Abstract
The use of FDG PET/CT in the assessment of oncologic disease is becoming increasingly widespread. The advent of PET/CT however presents some additional challenges; recent reports have emphasized the need to detect incidental findings which can confer additional health risks. We report a case of a pulmonary embolus with pulmonary infarct secondary to a metastatic right atrial mass on a background of squamous cell carcinoma of the neck that demonstrated findings on both the PET and CT portions of the examination. The significance of standardized uptake value (SUV) in the differential diagnosis of chest lesions is discussed. This report highlights the possible co-existence of non-metastatic, but potentially life threatening abnormalities that may be present on the CT portion of such studies.
Abbreviations: CT, Computed tomography; CTPA, CT pulmonary angiography; FDG, 2-deoxy-2-(F-18) fluoro-D-glucose; FNA, Fine needle aspiration; MBq, Mega Becquerel; PE, Pulmonary embolism; PET, Positron emission tomography; PET/CT, Positron emission tomography/computed tomography; RA, Right atrium; SUV, Standardized uptake value
Case Report
A 47-year-old man presented with a rapidly enlarging left neck mass. He subsequently developed hoarseness, and difficulty swallowing solid foods, and weight loss of 30 pounds over 6 months. No other significant symptoms were present. Relevant past medical history included 30 pack-years of cigarette smoking. Given the rapid development of local symptoms, the primary care team initially managed the patient for a provisional diagnosis of lymphadenitis with a combination of antibiotics and steroids. This treatment failed to relieve the patient's symptoms or reduce the size of the mass. On subsequent referral to this institution, examination confirmed a palpable large left neck mass that was clinically inseparable from the thyroid gland; there was no appreciable lymph node enlargement. Flexible endoscopic examination revealed swelling and obstruction of the left piriform fossa without ulceration. The vocal cords were normal but the larynx was displaced to the right. A fine needle aspiration (FNA) at the outside hospital demonstrated atypical cells, possibly consistent with a low-grade cystic squamous cell carcinoma.
The patient proceeded to a staging FDG PET/CT and repeat FNA of the mass. The FDG PET/CT study was performed sixty minutes following the intravenous administration of 300 MBq (8 mCi) of 2-deoxy-2-(F-18) fluoro-D-glucose (FDG); sequential contrast-enhanced CT with PET imaging was performed from the base of skull to the iliac crest. Helical CT was performed with 5 mm collimation followed immediately by PET at multiple overlapping bed positions (5 minutes per bed position). Registered contrast-CT and attenuation corrected FDG PET and attenuation uncorrected FDG PET projection images were reviewed for interpretation.
The diagnostic PET/CT images showed 7 cm by 7 cm by 10 cm mass with mixed attenuation was noted in the left neck with extension into and invasion of the thyroid cartilage. An enlarged 2.5 cm level II lymph node was also present. Several ipsilateral sub-centimeter and borderline enlarged level III and IV lymph nodes were also detected. Both the mass and associated lymph nodes had markedly increased FDG uptake; in addition, the mass demonstrated central photopenia compatible with necrosis (maximum SUV of the neck mass of 17.0) (Figure 1A, Figure 1B, Figure 1C, Figure 1D). Thoracic imaging revealed intensely increased FDG uptake in the right atrium which correlated with an atrial soft tissue mass (maximum SUV of 10.2) (Figure 1C, Figure 1D, Figure 1E). A right upper lobe lung nodule with increased FDG uptake (maximum SUV of 4.0) was also observed (Figure 1C, Figure 1D). In addition, there was a filling defect within the left lower lobe pulmonary artery suspicious for a pulmonary embolism (maximum SUV of 2.3). A left lower lobe peripherally situated parenchymal abnormality with mildly increased FDG activity (maximum SUV of 2.1) was also noted (Figure 2A, Figure 2B, Figure 2C).
Figure 1A.
