Abstract
Objectives
To evaluate the feasibility of an RCT of a pedometer driven walking program and education/advice to remain active compared with education/advice only for treatment of chronic low back pain (CLBP).
Methods
Fifty-seven participants with CLBP recruited from primary care were randomly allocated to either: (1) education/advice (E, n=17) or (2) education/advice plus an 8-week pedometer driven walking program (EWP, n=40). Step targets, actual daily step counts, and adverse events were recorded in a walking diary over the 8 weeks of intervention for the EWP group only. All other outcomes (eg, functional disability using the Oswestry Disability Questionnaire (ODQ), pain scores, physical activity (PA) measurement etc.) were recorded at baseline, week 9 (immediately post intervention), and 6 months in both groups.
Results
The recruitment rate was 22% and the dropout rate was lower than anticipated (13% to 18% at 6 mo). Adherence with the EWP was high, 93% (n=37/40) walked for ≥6 weeks, and increased their steps/day (mean absolute increase in steps/d, 2776, 95% confidence interval [CI], 1996–3557) by 59% (95% CI, 40.73%–76.25%) from baseline. Mean percentage adherence with weekly step targets was 70% (95% CI, 62%–77%). Eight (20%) minor-related adverse events were observed in 13% (5/40) of the participants. The EWP group participants demonstrated an 8.2% point improvement (95% CI, −13 to −3.4) on the ODQ at 6 months compared with 1.6% points (95% CI, –9.3 to 6.1) for the E group (between group d=0.44). There was also a larger mean improvement in pain (d=0.4) and a larger increase in PA (d=0.59) at 6 months in EWP.
Discussion
This preliminary study demonstrated that a main RCT is feasible. EWP was safe and produced a real increase in walking; CLBP function and pain improved, and participants perceived a greater improvement in their PA levels. These improvements require confirmation in a fully powered RCT.
Keywords: pedometer-driven walking, advice and education, chronic low back pain, randomized controlled trial, feasibility trial
Low back pain (LBP) has high lifetime prevalence with nonspecific LBP representing the majority of cases.(1) Relapses in pain (60%) and work absences (33%) are common,(1) making LBP one of the most costly conditions in the United Kingdom (total cost of £STG12,300 million, with the cost of informal care and production losses related to LBP contributing £STG10,668 million of this total figure).(2) Current research evidence supports the use of exercise- based treatment approaches for chronic low back pain (CLBP; pain persisting for at least 12 wk); in the United Kingdom, supervised group-based exercise programs are recommended, along with advice to stay physically active.(3) There is no specific guidance on how to self-direct participants to maintain or increase their physical activity (PA), although Savigny et al(3) refer to the National Institute for Clinical Health and Excellence (NICE) guidance on methods to increase PA.(4) One of the interventions identified in this review (NICE, 2006), was pedometer-driven walking, which can incorporate features considered important for increasing PA, that is, professional guidance, self-direction, and on-going professional support.(5)
The evidence to support walking in people with CLBP is promising(6,7); however, it is not yet clear what approach works best. Hartvigsen et al(8) showed that supervised Nordic walking was as effective as unsupervised Nordic walking, and a trial by some of the current research team(9) is currently investigating the effects of a structured walking program according to ACSM guidelines (30 min, 5 times/ wk). Other approaches for promoting PA have advocated the use of step targets driven by a pedometer, for example, 10,000 steps, which can be accumulated throughout the day in bouts of at least 10 minutes, and where 3000 steps approximates to 30 minutes of walking.(10) It is not clear whether such an approach would be suitable for people with LBP, for example, in terms of how many steps they should or could accumulate, and what risks might be associated with this approach for this clinical population. No trial to date has investigated the use of a pedometer driven walking program in people with CLBP.(7)
Therefore as a first step, before implementing a main RCT, we designed a preliminary study to test the feasibility of delivering a pedometer-driven walking program as an adjunct to a standard education/advice session in people with CLBP.(11) Our specific objectives were to:
Assess recruitment and adherence rates in education/ advice (E) and education/advice plus pedometer-driven walking program (EWP) groups.
Determine the incidence of adverse events (AEs), including musculoskeletal injuries, and level of overall satisfaction in both groups.
Make between and within group comparisons and estimate effect sizes for changes in functional disability, PA levels, stage of change, fear avoidance, self-efficacy, health-related quality of life, psychosocial beliefs, general health, and participant satisfaction.
MATERIALS AND METHODS
Ethical approval for the trial was granted by The Office for Research Ethics Committees (Northern Ireland) [Ref No. 09/NIR01/49]. All participants gave informed written consent before participation.
