Abstract
The cucumber (Cucumis sativus L.) exhibits extensive variations in fruit size and shape. Fruit length is an important agronomic and domesticated trait controlled by quantitative trait loci (QTLs). Nonetheless, the underlying molecular and genetic mechanisms that determine cucumber fruit length remain unclear. QTL-seq is an efficient strategy for QTL identification that takes advantage of bulked-segregant analysis (BSA) and next-generation sequencing (NGS). In the present study, we conducted QTL mapping and QTL-seq of cucumber fruit length. QTL mapping identified 8 QTLs for immature and mature fruit length. A major-effect QTL fl3.2, which explained a maximum of 38.87% of the phenotypic variation, was detected. A genome-wide comparison of SNP profiles between two DNA bulks identified 6 QTLs for ovary length. QTLs ovl3.1 and ovl3.2 both had major effects on ovary length with a △ (SNP-index) of 0.80 (P < 0.01) and 0.74 (P < 0.01), respectively. Quantitative RT-PCR of fruit size-related homologous genes localized in the consensus QTL FL3.2 was conducted. Four candidate genes exhibited increased expression levels in long fruit genotypes. Our results demonstrated the power of the QTL-seq method in rapid QTL detection and provided reliable QTL regions for fine mapping of fruit length-related loci and for identifying candidate genes.
Cucumber, Cucumis sativus L. (2n = 2x = 14), originates from the southern Himalayas and has been cultivated in India for at least 3000 years. Cucumber fruits exhibit extensive variation in shape or size between and within cultivated varieties resulting from long-term domestication. Recent studies categorized cucumbers into 4 geographic groups and 6 market classes based on various phenotypes and consuming properties, with fruit length being one of the most prominent traits1,2. For example, wild cucumbers typically bear small spheroid fruits ~4 cm in length. In contrast, North China fresh market cucumber fruits are at least 25 cm in length, and the European greenhouse cucumber is an intermediate type at 10 to 15 cm2,3.
Numerous studies have been performed to demonstrate the genetic and molecular basis controlling cucumber fruit length. It is now widely accepted that fruit length is determined by quantitative trait loci (QTLs) and easily influenced by cultivation conditions and environment. The first QTL mapping for cucumber fruit length was reported by Kennard and Havey, who used F3 populations from a cross between GY14 and P14328604. With an F2 population and 224 RILs derived from a narrow cross of S94 (North China type) and S06 (European greenhouse type), Yuan et al.5,6 identified 6 and 7 QTLs for immature fruit length (FL), respectively. Miao et al.7 mapped 6 QTLs for FL and MFL (mature fruit length) on LG1, LG5 and LG6, with 5 QTLs identified in both growing seasons. Nevertheless, a previous study found that QTLs for fruit shape (e.g., fruit length and diameter) were less consistent across years and spacing than earliness and yield components8. Resequencing of 115 cucumber lines generated a genomic variation map that characterized 5 QTLs for fruit length, and Csa3G199660 localized within QTL fl3.1 was considered as a candidate gene1. More recently, we constructed a SNP (single-nucleotide polymorphism)-based saturated genetic map using specific-length amplified fragment (SLAF) sequencing and identified 6 QTLs for FL and MFL with an F2 population9. Using RIL populations developed from North China type × semi-wild cucumber inbred lines, Bo et al.10 detected 5 QTLs for MFL (fl1.1, fl3.1, fl4.1, fl6.1, and fl7.1). Weng et al.2 performed QTL analysis with three QTL models and multiple populations, detecting 29 consistent and distinct QTLs for fruit size/shape in different developing stages. Analysis of the available fruit size QTLs resulted in 12 consensus QTLs and a dynamic view of genetic control of cucumber fruit development. However, given the complex genetic basis of quantitative traits plus influences caused by different mapping population and environments, additional efforts are still needed to screen for reliable QTL regions and identify candidate genes.
Cucumber fruits generally develop from an enlarged inferior ovary followed by increases in cell division and cell expansion after fertilization or fruit set before ripening. The process of fruit elongation begins almost immediately after pollination and is typically completed in 12 to 16 days11. To understand the molecular events during early fruit development, transcriptome profiling was conducted with different cucumber lines, associating cucumber kinesin genes as well as microtubule and cell cycle related genes with fruit size/development regulation3,12,13. Compared with the knowledge of molecular biology of early fruit development in cucumber, insights into the genetic basis were very limited. Weng et al.2 conducted QTL mapping of ovary length (OvL), diameter (OvD) and ovary number (OvN) as well as the relationships among OvL, FL and MFL. The results indicate that factors regulating fruit length are largely determined pre-anthesis and that OvL is a good predictor for FL and MFL. Three QTLs for OvL and four QTLs for OvD were mapped on all seven cucumber chromosomes, with one QTL for each chromosome. Nevertheless, the three QTLs for OvL together could only explain 28.8 to 38.0% of phenotypic variations; thus, it is possible that additional QTLs not detected exist in the RIL population.
Traditional QTL mapping typically requires a segregating population between two parents showing contrast phenotypes of target traits and the selection of polymorphic DNA markers for linkage analysis. The process of polymorphic marker screening and genotyping is often labour-intensive and time-consuming14. QTL-seq is proposed as an efficient strategy for rapid identification of QTLs, which takes advantage of bulked-segregant analysis and high-throughput genotyping using next-generation sequencing (NGS). This approach has been applied to detecting QTLs in rice, cucumber, tomato, and chickpea15,16,17,18,19. Previously we constructed a high-density cucumber genetic map compromising 1800 SNPs using F2 populations derived from CC3 × NC76. In this study, we examined the correlation coefficient between FL and MFL and between OvL and MFL with F3 family and RIL populations from the same cross. Traditional QTL mapping and QTL-seq were performed at different development stages over three years. A total of 14 QTLs were detected for fruit length on five cucumber chromosomes. Integration of QTL information from the present study allowed us to identify consensus QTLs for fruit length in the cucumber. Genome-wide identification of fruit size homologous genes was performed using 74 fruit size-related sequences in melon. The expression levels of candidate genes localized in a consensus QTL region on chromosome 3 were analysed by quantitative RT-PCR.
Results
Phenotypic evaluations of fruit length in CC3 × NC76 populations
In the present study, two cucumber inbred lines, ‘CC3’ and ‘NC76’ (Fig. 1a), were crossed to develop segregating populations for QTL analysis of fruit length. Phenotypic data of FL and MFL were collected using F3 families in spring 2013 and autumn 2014, and OvL and MFL data of F7 RIL populations (Fig. 1b) were collected in spring 2015. Detailed phenotypic data, frequency distribution among tested materials, and results from statistical analysis of fruit length are presented in Supplementary Tables S2 and S3.
Figure 1. Fruits of CC3, NC76 and RIL populations.

(a) Ovaries of the maternal CC3 (left) and paternal NC76 (right) on day-of-anthesis; (b) Extreme long and short mature fruits harvested from RIL populations.