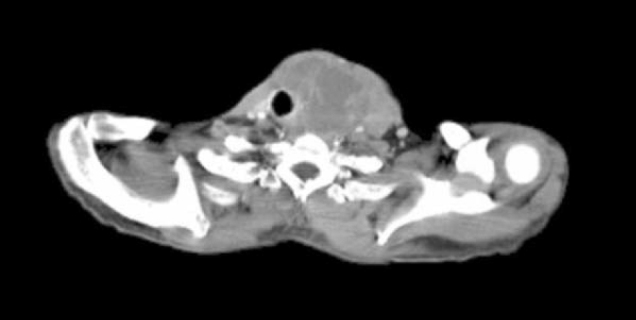
47-year-old man with metastatic head and neck cancer. Axial CT at the level of the thyroid cartilage shows that it has been destroyed by a large heterogenous tumor mass on the left.
Figure 1B.
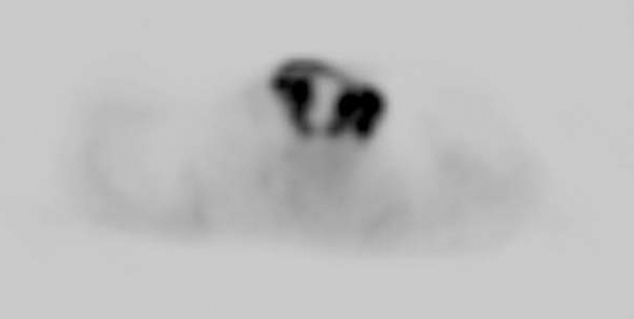
47-year-old man with metastatic head and neck cancer. PET image corresponding to Figure 1A, Figure 1B, Figure 1C, Figure 1D, Figure 1E A demonstrates peripheral marked FDG uptake (SUV max = 17) with central photopenia secondary to necrosis.
Figure 1C.
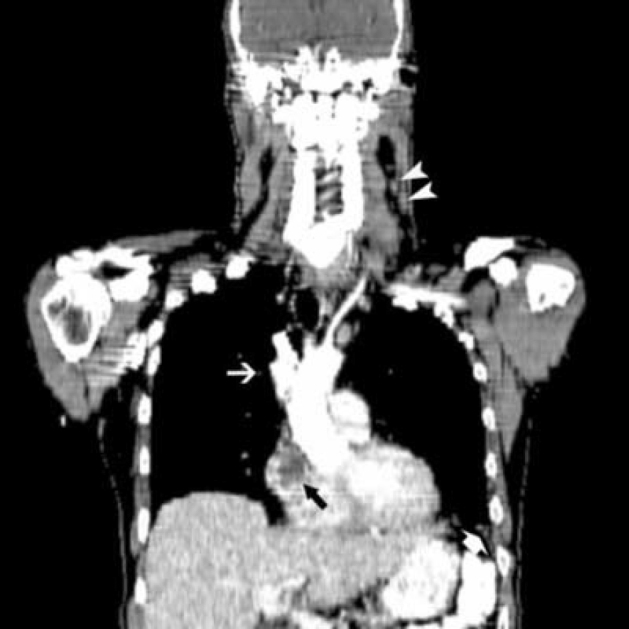
47-year-old man with metastatic head and neck cancer. Coronal reformatted CT shows a right atrial mass (black arrow), right upper lobe pulmonary nodule (white arrow), left cervical nodal disease (white arrowheads), and left lower lobe infarct (short white arrow).
Figure 1D.