Study Population
Individuals diagnosed with nonspecific CLBP who fulfilled the eligibility criteria (Table 1) were recruited from primary care practice referral lists of 2 hospital physiotherapy departments (the Robinson Memorial Hospital, Ballymoney and the Fort Centre, Coleraine) or through retrospective searches of local primary care practices (for further details see McDonough et al(11)).
TABLE 1.
Inclusion and Exclusion Criteria
| Inclusion | Exclusion |
|---|---|
| Males, and females aged 18 y or over | Any spinal surgery in the past 12 mo |
| LBP with/without radiation persisting for > 12 wk | Evidence of nerve root, spinal cord, or cauda equina compression, severe spinal stenosis indicated by signs of neurogenic claudication, grade 3 to 4 spondy lolisthesis (grade 1 to 2 spondy lolisthesis eligible for inclusion), fibromyalgia, or systemic/inflammatory disorder |
| Capable of participating in home-based exercise as indicated by their GP (home- based, waiting intervention) | Any other current musculoskeletal injury or contra indication to increasing PA levels, including any cardio respiratory or other medical condition limiting exercise tolerance |
| Fluency in English (verbal and written) | LBP caused by involvement in a road traffic accident in the last 12 mo or ongoing litigation History of serious psychological or psychiatric illness (mild depression eligible for inclusion) Current pregnancy |
LBP indicates low back pain; GP general practitioner; PA. physical activity.
Randomization
Consenting participants were randomized using a computer-generated random allocation sequence, to either the education/advice group (E) or the education/advice and walking group (EWP), with a 1:2 allocation ratio in favor of walking. This was to ensure as much information as possible was gathered regarding adverse events or other side effects. The trial statistician, who was not involved in the administration of treatment or collection of outcomes, generated the schedule for the random allocation sequence, which was held in a secure cabinet. To investigate whether treatment preference had any influence on outcomes, each participant was asked which treatment he/she would prefer to receive before randomization. Because of the nature of the interventions, it was not possible to blind participants or treatment providers (see McDonough et al(11) for more details).
Physiotherapists
Treatment was provided by 2 chartered physiotherapists, who undertook 2½days of training over a 2- month period, supplemented by additional support on site.(11) Training principally consisted of: implementation of the 5As model (see below); a background to brief motivational interviewing; main messages in The Back Book, and how to transmit the right message in response to specific patient behavior; recording of adverse events; motivation/ self-efficacy for PA; using a pedometer and the readiness to change questionnaire; setting tailored goals and grading activity on a weekly basis; and relevant follow-ups for each group.
Interventions
In week 1, both groups (E and EWP) received a single, one-to-one session (approximately 1 h) with a physiotherapist who completed a brief physical examination and gave standardized education/advice to remain active using “The Back Book.”(12) In addition, the EWP group also commenced a graded pedometer-driven walking program structured around the 5A’s framework, which was initially developed for brief smoking cessation advice, and subsequently used to facilitate change in other health-related behaviors, including PA.(13) The 5 components of the 5As used were: (1) ask/assess the patient concerning current health behaviors, barriers to PA etc.; (2) advise the patient in a nonjudgmental manner on benefits and means of increasing their PA; (3) agree with the participant the need for change, and to mutually define walking goals; (4) assist with feedback on progress and strategies to address barriers; and (5) arrange for regular feedback by telephone to support the participant.
As part of the assist stage, each participant (accompanied by the physiotherapist) completed a 10 minute “self efficacy walk” during which the participant recorded their step count on the pedometer, for example, 600 steps in 10 minutes; this demonstrated to the participant their ability to increase PA, and assisted in setting of realistic, achievable goals for increasing PA during the program.
Pedometer
At week 1 each participant completed a 20-step test with the physiotherapist to ensure that the pedometer was recording, and that they could use it correctly (Yamax Digiwalker CW-701, Yamax, Japan). They were then asked to familiarize themselves with wearing the pedometer and recording their daily steps in a walking diary for the subsequent 7 days. At the week 2 appointment the step target for the subsequent week was agreed between the physiotherapist and the participant by referring to (1) the mean daily step count for the previous week calculated from the walking diary, and (2) the number of steps accumulated during the 10-minute “self-efficacy walk.”(14) There were no further face-to-face appointments: between weeks 3 and 8 the physiotherapist phoned the participant at a prearranged time, each week, to discuss their progress, document their mean daily step count (recorded in diaries) for the previous week, and to agree to a new daily step target for the subsequent week. In this way the EWP was tailored to the individual and progressed on a weekly basis.