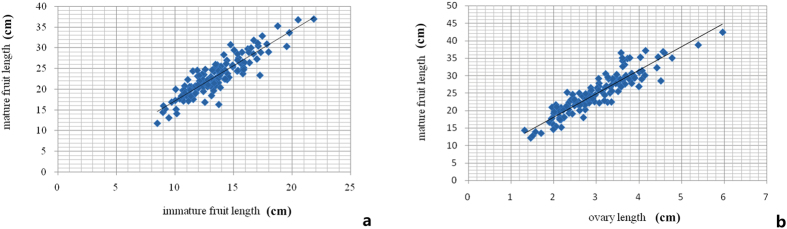
We calculated correlation coefficients among OvL, FL and MFL from two segregating populations in three growing environments. The correlation coefficients between FL and MFL in spring 2013 and autumn 2014 were 0.852 and 0.813, respectively (Supplementary Table S3). Different environmental conditions were likely the main factors leading to differences in correlation from two seasons. The correlation coefficient was calculated to be 0.896 using the average fruit length data of the two seasons (Fig. 2a), suggesting that fruit length at immature stage is significantly correlated with that at mature stage. Considering ‘immature’ as a dynamic period and given that fruit length is constantly changing, ovaries and mature fruits are in a relatively stable stage at the start and end of cucumber fruit development process, thus generating more effective phenotypic data. Thereby, correlation coefficients between OvL and MFL in the same RIL populations in spring 2015 were calculated. We observed a significant correlation between OvL and MFL, with a coefficient value of 0.917 (Fig. 2b). In the melon, the ovary and mature fruit morphology also have a high correlation, and fruit shape is predominantly determined pre-anthesis20,21. Phenotypic analysis in the present study suggests that cucumber early fruit development has a direct impact on final fruit length and that common QTL regions play important roles in determining cucumber fruit length, despite the chaos caused by differences in growing environments, nutrient conditions and pollination quality.
Figure 2. Correlation coefficiency among immature fruit length, mature fruit length and ovary length.
(a) Correlation coefficiency between immature length and mature fruit length using the means of F3 families in spring 2013 and autumn 2014. The X-axis represents immature fruit, and the Y-axis represents mature fruit length; (b) Correlation coefficiency between mature fruit length and ovary length using F7 RILs in spring 2015. The X-axis refers to ovary length on day-of-anthesis, and the Y-axis refers to mature fruit length.
QTL-mapping
We previously constructed a SNP-based genetic map by resequencing two parents (CC3 and NC76) and F2 populations using SLAF-seq9. To correct genotyping errors from NGS data, Highmap22 was employed to re-genotype the SNPs in this study. Although all the 1800 SNPs were preserved, the positions and orders of some SNPs on linkage map were corrected, generating a genetic map with better synteny when aligned to 9930 reference genome (Supplementary Fig. S1). QTL analysis of FL and MFL was performed using R/qtl (http://www.rqtl.org/) and MapQTL®6 (https://www.kyazma.nl/index.php/mc.MapQTL/) software packages to produce consensus QTL regions.
In total, eight QTLs were identified for FL and MFL in four cucumber chromosomes (chromosomes 1, 3, 4 and 6). Information for QTLs detected in QTL mapping is provided in Table 1. Figures for all QTLs with LOD sores were presented in Supplementary Fig. S2. Three QTLs, mfl1.1, mfl3.2, and fl3.2 were detected using two software packages in both growing seasons with slightly different physical intervals of each QTL locus. For QTL fl3.2, despite the slight shift of start and end locations detected with MapQTL in two seasons, the high phenotypic variation explained by this locus (>36%) and the same chromosome regions identified using R/qtl in both seasons suggest that this locus has a major effect on cucumber immature fruit length. There were four minor-effect QTLs (fl1.1, fl3.1, mfl3.1, and fl4.1) detected exclusively with R/qtl in either spring 2013 or autumn 2014, in which three QTLs for FL were noted. QTL fl6.1 was detected with two software packages in both seasons except for R/qtl in spring 2013. The chromosome interval of QTL fl3.2 (22.71–28.20 Mb) overlapped with that of mfl3.2 (25.37–30.54 Mb) in a 2.83 Mb region, explaining at least ~30.87% of fruit length variation. Thus, fl3.2 and mfl3.2 might simultaneously control elongation of immature and mature fruit. QTLs located in close chromosome regions were considered as a consensus QTL interval, and the maximum span of flanking markers of the QTL locus was obtained. For example, QTL mfl1.1 on chromosome 1 spanned a physical interval from 13.30 to 17.66 Mb.
Table 1. Summary of QTLs detected for immature and mature fruit length using MapQTL and R/qtl in F3 families in spring 2013 and autumn 2014.
| QTL | Chr | Data for QTL analysis | QTL package | 1.5 LOD interval (CM) |
Chromosome locations (Mb) |
Peak location (Mb) | Interval(Mb) | LOD score | % Expl. | SNP NO. | Consistently QTL | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left | Right | start | end | ||||||||||
| fl1.1 | 1 | 2014, autumn | R/qtl | 35.83 | 41.91 | 7.27 | 9.18 | 8.78 | 1.92 | 5.95 | * | 19 | FS1.1, Weng et al.2 |
| mfl1.1 | 1 | 2013, spring | R/qtl | 61.31 | 70.50 | 13.30 | 16.46 | 13.51 | 3.16 | 11.73 | * | 8 | FS1.1, Weng et al.2; fl1.1, Cheng et al.28; mfl1.1, Bo et al.10; mfl1.2, Wei et al.9 |
| 1 | 2013, spring | MapQTL | 64.35 | 74.70 | 13.47 | 17.43 | 16.48 | 3.97 | 6.23 | 17.34 | 12 | ||