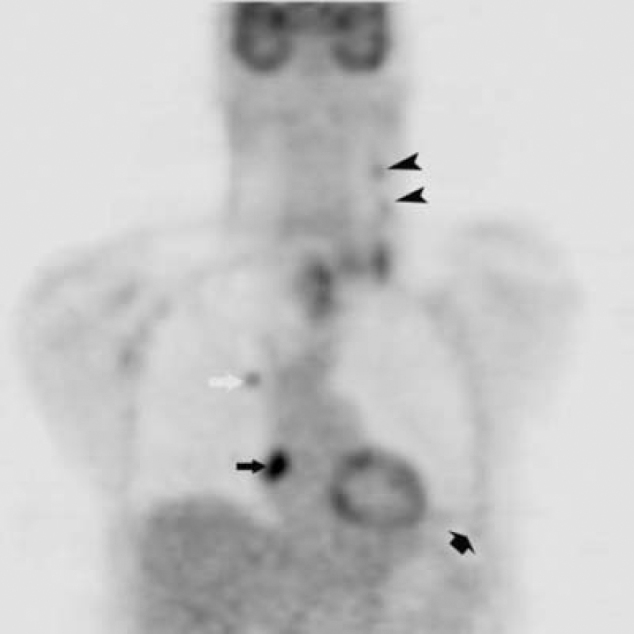
47-year-old man with metastatic head and neck cancer. Coronal reformatted PET corresponding to Fig. 1C shows a right atrial mass (black arrow)(SUV max = 10.2), right upper lobe pulmonary nodule (white arrow) (SUV max = 4.0), left cervical nodal disease (black arrowheads), and left lower lobe infarct (short black arrow) (SUV max = 2.1).
Figure 1E.
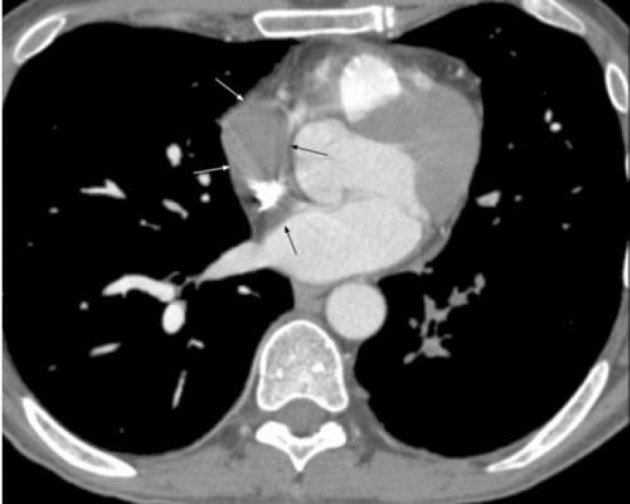
47-year-old man with metastatic head and neck cancer. CT pulmonary angiogram axial image at the level of the right inferior pulmonary vein shows a right atrial mass with constriction of the SVC inflow.
Figure 2A.
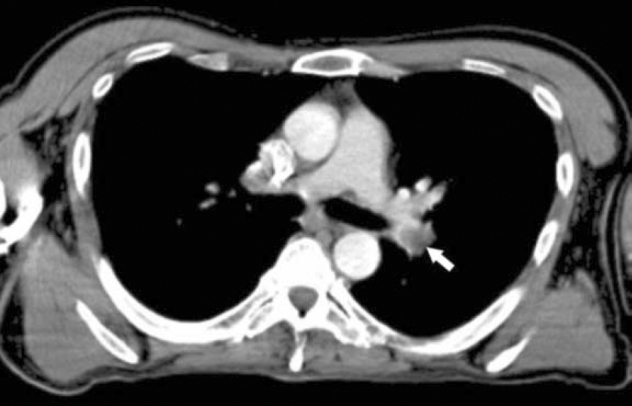
47-year-old man with metastatic head and neck cancer. Axial PET/CT at the level of the left pulmonary artery bifurcation shows a pulmonary embolus with left lower lobe pulmonary artery (arrow).
Figure 2B.