Outcome Measurements
Adherence for EWP only
Participants in the EWP group recorded target steps/ day, actual steps/day, and adherence with the walking program (see data analysis for more detail) in a step diary, completed daily by participants over the 8-week intervention period. Actual steps were verified each week by the physiotherapist asking the participant to read out the values for the last 7 days using the memory function of the pedometer.
EWP and E
All other outcome measures were assessed by a blinded assessor at baseline, week 9, and at 6 months. The primary outcome measure was functional disability using the Oswestry Disability Questionnaire (ODQ), which measures pain and physical function in various activities of daily living with values ranging from 0 (best health state) to 100 (worst health state). Other outcomes included quality of life (EuroQol, EQ-5D); average LBP over the last week on a numerical rating scale; PA (ActivPAL professional PA logger, PAL Technologies, Glasgow, UK; International PA Questionnaire short form [at baseline only], and modified global rating of change in PA and importance of this change); fear avoidance beliefs (Fear-Avoidance Beliefs Questionnaire); back beliefs (Back Beliefs Questionnaire); self-efficacy (PA Self-efficacy Scale); and state of change were also collected. In addition, participant expectation and satisfaction (Baseline and Exit Questionnaires) were assessed. Further details on the psychometric properties of these outcome measures are provided in the study protocol.(11)
Data Analysis
Intention-to-treat analysis was carried out using the Statistical Package for Social Sciences (SPSS) version 17.0 (SPSS Inc., Chicago, IL). Missing data were replaced using the last observation carried forward.(15) Change scores were calculated by subtracting the outcome data at week 9 or 24, as appropriate, from the baseline value. Since this was a feasibility study, significance tests were not performed and no formal hypothesis testing is reported. The treatment effect with 95% confidence interval (CI) was estimated at each follow-up time point for the clinical outcomes, and 95% CIs included to show the range of variation; for each individual participant, those achieving a minimal clinically important difference of either 10% points (absolute change) or 30% (percentage change) from baseline on ODQ, was calculated at 6 months.(16)
Between group effect sizes were calculated (using http://uccs.edu/Bfaculty/lbecker) as recommended by Pallant. (17) Specifically, these were calculated at each time point: EWP as the treatment group, M1, and E as control, M2, and interpreted using Cohen d values where 0.2 is a small effect, 0.5 is a medium effect, and 0.8 is a large effect.(18)
Accelerometer data were downloaded and transformed to Microsoft Office Excel format using ActivPAL Professional (ActivPAL3: Version 6.1.2). As there have been no validated methods to distinguish periods of nonwear for the ActivPAL device, we have adapted a previously reported method.(19–20) A valid day was defined as a 24-hour period, which exhibited a minimum of 10 hours where the device was worn.(21) Wear time was assessed as the number of hours in which at least 1 bout of upright activity was recorded within a 60-minute period. This was evaluated on an hour by hour basis, in an attempt to exclude individuals who did not wear the device for extended periods of time. Individual records were included if they contained at least 3 days of valid data, including at least 1 weekend day.(21) The device recorded periods of sedentary (sitting/ lying), upright (standing and walking) activity, numbers of steps during upright activities, and step cadence.(22) Number of steps taken in light (<100 steps/min) or moderate/vigorous (>100 steps per min) intensity activity were calculated by summing the average number of steps per day above and below 100 steps/minute. This cut-off was chosen based on the recommendations of previous research.(23,24)
Analysis of Pedometer Data (EWP Only)