| 1 | 2014, autumn | R/qtl | 69.35 | 74.02 | 13.41 | 17.66 | 15.15 | 4.25 | 10.12 | * | 9 | ||
| 1 | 2014, autumn | MapQTL | 71.99 | 73.35 | 16.48 | 16.84 | 16.52 | 0.36 | 5.94 | 20.08 | 4 | ||
| fl3.1 | 3 | 2013, spring | R/qtl | 41.85 | 50.10 | 9.76 | 10.17 | 10.12 | 0.41 | 7.11 | * | 9 | FS3.1, Weng et al.2 |
| fl3.2 | 3 | 2014, autumn | MapQTL | 118.18 | 139.49 | 22.71 | 28.20 | 26.37 | 5.50 | 12.81 | 36.76 | 23 | FS3.2, Weng et al.2;fl3.2, Yuan et al. 2008;mfl3.1, Wei et al.9 |
| 3 | 2013, spring | MapQTL | 118.52 | 136.51 | 22.72 | 27.67 | 26.37 | 4.95 | 15.59 | 38.78 | 27 | ||
| 3 | 2013, spring | R/qtl | 121.70 | 130.14 | 25.37 | 27.02 | 26.37 | 1.65 | 13.80 | * | 20 | ||
| 3 | 2014, autumn | R/qtl | 121.70 | 130.14 | 25.37 | 27.02 | 25.83 | 1.65 | 15.69 | * | 20 | ||
| mfl3.1 | 3 | 2013, spring | R/qtl | 29.28 | 38.40 | 7.57 | 9.03 | 8.09 | 1.45 | 6.36 | * | 13 | FS3.1, Weng et al.2 |
| mfl3.2 | 3 | 2013, spring | R/qtl | 121.70 | 130.14 | 25.37 | 27.02 | 25.86 | 1.65 | 18.38 | * | 20 | FS3.2, Weng et al.2;fl3.2 Yuan et al. 2008;mfl3.1, Wei et al.9 |
| 3 | 2013, spring | MapQTL | 123.05 | 137.32 | 25.54 | 28.10 | 26.37 | 2.56 | 14.16 | 36.16 | 32 | ||
| 3 | 2014, autumn | MapQTL | 135.70 | 142.67 | 27.51 | 29.98 | 26.37 | 2.48 | 10.05 | 30.87 | 15 | ||
| 3 | 2014, autumn | R/qtl | 136.85 | 143.48 | 27.98 | 30.54 | 29.98 | 2.57 | 12.99 | * | 12 | ||
| fl4.1 | 4 | 2014, autumn | R/qtl | 36.13 | 39.31 | 11.45 | 11.71 | 11.54 | 0.26 | 4.34 | * | 4 | FS4.1, Weng et al.2;fl4.1 Yuan et al. 2008;mfl4.1, Wang et al.29;fl4.1, Bo et al.10 |
| fl6.1 | 6 | 2013, spring | MapQTL | 31.78 | 41.24 | 11.11 | 15.50 | 14.13 | 4.39 | 6.43 | 17.76 | 26 | fl6.1, Bo et al.10; fl6.1, Wei et al.9 |
| 6 | 2014, autumn | R/qtl | 33.47 | 36.17 | 11.64 | 14.13 | 12.72 | 2.49 | 4.76 | * | 22 | ||
| 6 | 2014, autumn | MapQTL | 35.50 | 37.86 | 12.72 | 14.83 | 13.99 | 2.11 | 5.10 | 16.49 | 17 | ||
QTL-seq
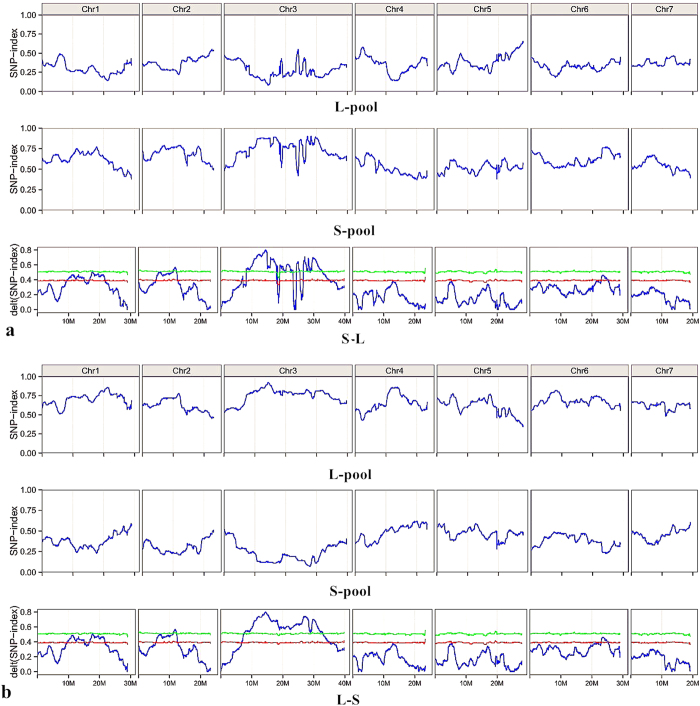
Illumina high-throughput sequencing generated 13,324,160,800 and 10,859,863,600 short reads (100 bp in length) from the L-pool and the S-pool, with a coverage of 91.34% (42.69-fold genome coverage) and 92.28% (34.22-fold genome coverage), respectively. The minimum Q20% was 93.84% of the S-pool, and the maximum was 94.26% of the L-pool. The lowest sample coverage was 91.34% of the L-pool. The effective sequencing depths for CC3 and NC76 were 16.41-fold and 21.04-fold genome coverage, respectively, which guarantees the accuracy of subsequent analysis. The results from QTL-seq are presented in Table 2. Sequence data were trimmed and filtered prior to analysis. Two paternal lines, CC3 and NC76, in the present study were resequenced because no reference genome sequences were available for these two cucumber lines. First, short reads from both parents were aligned to the 9930 genome to obtain two consensus sequences, which were used as reference sequences in the subsequent analysis. Second, reads obtained from two DNA-bulks (L-pool and S-pool) were aligned to consensus sequences to identify single nucleotide polymorphisms (SNPs). To identify genomic regions associated with fruit length, we evaluated the proportion of SNP bases available between L-bulk and S-bulk. The SNP-index was calculated for each SNP identified in the genome, and the average SNP-index within a 1 Mb window size was computed using a 10 kb step increment. SNP-index graphs for L-pool and S-pool were plotted by aligning an average SNP-index against the position of each sliding window in the CC3 reference genome (Fig. 3a). The Δ (SNP-index) was calculated and plotted using the information from two graphs for L-bulk and S-bulk (Fig. 3a). Δ (SNP-index) = 1 means that the bulked DNA exclusively comprises the NC76 genome (S–L). To ensure the accuracy of results, graphs of SNP-index and Δ SNP-index using the genome sequence of NC76 as a reference genome were also generated (Fig. 3b), whereby Δ (SNP-index) = 1 means that the bulked DNA exclusively comprises the CC3 genome (L–S).
Table 2. Summary of QTL sequencing data for each sample.
| Name | Sample Clean Base (bp) | Clean Q20 (%) | Clean GC Content (%) | Sample coverage rate (%) | Map reads rate (%) | Uni hit reads rate (%) | Sequencing depth | Effective depth |
|---|---|---|---|---|---|---|---|---|
| L-pool | 13324160800 | 94.26 | 38.1 | 91.34 | 61.98 | 54.43 | 65.73 | 42.69 |
| S-pool | 10859863600 | 93.84 | 37.99 | 92.28 | 61.03 | 52.54 | 53.58 | 34.22 |
| CC3 | 5075004600 | 94.09 | 38.69 | 92.86 | 62.55 | 54.59 | 25.04 | 16.41 |
| NC76 | 6472593400 | 94.01 | 38.22 | 93.31 | 62.93 | 54.47 | 31.93 | 21.04 |
Figure 3. SNP-index graphs of L-pool, S-pool and Δ (SNP-index) graphs from QTL-seq analysis.
The Δ (SNP-index) plot with statistical confidence intervals under the null hypothesis of no QTL (green, P < 0.01; red, P < 0.05). The X-axis represents the position of seven chromosomes, and the Y-axis represents the SNP-index. The SNP-index was calculated based on a 1 Mb interval with a 10 kb sliding window. a: using CC3 genome sequence as the reference genome; b: using NC76 genome sequence as the reference genome.