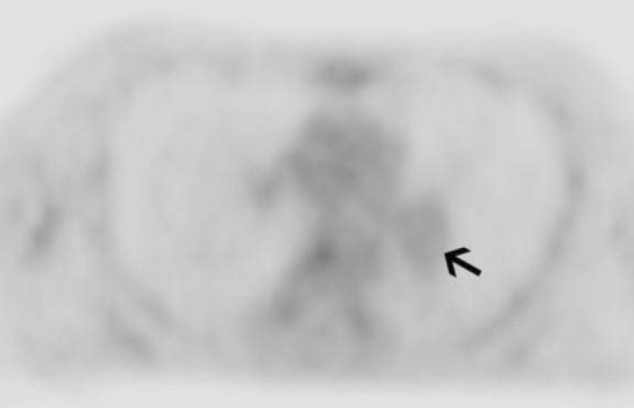
47-year-old man with metastatic head and neck cancer. PET image (SUV max = 2.3) corresponding to Fig. 2A indicates pulmonary embolism (arrow) which has similar FDG uptake to background and is therefore a bland thrombus rather than tumor thrombus.
Figure 2C.
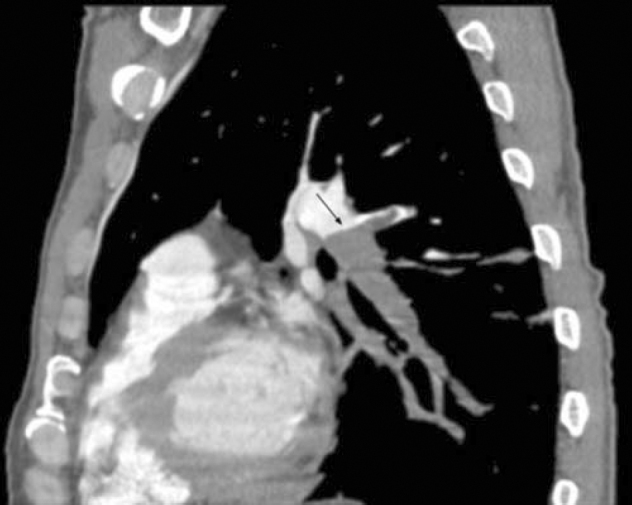
47-year-old man with metastatic head and neck cancer. Parasagittal CT pulmonary angiogram reformat shows pulmonary emboli within lingula and left lower lobe pulmonary arteries (arrows).
In view of the PET/CT findings in the chest, the patient underwent a CT pulmonary angiogram (CTPA). The CTPA protocol included indirect CT venography of the pelvic and lower extremity veins using 1.25 mm collimation. The imaging was performed following intravenous injection of 100 ml of contrast material. Findings confirmed lingula and left lower pulmonary artery emboli, and a left lower lobe wedge shaped parenchymal abnormality compatible with an infarct. No thrombus was noted in the pelvis or lower limb veins. A right atrial soft tissue mass was also noted, as were two right upper lobe pulmonary nodules, suspicious for metastases.
A subsequent FNA of the neck mass confirmed poorly differentiated squamous cell carcinoma. The final diagnosis was squamous cell carcinoma of the hypopharynx with a T4, N2b, M1 FDG PET/radiological staging.
Discussion
In the initial era of PET imaging, normal tissue was often misinterpreted as disease due to altered metabolic activity and uptake. The classic example of this was “brown fat”, however, muscle uptake and partial subcutaneous injections with lymphatic uptake also proved problematic. The advent of PET/CT significantly improved our diagnostic accuracy by enabling the identification of normal structures as the cause of the uptake. However, the routine staging of malignant disease has evolved to often include PET/CT in a number of tumor types. Its application has led to considerable improvement in the diagnosis and hence, subsequent management of these patients. As with any imaging modality however, caution is often required in the interpretation of the findings. Potential confusion can arise with the presence of differing pathology but similar radiological features. Reports have documented the occurrence of abdominal aortic aneurysm, pneumothorax, pneumoperitoneum, tumor invasion/compression of airways, massive pleural/pericardial effusion, large osteolytic lesions with impending fracture, and tumor invasion of the spinal canal as findings [1].