Data were extracted from each participant’s step diary and from the physiotherapist’s telephone-based record; where appropriate missing data were replaced using last observation carried forward.(15) The absolute change in steps/day during the intervention period was calculated (steps/d at week 8-steps/d at baseline), and the change relative to baseline calculated by dividing by baseline steps/ day. The absolute change in target steps/day was also calculated (target steps/d at week 8- steps/d at week 2) and the change relative to baseline calculated by dividing by week 2 steps/day, and in both cases multiplied by 100 to convert to a percentage. Adherence with the walking program was calculated by dividing the number of participants who walked for all 8 weeks, as defined in their step diary, by the total number recruited to the EWP (n=40). Percentage adherence with the weekly step targets was calculated by examining the target steps/day and the actual steps/day recorded for each person, and counting the number of weeks that they met their target, then dividing this by the total number of weeks that a target was set. Reports of all adverse events, both those related and unrelated to the pedometer-driven walking program, were recorded.(25)
RESULTS
Recruitment and Follow-Up
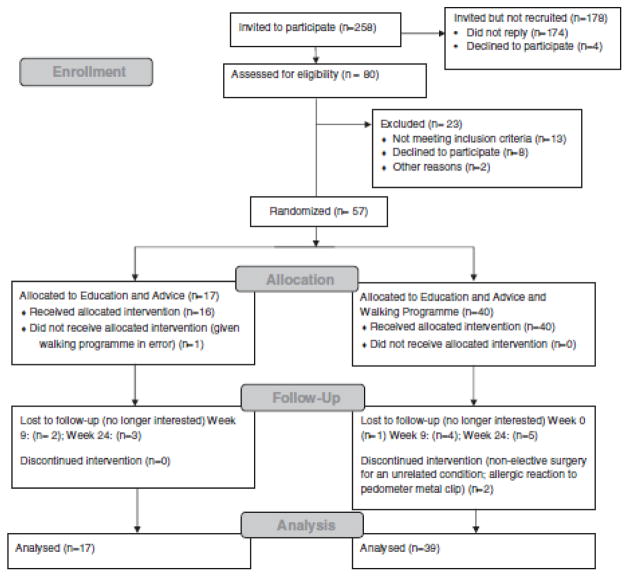
Participant flow and retention are shown in Figure 1. Of those invited to participate (n=258), 80 were assessed for eligibility, with n=57 recruited (22% recruitment rate) and randomized (n=40 to EWP; n=17 to E). All data were included in the adherence analysis of the pedometer steps/day for EWP. However, a single participant from this group was excluded from the exploratory comparative analysis as the participant did not provide data for the primary outcome measure (OQD) at baseline or any of the other time points. Therefore data were analyzed for n=39 in EWP group and n=17 in the E group at baseline, and follow-up data were available for 86% of these participants at 6 months.
FIGURE 1.
CONSORT 2010 flow diagram.
Adherence With Walking (EWP Only)
Of the 40 individuals recruited to EWP, 2 did not start the program, and 1 stopped after week 1 as they felt that they could not wear the pedometer at work. Of the 37 who did participate, their step counts increased from a mean value of 5563 steps/day (95% CI, 4915–6211 steps) to a mean value of 8339 steps/day (95% CI, 7371–9308 steps), or an absolute increase of 2776 steps/day (95% CI, 1996–3557 steps), which equated to a 59% increase (95% CI, 40.73%– 76.25%) in daily step counts. Adherence with the EWP was good: 93% walked for at least 6 weeks, 73% (29/40) walked all 8 weeks, 2 participants walked 7 weeks, and 6 participants walked 6 weeks. Percentage adherence with the weekly step/day targets was 70% (95% CI, 62%−77%) overall.
Adverse Events Related to Walking (EWP Only)
During the course of the trial, 14 (35%) participants reported 20 AEs. There were n=12 unrelated adverse events in 10 people (n=10 minor illness such as colds or flu reported; n=1 road traffic collision; n=1 lateral ankle sprain); and n=8 related adverse events in 5 people (n=6 increase in pain: n=4 lower limb and n=2 back; n=2 allergic reaction to the metal clip of the pedometer). Reports of leg or back pain were confined to either the first 1 to 2 weeks or week 5, lasted only for a couple of days, and were probably due to an increase in (unaccustomed) exercise. Those reporting pain persisted with the EWP, and in discussion with the physiotherapist temporarily reduced their steps/day target until the pain subsided. Only 1 related AE led to an individual stopping the walking program (reported allergic reaction to the pedometer clip).
Exploratory Comparative Analysis of EWP and E: Baseline Characteristics
Table 2 presents the baseline characteristics of the 56 participants included in the comparative analyses. The mean age of participants, BMI, and duration of CLBP was similar for both groups. There were a greater percentage of men in the EWP group (54%) than the E group (24%), and greater numbers were employed in the EWP (72% vs. 47%). The mean baseline scores for the ODQ were similar in each group, and indicated that both represented a moderately functionally disabled CLBP population. In terms of PA, subjective measures indicated that both groups were highly active (over 3000 m/min/wk),(26) in contrast, objective measurement of number of steps indicated that both groups were somewhat active.(27)
TABLE 2.