DNA sequences of L-bulk and S-bulk were expected to be identical except for regions harbouring QTLs relevant to ovary length where unequal contributions from CC3 and NC76 paternal genomes may exist. In total, we identified six QTLs underlying ovary length in RIL populations (ovl1.1, ovl1.2, ovl2.1, ovl3.1, ovl3.2, and ovl6.1) across four cucumber chromosomes (Table 3). Four QTLs were detected using both parent genome sequences, whereas two QTLs were only detected with the NC76 reference genome. In addition, sequence alignment using different paternal reference genomes may result in different chromosome locations. Statistical confidence intervals of Δ (SNP-index) were calculated for all the SNP positions with given read depths under the null hypothesis of no QTL (see Materials and Methods). The chance that Δ (SNP-index) becomes higher than 0.52 as observed for the chromosomal region of 10.73–12.76 Mb is P < 0.01 under the null hypothesis. Likewise, the chance that Δ (SNP-index) becomes higher than 0.48 as observed for the chromosomal region of 16.95–20.20 Mb is 0.01 < P < 0.05 under the null hypothesis. The six Δ (SNP-index) peaks for OvL were statistically significant (the peaks for ovl2.1, ovl3.1 and ovl3.2, P < 0.01; and the peaks for ovl1.1, ovl1.2 and ovl6.1, 0.01 < P < 0.05). QTL ovl3.2, spanning a physical interval of 25.97 to 30.55 Mb on chromosome 3, was a major-effect QTL with a Δ (SNP-index) of 0.74 (P < 0.01). Another QTL that had a major effect on the ovary length was ovl3.1 ranged from 11.12 Mb to 16.45 Mb with a peak ΔSNP-index value of 0.80 (P < 0.01).
Table 3. Summary of QTLs detected for ovary length with QTL-seq in RIL populations in spring 2015.
| QTL | Cucumber Chr. | Chromosome locations (Mb) | Interval (Mb) | △SNP-index | Parent for QTL-seq analysis | ||
|---|---|---|---|---|---|---|---|
| start | end | min | max | ||||
| ovl1.1 | 1 | 10.42 | 12.09 | 1.67 | 0.48 | 0.49 | NC76 |
| ovl1.2 | 1 | 16.92 | 18.83 | 1.91 | 0.48 | 0.51 | CC3 |
| 1 | 16.95 | 20.20 | 3.25 | 0.48 | 0.51 | NC76 | |
| ovl2.1 | 2 | 10.73 | 12.76 | 2.03 | 0.52 | 0.57 | CC3 |
| 2 | 10.77 | 12.76 | 1.99 | 0.52 | 0.57 | NC76 | |
| ovl3.1 | 3 | 11.12 | 16.45 | 5.48 | 0.71 | 0.80 | NC76 |
| 3 | 11.25 | 15.45 | 4.20 | 0.71 | 0.80 | CC3 | |
| ovl3.2 | 3 | 25.97 | 30.55 | 4.58 | 0.70 | 0.74 | NC76 |
| ovl6.1 | 6 | 22.55 | 25.12 | 2.57 | 0.42 | 0.46 | CC3 |
| 6 | 22.57 | 24.75 | 2.18 | 0.44 | 0.50 | NC76 | |
Comparison of QTL detection using two NGS-based methods
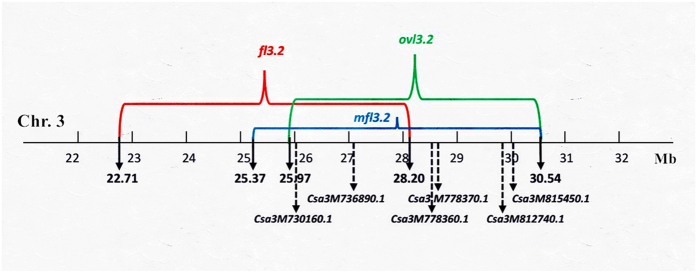
The two strategies employed in the present study take advantage of high-throughput NGS. QTL-seq of 2015 RIL trails identified 6 QTLs for ovary length (ovl1.1, ovl1.2, ovl2.1, ovl3.1, ovl3.2, and ovl6.1). QTL mapping of 2013 and 2014 F3 families using a revised saturated SNP-based genetic map detected 3 QTLs for mature length (mfl1.1, mfl3.1 and mfl3.2). RIL trails are more suitable for detecting minor-effect QTLs, which may partly explain why more QTLs were identified when using RILs. Two overlapping regions were noted between chromosomal intervals of mfl1.1 (13.30–17.66 Mb) and ovl1.2 (16.92–20.20 Mb) as well as mfl3.2 (25.37–30.54 Mb) and ovl3.2 (25.97–30.55 Mb). Although the QTL interval of ovl1.1 (10.42–12.09 Mb) did not overlap with mfl1.1, it was found to be located between fl1.1 (7.27–9.18 Mb) and mfl1.1, where a dozen QTLs were detected for fruit length and defined as FS1.1 and FS1.2 by Weng et al.2. Given that the genomic regions of fl3.2 (22.71–28.20 Mb), mfl3.2 and ovl3.2 that control fruit length at different development stages overlapped (as shown in Fig. 4), we defined QTL FL3.2 as a consensus QTL that potentially played a consistent role in cucumber fruit elongation through all growing phases. The maximum interval was obtained for FL3.2 ranging from 22.71 to 30.55 Mb (7.84 Mb), whereas the actual overlapping region spanned 2.33 Mb (25.97–28.20 Mb). However, differences in QTL detection were present in several cases. The QTL interval of ovl3.1 did not overlap with mfl3.1; QTLs ovl2.1 and ovl6.1 were only detected for OvL. A high correlation coefficieny was observed between OvL and MFL using phenotypic data from 2015 RIL trails. Whereas QTL-seq of OvL was conducted with 2015 RILs, QTL mapping of MFL was conducted with 2013 and 2014 F3 families. Different mapping populations and QTL analysis methods possibly contributed to the differences in chromosomal locations of QTLs for OvL and MFL.
Figure 4. The co-localization of six fruit size homologues within chromosomal interval of the consensus QTL FL3.2.
Chromosomal intervals of ovl3.2 (green), fl3.2 (red) and mfl3.2 (blue) are given in Mb.
