391. doi: 10.2214/ajr.164.2.7839976. [PubMed] [DOI] [PubMed] [Google Scholar]
- 21.Moertel CG, Dockerty MB, Judd ES. Carcinoid tumors of the vermiform appendix. Cancer. 1968;21:270–278. doi: 10.1002/1097-0142(196802)21:2<270::aid-cncr2820210217>3.0.co;2-9. [PubMed] [DOI] [PubMed] [Google Scholar]
- 22.Spunt SL, Pratt CB, Rao BN. Childhood carcinoid tumors: The St Jude Children's Research Hospital experience. J Pediatr Surg. 2000;35(9):1282–1286. doi: 10.1053/jpsu.2000.9297. [PubMed] [DOI] [PubMed] [Google Scholar]