Baseline Demographics and Clinical Characteristics (n = 56)
| Outcome Measure | Education and Advice (N = 17) | Education and Advice and Walking Program (N = 39) |
|---|---|---|
| Baseline demographic characteristics (mean [± SD]) | ||
| Age (y) | 51 (42–60) | 48 (43–53) |
| Sex (m:f) | 4:13 | 21:18 |
| Duration of low back pain (y) | 11.1 (7.7–14.5) | 10.3 (6.5–14.0) |
| BMI | 28.1 (24.1–32.1) | 28.9 (27.2–30.5) |
| Employed | 47% (8/17) | 72% (28/39) |
| Baseline clinical characteristics (mean [± 95% CI]) | ||
| Oswestry Disability Questionnaire (ODQ expressed as %) | 27.7(23.3–32.2) | 31.9 (26.6–37.2) |
| International PA Questionnaire (IPAQ in m/min/wk) | 3029 (1687–4371) | 5832 (3285–8079) |
| ActivPAL daily step count | 7678 (6284–9071) | 7826 (6767–8886) |
| ActivPAL cadence < 100 steps/min | 3952 (3159–4744) | 4273 (3617–4929) |
| ActivPAL cadence > 100 steps/min | 3726 (2214–5238) | 3554 (2930–4178) |
| ActivPAL time spent stepping (h/d) | 1.59 (1.34–1.83) | 1.67 (1.45–1.90) |
| ActivPAL time spend standing (h/d) | 4.46 (3.35–5.57) | 4.25 (3.84–4.65) |
| ActivPAL time spent sitting/lying (h/d) | 17.9 (16.6–19.2) | 18.1 (17.6–18.6) |
| Euroqol Weighted Health Index(EQ-5D, range of values: −0.59 to 1.0) | 0.64 (0.53–0.75) | 0.58 (0.49–0.66) |
| Physical Activity Self-Efficacy Scale (PASES, range of values 0–5) | 2.7 (2.2–3.2) | 2.5 (2.3–2.8) |
| Fear Avoidance Beliefs Questionnaire for Physical Activity (FABQ- PA, range of values 0–24) | 15.1 (11.8–18.4) | 16.0 (13.9–18.0) |
| Back Beliefs Questionnaire (BBQ, range of values 9–45) | 28.9 (25.4–32.3) | 28.1 (25.8–30.5) |
| Euroqol (EQ5D Visual Analogue Scale—Health State, range of values (0–100) | 59.4 (49.6–69.2) | 68.5 (62.7–74.3) |
| Numerical Rating Scale for average pain over last week (NRS. range of values 0–10) | 4.6 (3.6–5.5) | 5.4 (4.8–6.0) |
| Modified Global Rating of Change (MGROC for Physical Activity, range of values −7–7) | −2.6 (−4.0 to −1.3) | −3.3 (−4.4 to −2.2) |
| Modified Global Rating of Change (MGROC importance of change, range of values 0–7) | 3.3 (2.1–4.5) | 4.3 (3.7–5.0) |
| Readiness to change (n [%]) | ||
| Precontemplation | 0 | 1(3) |
| Contemplation/preparation | 9(53) | 24 (61) |
| Action/maintenance | 8(47) | 14 (36) |
Treatment Effect
All included participants (except for single individual in the E group described, Fig. 1) received treatment as allocated. Table 3 shows the summary statistics for both groups and Table 4 presents the mean change scores (±95% CI) from baseline for all outcome measures plus the between-group effect size values.
TABLE 3.
Summary Statistics at Each Time for Both Interventions Expressed as Mean (± 95% CI) Except for Readiness to Change (n [5]).