Analysis of genome-wide homologous genes for fruit size
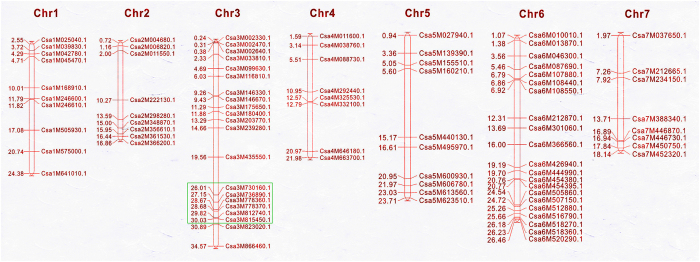
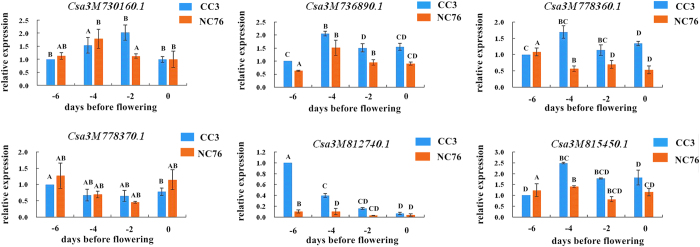
A total of 74 homologues of fruit size-related genes were identified in the melon genome using tomato or Arabidopsis thaliana gene family sequences as queries23. With the hypothesis that gene families associated with fruit size may have similar roles in regulating fruit morphology, especially in phylogenetically close species10, we used the 74 melon fruit size-related sequences as queries to blast against the 9930 cucumber genome sequence24 (Version 2). Homologues of all 74 gene sequences were identified in the cucumber genome. Optimal alignment gene homologues and associated information of each homologous gene are presented in Supplementary Table S4. The distribution of homologous genes on cucumber chromosomes was shown in Fig. 5. To further understand the function of these homologs, gene ontology (GO) term enrichment analysis (P ≤ 0.05) was performed. The most enriched GO terms were ‘cellular process’ in biological process (GOBP) group (green in Fig. S3), ‘cell part’ in molecular function (GOMF) group (blue in Fig. S3) and ‘binding’ in cellular component (GOCC) (red in Fig. S3), respectively. Six genes (Csa3M730160.1, Csa3M736890.1, Csa3M778360.1, Csa3M778370.1, Csa3M812740.1, and Csa3M815450.1) from predicted fruit-size homologues co-localized within the chromosomal region of the consensus QTL FL3.2; thus, these genes are considered as possible candidate genes for fruit length. GO terms were assigned to three candidate genes (Csa3M730160.1, Csa3M736890.1, and Csa3M812740.1) (Supplementary Table S4). Csa3M812740.1 was involved in multiple biological processes (GOBP) such as embryo morphogenesis, floral organ development and cell growth, etc. Gene annotation of the six fruit size homologues using Cucurbit genomics database (http://www.icugi.org) was shown in Supplementary Table S4. Furthermore, six primers pairs were designed based on cDNA sequences to perform qRT-PCR analysis. Expression levels of all candidate genes in ovaries of 0, 2, 4, and 6 days before flowering from both parents were measured. Six genes except for Csa3M778370.1 and Csa3M815450.1 exhibited increased expression levels in CC3 compared with NC76, especially on ovaries of 2 and 4 days before flowering, including Csa3M730160.1 and Csa3M736890.1 located in the 2.23Mb overlapping interval of FL3.2 (Fig. 6). Csa3M778370.1 exhibited no obvious regularity in expression patterns. Csa3M812740.1 had significantly increased expression in the ovaries of CC3, corresponding to its involvement in multiple biological processes that determine the development of ovary such as embryo morphogenesis, floral organ development and cell growth, etc. Gene annotation suggested that Csa3M730160.1 and Csa3M778360.1 encoded members from ovate family protein (OFP); Csa3M736890.1 encodes a member from IQ domain family. The functions of Csa3M778370.1 and Csa3M815450.1 are still unknown.
Figure 5. Distribution of homologous genes for fruit size detected on seven cucumber chromosomes.
Physical locations for fruit size homologues on cucumber chromosomes (Chr. 1–7, on the top). Chromosomal distances (Mb) are on the left, and gene names are on the right. The six homologous genes co-localized within QTL FL3.2 are depicted by green box.
Figure 6. qRT-PCR results of six candidate genes localized in chromosome regions of the consensus QTL FL3.2.
The blue bars represent CC3, and the red bars represent NC76; The X-axis indicates 0, 2, 4, 6 days before anthesis, whereas the Y-axis indicates expression levels. The alphabets (A–D) represent significant levels of gene expression.
Discussion
Early fruit development of many horticultural crops, including the cucumber, can be divided into three phases: development of the ovary, cell division, and cell expansion25. The increase in cucumber fruit size is often mirrored by the increase in cell number and size26. Insight into the genetic and molecular mechanisms of early fruit development is important to understand the factors determining fruit yield and quality in cucumber production. Cell division typically occurs approximately one week post-anthesis, whereas cell enlargement persists throughout the process of fruit development27. In the melon, the ovary and mature fruit morphologies exhibit a high correlation20,21, and fruit shape is predominantly determined pre-anthesis. In the present study, correlation analysis was conducted based on ovary, immature and mature fruit length in F3 families and RIL populations derived from CC3 × NC76 in 2013, 2014 and 2015. The correlation coefficient between FL and MFL was 0.896, whereas that of OvL and MFL was 0.917 in RILs. The high correlation coefficient, especially between OvL and MFL, suggests that early fruit development may directly determine final fruit length. These results were consistent with that of Weng et al.2, in which strong positive correlations were also observed among OvL, FL, MFL and OvN using F2, F3 families and RIL populations derived from 9930 × Gy14. Thus, dynamic changes in fruit length during fruit development are consistent, i.e., the fruit length of NC76 was consistently shorter than that of CC3 through all growing stages. OvL could serve as good indicator for FL and MFL in the QTL analysis of cucumber fruit length.
High-throughput NGS has revolutionized the approach to QTL analysis of complex traits in rapid marker discovery and map construction. We conducted rapid QTL detection of cucumber fruit length using two NGS-based strategies. QTL mapping requires a linkage map with abundant markers, whereas QTL-seq does not require the construction of segregation populations or screens for polymorphic markers. However, QTL-Seq can provide a rough location of a particular QTL, and additional QTL analysis is still needed to refine locations and narrow chromosomal intervals. Highmap22 was employed to re-genotype the SNPs from a pre-constructed genetic map using SLAF-seq9. A revised SNP-based genetic map (1800 SNPs) was generated with better synteny when aligned to 9930 reference genome (Fig. S1). QTL mapping was performed in F3 families in spring 2013 and autumn 2014 using the revised SNP genetic map. QTL-seq was performed using 2015 RIL populations. In total, 14 QTLs associated with OvL, FL and MFL were detected on all cucumber chromosomes except for chromosomes 5 and 7. Given the high correlation coefficiency between OvL and MFL from 2015 RIL trails, corresponding correlated chromosomal intervals of QTLs for OvL and MFL are expected. Indeed, two overlapping regions were identified between QTL intervals of mfl1.1 and ovl1.2, and mfl3.2 and ovl3.2. We defined a consensus QTL FL3.2 for OvL, FL and MFL that harbors ovl3.2, fl3.2 and mfl3.2, which was considered as a major-effect QTL for cucumber fruit length. This result is consistent with that of our previous study that identified two major QTLs (fl3.2 and mfl3.2) for fruit length. However, there are several cases that QTL locations did not overlap such as ovl3.1 and mfl3.1. One reasonable explanation is that different environment conditions, populations and QTL analysis methods affect the results. In addition, although OvL and MFL are highly correlated, they are considered as different traits.