A number of observations and lessons can be drawn from this case report. The relative acute development of the neck mass could be suggestive of an infective or inflammatory pathology. The constitutional symptoms (marked weight loss) together with the presence of hoarsening of voice are strongly suggestive of underlying neoplasm. The marked increased metabolic activity associated with central necrosis and a neck mass that is seen invading adjacent structures on FDG PET/CT further confirms this as a malignant neoplasm and unlikely to be an inflammatory in origin.
In addition to a large neck mass revealed by subsequent FDG PET/CT scanning, impalpable neck nodes on the ipsilateral side were also noted, increasing the staging to a N2b category (although not of significant implication in this particular patient's case in the context of M1 stage disease). Such capability of detecting sub-centimeter metabolically active nodal disease is a feature unique to functional imaging and essentially questions the validity of using solely size criteria in the assessment of nodal disease [2]. However the detection of sub-centimeter nodes is limited by partial volume effect and is at the limits of resolution of the current generation of PET scanners. Metabolically active nodes could also be due to inflammatory disease and this needs to be kept in mind when reporting FDG PET/CT, at times these nodes require histological confirmation if management is going to be altered.
The mildly increased FDG uptake in the pulmonary infarct was an important finding as the patient had a pulmonary embolus that needed immediate treatment. However, the key to the diagnosis was the differential uptake in the infarct versus the tumor and the presence of intra-luminal filling defects in the pulmonary artery. These pathologic processes clearly had different metabolic signatures/SUV (Fig. 1D). The right upper lobe lung nodule was metabolically active, likely due to metastatic disease (the thin slice CTPA however detected 2 right upper lobe nodules, one of which was not detected on FDG PET/CT as it was beyond the resolution of this modality); however the left lower lobe parenchymal wedge abnormality demonstrated features morphologically and metabolically different to the right lung lesions. This should alert the interpreter to an alternative diagnosis [3, 4]. In this case, the CT study was a contrast-enhanced study which on further inspection demonstrated a filling defect in the left pulmonary artery. Subsequent CTPA confirmed this to be an embolus with an associated lung infarct [3].
Cardiac uptake can be variable and is associated with the patient's fasting status. However, focal increased uptake can be abnormal and requires further assessment. In this case, the abnormality proved to be a right atrial soft tissue mass. The metabolic signature of the mass was similar to the neck mass and would therefore suggest a tumor thrombus. Benign causes of increased atrial uptake of FDG include atrial fibrillation, physiologic activity in the right atrial appendage, central venous catheters, lipomatous hypertrophy of the interatrial septum, or a non neoplastic thrombus. There have been documented cases of cardiac metastases from head and neck tumors, especially squamous cell carcinoma of the glottis and sub-glottic area (5, 6). In our case, the patient had no lower limb symptoms and the contrast CT did not detect a thrombus apart from in the left pulmonary artery; hence the atrial mass is likely to be a tumor. It is conceivable that the left pulmonary artery thrombus was bland rather than tumor or the partial volume effects had caused overall apparent reduction in activity. Tumor thrombus and inflammatory thrombus are known to have increased uptake of FDG [7, 8, 9]. In a majority of cases, particularly in the practice of a generalist the detection of an atrial abnormality can be quite difficult; it was the increased activity on the PET scan that initially alerted us to the abnormality in this case.
Teaching Points
1. Many benign processes are FDG avid and need to be considered in the differential diagnosis of disease in the chest even in patients with malignant disease (i.e. sarcoid, histoplasmosis, pneumonitis including pulmonary infarcts).
2. Careful review of the CT component of FDG PET/CT is mandatory to assess for significant and potentially life threatening processes.
3. Suspected cardiac metastases can be difficult to detect and assess, particularly from a primary that is not within the thorax or abdomen. This case demonstrates the use of FDG PET in the detection and evaluation of the cardiac metastases/mass.