| Outcome Measure | Group | Baseline | Week 9 | 6 mo |
|---|---|---|---|---|
| Oswestry Disability Questionnaire (ODQ expressed as %) | E Group | 27.7 (23.3–32.2) | 26.8 (19.4–34.2) | 26.2 (18.5–33.8) |
| EWP Group | 31.9 (26.6–37.2) | 26.4 (21–31.9) | 23.7 (18.4–29.0) | |
| Fear Avoidance Beliefs Questionnaire for Physical Activity (FABQ-PA, range of values 0–24) | E Group | 15.1 (11.8–18.4) | 9.9 (6.7–13.1) | 10.3 (6.6–14.0) |
| EWP Group | 16.0 (13.9–18.0) | 10.7 (8.4–12.9) | 11.5 (9.2–13.9) | |
| Back Beliefs Questionnaire (BBQ, range of values 9–45) | E Group | 28.9 (25.4–32.3) | 25.9 (21.9–30.0) | 23.8 (20.3–27.3) |
| EWP Group | 28.1 (25.8–30.5) | 24.4 (21.8–26.9) | 25.2 (22.6–27.8) | |
| Numerical Rating Scale for average pain over last week (NRS, range of values 0–10) | E Group | 4.6 (3.6–5.5) | 3.9 (2.7–5.1) | 4.1 (2.8–5.3) |
| EWP Group | 5.4 (4.8–6.0) | 4.5 (3.7–5.4) | 3.8 (3.0–4.6) | |
| Euroqol Weighted Health Index(EQ-5D, range of values: −0.59 to 1.0) | E Group | 0.64 (0.53–0.75) | 0.70 (0.59–0.80) | 0.69 (0.60–0.78) |
| EWP Group | 0.58 (0.49–0.66) | 0.61 (0.51–0.70) | 0.63 (0.53–0.72) | |
| Euroqol (EQ5D Visual Analogue Scale–Health State, range of values (0–100) | E Group | 59.4 (49.6–69.2) | 66.7 (55.7–77.7) | 62.5 (50.7–74.2) |
| EWP Group | 68.5 (62.7–74.3) | 70.4 (63.2–77.5) | 72.1 (65.8–78.4) | |
| Physical Activity Self-Efficacy Scale (PASES, range of values 0–5) | E Group | 2.71 (2.22–3.19) | 0.19 (−0.18 to 0.57) | 0.15 (−0.15 to 0.46) |
| EWP Group | 2.53 (2.25–2.82) | 0.05 (−0.14 to 0.24) | 0.06 (−0.12 to 0.23) | |
| Modified Global Rating of Change (MGROC for Physical Activity, range of values −7 to 7) | E Group | −2.65 (−4.00 to −1.29) | 0.94 (−0.35 to 2.23) | 0.00 (−1.70 to 1.70) |
| EWP Group | −3.31 (−4.38 to −2.23) | 1.92 (0.92–2.93) | 1.95 (0.92–2.98) | |
| Modified Global Rating of Change (MGROC importance of change, range of values 0–7) | E Group | 3.29 (2.08–4.51) | 2.76 (1.50–4.03) | 3.59 (2.18–5.00) |
| EWP Group | 4.33 (3.68–4.99) | 3.77 (3.12–4.42) | 3–21 (2.37–4.04) | |
| Readiness to change (n [%]) | ||||
| Precontemplation | E Group | 0 | 1 (6) | 1 (3) |
| EWP Group | 1 (3) | 1 (3) | 1 (6) | |
| Contemplation/preparation | E Group | 9 (53) | 7 (41) | 5(29) |
| EWP Group | 24 (61) | 13 (33) | 14 (36) | |
| Action/maintenance | E Group | 47 | 9 (53) | 11 [64) |
| EWP Group | 36 | 25 (64) | 24 (62) | |
TABLE 4.
Mean Unadjusted Change (±95 CI) Scores (From Baseline) for all Outcome Measures and Between-Group Effect Sizes
| Outcome Measure | Group | Week 9 | Between-Group Effect Size (Cohen d) | 6 mo | Between-Group Effect Size (Cohen d) |
|---|---|---|---|---|---|
| Oswestry Disability Questionnaire (ODQ) | E Group | −1.0 (−7.6 to 5.6) | −0.39 | −1.6 (−9.3 to 6.1) | −0.44 |
| EWP Group | −5.5 (−8.8 to −2.2) | — | −8.2 (−13 to −3.4) | — | |
| Fear Avoidance Beliefs Questionnaire for Physical Activity (FABQ-PA, range of values 0–24) | E Group | −5.2 (−8.6 to −1.9) | −0.02 | −4.8 (−8.2 to −1.4) | 0.06 |
| EWP Group | −5.3 (−7.4 to −3.1) | — | −4.4 (−6.8 to −2.0) | — | |
| Back Beliefs Questionnaire (BBQ, range of values 9–45) | E Group | −2.9 (−6.5 to 0.6) | −0.14 | −5.1 (−7.9 to −22) | 0.36 |
| EWP Group | −3.8 (−5.7 to −1.9) | — | −2.9 (−5.1 to −0.8) | — | |
| Numerical Rating Scale for average pain over last week (NRS. range of values 0–10) | E Group | −0.7 (−1.6 to 0.2) | −0.1 | −0.5 (−1.8 to 0.8) | −0.4 |
| EWP Group | −0.9 (−1.6 to −0.1) | — | −1.6 (−2.6 to −0.6) | — | |
| Euroqol Weighted Health Index (EQ-5D, range of values: −0.59 to 1.0) | E Group | 0.06 (−0.05 to 0.17) | −0.13 | 0.05 (−0.09 to 0.18) | 0 |
| EWP Group | 0.03 (−0.06 to 0.11) | — | 0.05 (−0.04 to 0.14) | — | |
| Euroqol (EQ5D Visual Analogue Scale—Health State, range of values (0–100) | E Group | 7.3 (−1 to 15.6) | −0.32 | 3.1 (−9.1 to 15.3) | 0.03 |
| EWP Group | 1.9 (−3.8 to 7.5) | — | 3.6 (−1.3 to 8.5) | — | |
| Physical Activity Self-efficacy Scale (PASES, range of values 0–5) | E Group | 0.19 (−0.18 to 0.57) | −0.2 | 0.15 (−0.15 to 0.46) | −0.16 |