A number of fruit length QTLs have been identified in previous studies and are distributed on all seven chromosomes2,4,5,6,8,9,10,28,29. Assuming that QTLs at the same or close chromosome locations belonged to the same QTL locus, those from different studies may share common genetic mechanisms underlying fruit elongation. Weng et al.2 identified 12 consensus QTLs for fruit size by the synthesis of information from 29 consistent and distinct fruit size QTLs. The four QTLs on chromosome 1 in the present study were mapped in the consensus QTL region of FS1.1 and FS1.2 for fruit size (Table 1). Similarly, QTL ovl6.1 for ovary length localized in consensus QTL regions for FS6.1 and FS6.2 which harboured QTLs for FL identified by several studies2,6,7,27. The QTL for FL on chromosome 4 (fl4.1) was close to several QTLs detected by three previous studies2,6,10. QTL fl6.1 has a highly consistent chromosome interval in different mapping populations and environmental conditions9. Although ovl3.2 in the present study has a different chromosome location from that detected by Weng et al.2, both were located in consensus QTL FS3.2. We detected two QTLs for ovary length (ovl1.1 and ovl1.2) in the RIL populations. One QTL (qOvL1.1) was detected by Weng et al.2, which was close to the chromosome location of ovl1.1. Previously, cucumber chromosome 2 has only one QTL identified for immature fruit length, whereas we detected a QTL for ovary length (ovl1.2) located within chromosome intervals of FS2.1. QTLs fl3.1 and mfl3.1 were localized in the QTL region of FS3.1, whereas ovl3.1 was close to a QTL for immature fruit length (fl3.1) identified by Wang et al.29. Many factors could influence results of QTL mapping from different studies for the same trait, such as different mapping populations (F2, F3 and RIL), the season, different growth stages and environment conditions. However, a more reasonable explanation is that cucumber fruit size has undergone domestication or diversifying selection for different taxonomic groups or specialized market classes. Thus, the underlying genes/QTLs for the same trait may be differentially expressed in cucumber lines of different market classes.
The molecular and genetic mechanisms underlying fruit development in the tomato (Solanum lycopersicum) have been extensively studied, with several genes/QTLs for fruit size or shape being fine-mapped and cloned30,31,32. Four genes regulating fruit shape (SUN and OVATE) and weight (CNR/FW2.2 and SlKLUH/FW3.2) are associated with fruit elongation. SUN encodes a protein that belongs to the IQ domain family; OVATE encodes a protein in the ovate family protein (OFP). CNR/FW2.2 encodes one of the cell number regulators (CNR). Putative orthologues of genes regulating tomato fruit morphology have been proposed as candidate genes in other species such as pepper and cherry33,34,35. With the assumption that fruit morphology in different taxa could be controlled by genes from certain ancestral gene families, Monforte et al.23 identified 74 homologues of the CNR, CYP78A, OFP, SUN, WOX, and YABBY gene families for fruit shape and size in the melon genome. We used the 74 melon gene sequences as queries to BLAST against the 9930 cucumber genome sequence24 (Version 2). Homologues of all 74 gene sequences were identified in the cucumber genome, and this finding is consistent with results reported by Bo et al.10. We employed a different E value and retained all the 87 genes detected; the blast frequency was calculated for each gene as another reference besides identity. The consensus QTL FL3.2 compromised six homologous genes for fruit size, whereas GO terms were assigned to three of them (Csa3M730160.1, Csa3M736890.1, and Csa3M812740.1) (Supplementary Table S4). Notably, all six predicted genes co-localized in the QTL region detected for ovl3.2. Expression levels of six genes except for Csa3M778370.1 and Csa3M815450.1 were higher in CC3 compared to NC76, especially 2 and 4 days before flowering (Fig. 6). Csa3M812740.1 had significantly increased expression in the ovaries of CC3, which corresponded to its involvement in multiple biological processes such as embryo morphogenesis, floral organ development and cell growth, etc. Gene annotation using Cucurbit genomics database (http://www.icugi.org) suggests that Csa3M730160.1 and Csa3M778360.1 encode members from OFP family; Csa3M736890.1 encodes a member from IQ-domain family. The functions of Csa3M778370.1 and Csa3M815450.1 are lacking. The increased levels that exhibited by the four cucumber homologous genes in CC3 and corresponding functions implicate the putative involvement in regulating cucumber fruit length. However, additional work is still needed to validate the roles of homologous genes in determining cucumber fruit size. Seven kinesin genes (CsKF1 to CsKF7) in the cucumber genome were associated with rapid cell production and cell expansion via transcriptome analysis of cucumber lines with different fruit sizes13. Weng et al.2 anchored 74 fruit size homologous genes and the seven kinesin genes onto a cucumber SNP map, most of which are located within the QTL regions detected from the present and previous studies. Nevertheless, one QTL locus often harbours multiple gene homologs. Genomic intervals of QTL loci still need to be narrowed down to determine candidate genes for cucumber fruit length.The results from the present study demonstrate the power of combined QTL mapping and QTL-seq method in multiple traits and provide insights into the genetic mechanisms underlying the cucumber fruit length.
Materials and Methods
Plant materials and phenotypic data collection
Two cucumber inbred lines, ‘CC3’ and ‘NC76’, were used to develop segregating populations for QTL analysis in this study (Fig. 1a). CC3, the maternal line, is a typical North China fresh market type with slim immature fruits and a length of 20~30 cm at commercial harvest stage. NC76, the paternal line, is a US slicing cucumber that bears short immature fruits of 7 to 10 cm in length at commercial harvest stage. In this study, a single F1 plant from the cross between CC3 and NC76 was self-pollinated to produce 148 F2, and 133 F2-derived F3 families were used in QTL mapping. In total, 135 F7 recombinant inbred lines (RILs) developed through single seed descent were used for QTL-seq (Fig. 1b).
Two parental lines (CC3 and NC76), F3 families and F7 RILs were grown in a greenhouse at Jiangpu Cucumber Research Station of Nanjing Agricultural University (JCRSNAU), Nanjing, China. The soil media was 25% peat + 25% cinder + 50% perlite. For the evaluations of immature and mature fruit length (FL and MFL), six plants randomly selected from F3 families were grown in July 2014, and three plants from each F7 RILs were planted to measure OvL and MFL in March 2015. Ovaries were hand-pollinated to produce immature and mature fruits, and three fruits from the sixth node of each plant were measured for fruit length (OvL and MFL) using digital callipers. The traits were measured according to the standards published by Yuan et al.6. All measurements were obtained on individual plant and averaged within each serial number. Subsequent data were analysed with analysis of variance and partial correlations using Microsoft Excel 2013.
DNA isolation
Genomic DNA was extracted from young healthy leaves using the cetyltrimethyl ammonium bromide (CATB) method36. DNA concentration and quality were examined with the Qubit® 2.0 fluorometer (Invitrogen-Molecular Probes, Eugene, OR) and 1% agarose gel electrophoresis with a standard lambda DNA and a ND-1000 spectrophotometer (NanoDrop, Wilmington, DE, USA).