Conclusion
Whilst FDG PET/CT has undoubtedly refined cancer detection and management, it has created additional challenges. Several, potentially significant and life-threatening incidental findings on the CT portion of the examination may exist. It is imperative for the interpreting physician to be aware of these entities and to report them appropriately. The lungs can be a frequent site of false positive findings in the context of neoplasia. In such circumstances, underlying pathology predominantly takes the form of metastatic or inflammatory disease although pulmonary infarcts are an important pathology to consider. Meticulous care in the interpretation of the PET and CT images are essential steps in avoiding the hazards of over calling such abnormalities as metastatic. The correlative studies allow the interpreter to accurately visualize the PET abnormality on CT and hence derive an accurate conclusion.
Footnotes
Published: August 13, 2007
Contributor Information
Prakash Manoharan, Email: prakashmanoharan@yahoo.co.uk.
Richard K.J. Brown, Email: rkjbrown@med.umich.edu.
References
- 1.Schoder H, Yeung H.W.D., Larson S.M. CT in PET/CT: Essential Features of Interpretation. J Nuc Med. 2005;46(8):1249–1251. [PubMed] [PubMed] [Google Scholar]
- 2.Yen TC, Chang YC, Chan SC. Are dual-phase 18F-FDG PET scans necessary in nasopharyngeal carcinoma to assess the primary tumour and loco-regional nodes? Eur J Nucl Med Mol Imaging. 2005;32(5):541–548. doi: 10.1007/s00259-004-1719-2. [PubMed] [DOI] [PubMed] [Google Scholar]
- 3.Hany TF, Huerberger J, Schulthess G.K. Iatrogenic FDG foci in the lungs: a pitfall of PET image interpretation. Eur Radiol. 2003;9:2122–2127. doi: 10.1007/s00330-002-1681-y. [PubMed] [DOI] [PubMed] [Google Scholar]
- 4.Asad S, Aquino SL, Piyavisetpat N. False-positive FDG positron emission tomography uptake in nonmalignant chest abnormalities. Am J Roentgenol. 2004;182:983–989. doi: 10.2214/ajr.182.4.1820983. [PubMed] [DOI] [PubMed] [Google Scholar]
- 5.Nakamura Y, Nakano K, Gomi A. A Metastatic Ball Tumor in the Right Atrium Originating from Esophageal Cancer: Report of a Case. Surg Today. 2005;35:145–148. doi: 10.1007/s00595-004-2895-1. [PubMed] [DOI] [PubMed] [Google Scholar]
- 6.Rivkin A, Meara J, Kasey K.U. Squamous cell metastasis from the tongue to the myocardium presenting as pericardial effusion. Otolaryngol Head and Neck Surg. 1999;120(4):592–595. doi: 10.1053/hn.1999.v120.a84489. [PubMed] [DOI] [PubMed] [Google Scholar]
- 7.Beadsmoore J, Cheow K, Sala E. Hepatocellular carcinoma tumour thrombus in a re-canalised para-umbilical vein: detection by 18-fluoro-2-deoxyglucose positron emission tomography imaging. Br J Radiol. 2005;78:841–844. doi: 10.1259/bjr/37052159. [PubMed] [DOI] [PubMed] [Google Scholar]
- 8.Kikuchi M, Yamamoto E, Shiomi Y. Internal and external jugular vein thrombosis with marked accumulation of FDG. BR J Radiol. 2004;77:888–890. doi: 10.1259/bjr/32956594. [PubMed] [DOI] [PubMed] [Google Scholar]
- 9.Miceli M, Atoui R, Walker R, Mahfouz T, Mirza N, Diaz J. Diagnosis of deep septic thrombophlebitis in cancer patients by fluorine-18 fluorodeoxyglucose positron emission tomography scanning: a preliminary report. J Clin Oncol. 2004;22:1949–1956. doi: 10.1200/JCO.2004.10.160. [PubMed] [DOI] [PubMed] [Google Scholar]