| EWP Group | 0.05 (−0.14 to 0 24) | — | 0.06 (−0.12 to 0.23) | — | |
| Modified Global Rating of Change (MGROC for Physical Activity, range of values −7 to 7) | E Group | 3.59 (1.60–5.58) | 0.39 | 2.65 (0.47–4.82) | 0.59 |
| EWP Group | 5.23 (3.74–6.72) | — | 5.26 (3.76–6.75) | — | |
| Modified Global Rating of Change (MGROC importance of change, range of values 0–7) | E Group | −0.53 (−1.89 to 0.83) | −0 1 | 0.29 (−1.19 to 1.78) | −0.56 |
| EWP Group | −0.56 (−1.30 to 0.17) | — | −1.13 (−1.80 to −0.46) | — |
For ODQ, FABQ-PA, BBQ, and NRS, a negative change score indicates improvement in outcome, whereas for EQ-5D and PASES a positive change score indicated improvement
Participants in the EWP group demonstrated a mean improvement of 8.2% points (95% CI −13 to −3.4) in the ODQ at 6 months compared with 1.6% points (95% CI, − 9.3 to 6.1) in the E group. The between-group difference, in favor of EWP, was medium at both time points (d=0.39 and 0.44). The number of participants achieving a minimal clinically important difference of 10% points over time for the ODQ was somewhat higher in the EWP group (56%) and E group (44%) at 6 months; results for 30% change (percentage change) were similar. In terms of average perceived pain, the between-group difference increased with time in favor of the EWP (d=0.1 at week 9 and d=0.4 at 6 mo, Table 4); however, the changes are unlikely to be clinically important (mean 1.6, 95% CI −2.6 to −0.6) compared with the E group (mean − 0.5, 95% CI − 1.8 to 0.8). For other outcomes similar improvements were observed in both groups over time with small differences only between the groups (eg, fear avoidance and self-efficacy). In contrast, the E group showed a greater improvement in EQ5D health state at 9 weeks (d=0.32) and in back beliefs at 6 months (d=0.36, medium difference).
Readiness to Change and PA
Both groups demonstrated a shift in their readiness to change at 6 months (over 60% in both groups were in the action/maintenance phase; Table 3). The EWP perceived that their PA levels had improved to a greater extent than the E group (d=0.39 and 0.59, at week 9 and 6 mo, respectively). The feasibility of using the ActivPAL device as an objective outcome measure for PA was explored in this trial. The missing data for this outcome (ranging from 23% at baseline to 47% at 6 mo) were higher than for the paper based outcomes (Fig. 1). The reasons for missing data were as follows: (1) accelerometer not worn: 2% at baseline; 18% at week 8; 23% at week 24; (2) displaying patterns of sedentary behavior consistent with not wearing the device: 11% at baseline; 5% at week 8; 11% at week 25; (3) insufficient days of recording (1 to 2 d) or no weekend data: 11% at baseline; 23% at week 8; 14% at week 24. The degree of missing data was similar for both arms of the trial at baseline (23%) and week 8 (46%), but was much greater in the EWP group at week 24 (60% vs. 18%), with the main reasons being that the device was not worn (n=11 in EWP) or that there were only 1 to 2 days of data (n=7 in EWP). Because of the high dropout rates, we felt it was not valid to analyze these data; however, baseline data are reported in Table 2.
Patient Expectation and Satisfaction
At the 6-month time point, the majority of participants were at least “somewhat satisfied” with their overall care (EWP: 75%; E: 100%), and for the treatment they received for CLBP (EWP: 62.5% vs. E: 87%, Table 5), they reported at least “some benefit” (EWP: 78.2% vs. E: 73.3%). The majority of participants were at least “somewhat satisfied” with the advice/information they received about their CLBP (EWP: 93.8% vs. E: 100%), whereas the majority found the treatment they received resulted in at least “some benefit” in reaching their treatment goals (EWP: 75% vs. E: 86.6%, Table 5). Although figures for the EWP group for patient satisfaction were lower, the small numbers involved here require further exploration in a larger trial before more definitive conclusions could be made concerning patient satisfaction with treatment.