QTL analysis
Using 148 F2 plants from CC3 × NC76, we previously constructed a saturated cucumber genetic map compromising 1800 SNPs. The F3 families used for fruit length data collection were derived from the same F2 population. In the present study, we re-genotyped the 1800 SNPs using Highmap22 software and corrected genotypes of some SNPs to generate a revised genetic map with the same number of SNPs. Details of genotype corrections and information of this SNP-based map are presented in Supplementary Table S1. The original SNP information is reported in Wei et al.9
QTL analysis was performed with R/qtl (http://www.rqtl.org/) and MapQTL®6 (https://www.kyazma.nl/index.php/mc.MapQTL/) using the revised F2 genetic map. QTL models adopted in R/qtl and MapQTL were CIM and MQM, respectively. Means of fruit length within each F3 family in spring 2013 and autumn 2014 were calculated and subjected to QTL analysis. The support intervals for map locations of QTL were calculated using a 1.5 likelihood-ratio statistic (LOD) drop interval. To declare the significance of QTL, LOD threshold was determined using a permutation test with 1000 repetitions (P = 0.05). The QTL was named according to its chromosome location and trait name. For example, fl1.1 and mfl3.1 refer to the first QTL for the length of immature and mature fruits on cucumber chromosomes 1 and 3, respectively.
QTL-seq
Equal amounts of DNA were sampled from individuals with extreme long (4.0–6.0 cm) or short (1.3–2.0 cm) ovaries (15 DNA samples for each extreme trait) and bulked to generate the ‘Longest’ pool (L-pool) and ‘Shortest’ pool (S-pool). Pair-end sequencing libraries with a read length of 100 bp and insert sizes of approximately 500 bp were subjected to whole-genome resequencing with Illumina HiSeq 2500. Short reads obtained from both parents and two DNA-bulks were aligned against the cucumber genome sequence (the 9930 reference genome) to obtain the consensus sequence using BWA software24,37. Reads of L-pool and S-pool were separately aligned to CC3 and NC76 consensus sequence reads to call SNPs with SAM tools software37.
We calculated the SNP-index and Δ (SNP-index) to determine candidate fruit length-related QTLs16,38. The SNP-index refers to the proportion of reads harbouring a SNP when aligned to genome sequences of either parent. The average SNP-index of SNPs in a certain genomic interval was calculated using a sliding window analysis with 1 Mb window size and 10 kb increment. Δ (SNP-index) is the difference between SNP-index of L-pool and that of S-pool. Using CC3 as reference genome, SNP-index was 0 when all short reads contain genomic fragments from CC3, and SNP-index was 1 when all short reads contain genomic fragments from NC76. To ensure the accuracy of QTL detection, genome sequence of NC76 was also used as reference, and the SNP-index was similarly calculated. A SNP-index of 0.5 indicates equal contribution from both paternal genomes. Thus, most short reads are from both parents, except regions that significantly contribute to certain phenotypic variation. Δ (SNP-index) presents a strengthened result because it can eliminate background interference. The SNP-index graphs and corresponding Δ (SNP-index) graph were plotted. Statistical confidence intervals of Δ (SNP-index) was calculated under the null hypothesis of no QTLs followed the description by Takagi H. et al.16.
Physical mapping and Gene Ontology (GO) annotation
Physical mapping of the genes homologous to fruit size onto the cucumber genome was performed by conducting BLASTN search of respective CDS sequences against the cucumber genome. Subsequently the genes were plotted onto the seven chromosomes according to their ascending order of physical position (bp), from the short arm telomere to the long arm telomere and ultimately the map was displayed using MapChart39.
We used Blast2GO40 to assign GO terms to cucumber genes. The GO term enrichment analysis was conducted for fruit size homologues in cucumber. The six homologous genes co-localized within QTL FL3.2 was annotated using Cucurbit genomics database (http://www.icugi.org).
Quantitative PCR (qPCR) analysis of candidate genes
Ovaries at 0, 2, 4, and 6 days before flowering from both parents were obtained and frozen in liquid nitrogen. Experiments were performed with two biological replicates (2 fruits from 2 plants) for each parent. Total RNA was isolated, and DNase I (Fermentas) digestion was performed for 30 min at 25 °C to remove DNA according to manufacturer’s instructions. cDNA was synthesized from 2 mg of total RNA using a cDNA Synthesis Kit (Fermentas). Quantitative real-time PCR was performed with a SYBR Premix Ex TaqTM Kit (TAKARA) in a Bio-Rad iQ1 real-time PCR system (Bio-Rad) as described by Li et al.41. The threshold cycle (Ct) value of each gene was investigated and normalized to the Ct value of Cs-Actin. To determine relative expression fold differences for each gene during different treatments, the 2−△△Ct formula was applied. PCR primers were designed with Primer Premier 5.0 software (Premier Biosoft International) to avoid the conserved region. Details of the primer sequences are presented in Supplementary Table 5. Three replicates were used for the qRT-PCR. Analysis of relative mRNA expression data was performed using the Δ Ct method.
Additional Information
How to cite this article: Wei, Q.-z. et al. Rapid identification of fruit length loci in cucumber (Cucumis sativus L.) using next-generation sequencing (NGS)-based QTL analysis. Sci. Rep. 6, 27496; doi: 10.1038/srep27496 (2016).
Supplementary Material
Acknowledgments
This research was partially supported by Key Program 31430075, General Programs 31272174 and 31471872 from the National Natural Science Foundation of China, the National Basic Research Program of China (973 Program:2012CB113900), the ‘863’ Project (2012AA100102), the ‘111’ Project (B08025), and the National Key Technology R&D Program of the Ministry of Science and Technology of China (2013BAD01B04-10).
Footnotes
Author Contributions J.-F.C. and Q.-F.L. conceived of the study and designed the experiments. Q.-F.L. and Q.-Z.W. conceived of the study, participated in its design, carried out data analysis and drafted the manuscript. F.W.Y., W.-Y.F., J.W. and J.L. helped with statistical collection and analysis. X.-D.Q. participated in data analysis. Y.-Z.W. helped collect statistics and draft the manuscript. All authors read and approved the final manuscript.
References
- Qi J. et al. A genomic variation map provides insights into the genetic basis of cucumber domestication and diversity. Nat. Genet. 45, 1510–1515 (2013). [DOI] [PubMed] [Google Scholar]
- Weng Y. et al. QTL mapping in multiple populations and development stages reveals dynamic quantitative trait loci for fruit size in cucumbers of different market classes. Theor. Appl. Genet. 128, 1747–1763 (2015). [DOI] [PubMed] [Google Scholar]
- Jiang L. et al. Transcriptomic analysis reveals the roles of microtubule-related genes and transcription factors in fruit length regulation in cucumber (Cucumis sativus L.). Sci. Rep. 5, 8031; 10.1038/srep08031 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Havey W. C. K. M. J. Quantitative trait analysis of fruit quality in cucumber QTL detection, Theor. Appl. Genet. 91, 53–61 (1995). [DOI] [PubMed] [Google Scholar]
- Yuan X. et al. Genetic mapping and QTL analysis of fruit and flower related traits in cucumber (Cucumis sativus L.) using recombinant inbred lines. Euphytica 164, 473–491 (2008a). [Google Scholar]
- Yuan X. et al. Genetic linkage map construction and location of QTLs for fruit‐related traits in cucumber. Plant Breeding 127, 180–188 (2008b). [Google Scholar]
- Miao H. et al. Mapping qtls for fruit-associated traits in Cucumis sativus L. Scientia Agricultura 24, 009 (2011). [Google Scholar]
- Dijkhuizen A. & Staub J. E. QTL Conditioning yield and fruit quality traits in cucumber (Cucumis sativus L.) effects of environment and genetic background. Journal of New Seeds 4, 1–30 (2002). [Google Scholar]
- Wei Q. et al. An SNP-based saturated genetic map and QTL analysis of fruit-related traits in cucumber using specific-length amplified fragment (SLAF) sequencing. BMC Genomics 15, 1158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bo K. et al. Molecular mapping reveals structural rearrangements and quantitative trait loci underlying traits with local adaptation in semi-wild Xishuangbanna cucumber (Cucumis sativus L. var. xishuangbannanesis Qi et Yuan). Theor. Appl. Genet. 128, 25–39 (2014). [DOI] [PubMed] [Google Scholar]
- Grumet R. & Kaori A. Transcriptional profiling of rapidly growing cucumber fruit by 454-pyrosequencing analysis. J. Amer. Soc. Hort. Sci. 135, 291–302. (2010). [Google Scholar]
- Ando K. et al. Transcriptome analyses of early cucumber fruit growth identifies distinct gene modules associated with phases of development. BMC Genomics 13, 518 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X. Y. et al. Characterization and expression profiling of cucumber kinesin genes during early fruit development: revealing the roles of kinesins in exponential cell production and enlargement in cucumber fruit. J. Exp. Bot. 64, 4541–4557 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvi S. & Tuberosa R. To clone or not to clone plant QTLs: present and future challenges. Trends Plant Sci. 10, 297–304 (2005). [DOI] [PubMed] [Google Scholar]
- Michelmore R. W. et al. Identification of markers linked to disease-resistance genes by bulked segregant analysis: a rapid method to detect markers in specific genomic regions by using segregating populations. Proc. Natl. Acad. Sci. 88, 9828–9832 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H. et al. QTL-seq: rapid mapping of quantitative trait loci in rice by whole genome resequencing of DNA from two bulked populations. Plant J 74, 174–183 (2013). [DOI] [PubMed] [Google Scholar]
- Lu H. et al. QTL-seq identifies an early flowering QTL located near Flowering Locus T in cucumber. Theor. Appl. Genet. 127, 1491–1499, (2014). [DOI] [PubMed] [Google Scholar]
- Illa-Berenguer E. et al. Rapid and reliable identification of tomato fruit weight and locule number loci by QTL-seq. Theor. Appl. Genet. 128, 1329–1342 (2015). [DOI] [PubMed] [Google Scholar]
- Das S. et al. Deploying QTL-seq for rapid delineation of a potential candidate gene underlying major trait-associated QTL in chickpea. DNA Res. 22, 193–203 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Périn C. et al. Genetic control of fruit shape acts prior to anthesis in melon (Cucumis melo L.). Mol Genet Genomics 266, 933–941 (2002). [DOI] [PubMed] [Google Scholar]
- Eduardo I. et al. Estimating the genetic architecture of fruit quality traits in melon using a genomic library of near isogenic lines. J Am Soc Hortic Sci 132, 80–89 (2007). [Google Scholar]
- Liu D. et al. Construction and analysis of high-density linkage map using high-throughput sequencing data. PLoS One 9, e98855 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monforte A. J. et al. The genetic basis of fruit morphology in horticultural crops: lessons from tomato and melon. J. Expt. Bot. 65, 4625–4637 (2014). [DOI] [PubMed] [Google Scholar]
- Huang S. et al. The genome of the cucumber, Cucumis sativus L. Nat. Genet. 41, 1275–1281 (2009). [DOI] [PubMed] [Google Scholar]
- Glenda G. et al. Fruits: A developmental perspective. Plant Cell 5, 1439 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boonkorkaew P., Hikosaka S. & Sugiyama N. Effect of pollination on cell division, cell enlargement, and endogenous hormones in fruit development in a gynoecious cucumber. Scientia Hortic. 116, 1–7 (2008). [Google Scholar]
- Marcelis L. & LRB H. E. Cell division and expansion in the cucumber fruit. J. Hortic. Sci. 68, 665–671 (1993). [Google Scholar]
- Cheng Z. C. et al. QTL Mapping of fruit length in cucumber. China Veg Issue 12, 20–25(2010) [Google Scholar]
- Wang M. et al. Quantitative trait loci associated with fruit length and stalk length in cucumber using RIL population. Act Bot Boreal Occident Sin 34, 1764–1770 (2014). [Google Scholar]
- Rodríguez G. R. et al. Distribution of SUN, OVATE, LC, and FAS in the tomato germplasm and the relationship to fruit shape diversity. Plant Physiol. 156, 275–285 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Knaap E. et al. What lies beyond the eye: the molecular mechanisms regulating tomato fruit weight and shape. Front. Plant Sci. 5, 10.3389/fpls.2014.00227 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azzi L. et al. Fruit growth-related genes in tomato. J. Expt. Bot. 66, 1075–1086 (2015). [DOI] [PubMed] [Google Scholar]
- Tsaballa A. et al. Multiple evidence for the role of an Ovate-like gene in determining fruit shape in pepper. BMC Plant Biol. 11, 46 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Franceschi P. et al. Cell number regulator genes in Prunus provide candidate genes for the control of fruit size in sweet and sour cherry. Mol. Breeding 32, 311–326 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G. et al. Fruit size QTL analysis of an F1 population derived from a cross between a domesticated sweet cherry cultivar and a wild forest sweet cherry. Tree Genet. Genomes 6, 25–36 (2010). [Google Scholar]
- Murray M. & Thompson W. F. Rapid isolation of high molecular weight plant DNA. Nucleic. Acids Res. 8, 4321–4326 (1980). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H. & Durbin R. Fast and accurate short read alignment with Burrows-Wheeler transform. Bioinformatics 25, 1754–1760 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abe A. et al. Genome sequencing reveals agronomically important loci in rice using MutMap. Nat. Biotechnol. 30, 174–178 (2012). [DOI] [PubMed] [Google Scholar]
- Voorrips R. MapChart: software for the graphical presentation of linkage maps and QTLs. J. Hered. 93, 77–78 (2002). [DOI] [PubMed] [Google Scholar]
- Conesa A. et al. Blast2GO: a universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 21, 3674–3676 (2005). [DOI] [PubMed] [Google Scholar]
- Li J. et al. A conserved phosphorylation site regulates the transcriptional function of ETHYLENE-INSENSITIVE3-like1 in tomato. J. Exp. Bot. 63, 427–439 (2012). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.