TABLE 5.
Exit Questionnaire
| n (%)
|
||
|---|---|---|
| Education and Advice | Education and Advice and Walking Program | |
| Were you satisfied with the overall care you received in this study? | ||
| Very satisfied | 13 (86.7) | 22 (68.8) |
| Somewhat satisfied | 2 (13.3) | 2 (6.3) |
| Neither satisfied nor dissatisfied | 0 | 8 (25.0) |
| Somewhat dissatisfied | 0 | 0 |
| Very dissatisfied | 0 | 0 |
| Were you satisfied with the treatment you received for your low back pain in this study’? | ||
| Very satisfied | 8 (53.3) | 13 (40.6) |
| Somewhat satisfied | 5 (33.3) | 7 (21.9) |
| Neither satisfied nor dissatisfied | 2 (13.3) | 10 (31.3) |
| Somewhat dissatisfied | 0 | 2 ((6.3) |
| Very dissatisfied | 0 | 0 |
| Were you satisfied with the advice/information you received about your low back pain in this study? | ||
| Very satisfied | 10 (66.7) | 22 (68.8) |
| Somewhat satisfied | 5 (33.3) | 8 (25.0) |
| Neither satisfied nor dissatisfied | 0 | 2 (6.3) |
| Somewhat dissatisfied | 0 | 0 |
| Very dissatisfied | 0 | 0 |
| Do you think that the treatment you received in this study benefited your low back pain? | ||
| Great benefit | 2 (13.3) | 6 (18.8) |
| Some benefit | 9 (60.0) | 19 (59.4) |
| No benefit | 4 (26.7) | 7 (21.9) |
| Do not know | 0 | 0 |
| How helpful in reaching your treatment goal(s) was the treatment you received in this study? | ||
| Great benefit | 2 (13.3) | 8 (25.0) |
| Some benefit | 11 (73.3) | 16 (50.0) |
| No benefit | 2 (13.3) | 8 (25.0) |
| Do not know | 0 | 0 |
| How easy /difficult was it for you to stick to your exercise program? | ||
| Very difficult | 1 (6.7) | 0 |
| Somewhat difficult | 3 (20.0) | 10 (31.3) |
| Neither difficult nor easy | 6 (35.3) | 13 (40.6) |
| Somewhat easy | 2 (13.3) | 5 (15.6) |
| Very easy | 3 (20.0) | 4 (12.5) |
| Do you think the treatment you received in this study changed the number of painkilling tablets you had to take? | ||
| Took more tablets | 0 | 0 |
| Took less tablets | 4 (26.7) | 11 (34.4) |
| Stopped taking tablets | 2 (13.3) | 5 (15.6) |
| No change | 8 (53.3) | 15 (46.9) |
| Did not take any tablets | 1 (6.7) | 1 (3.1) |
| Would you recommend the treatment you received in this study to a friend or colleague? | ||
| Yes | 14 (93.3) | 25 (78.1) |
| No | 1 (6.7) | 7 (21.9) |
| Would you be happy to receive this form of treatment again? | ||
| Yes | 12 (80.0) | 19 (59.4) |
| No | 3 (20.0) | 13 (40.6) |
| If you had to spend the rest of your life with your low hack pain as it is now, how would you feel about it? | ||
| Terrible | 2 (13.3) | 3 (9.4) |
| Unhappy | 4 (26.7) | 8 (25.0) |
| Mostly dissatisfied | 2 (13.3) | 3 (9.4) |
| Mixed (equally satisfied/dissatisfied) | 1 (6.7) | 11 (34.4) |
| Mostly satisfied | 4 (26.7) | 3 (9.4) |
| Pleased | 2 (13.3) | 2 (6.3) |
| Delighted | 0 | 2 (6.3) |
The responses to the exit questionnaire administered at week 24, not all participants answered all questions, and “n” values represent the number of responses for each question.
The Effect on Uptake of Physiotherapy at the End of the Intervention Period
Seventy-one percent (40/56) of participants were recruited by physiotherapy department waiting lists (referred from primary care), whereas the remainder were recruited directly from primary care practices. The former recruitment strategy offered the option of receiving standard physiotherapy care at the end of the intervention period (week 9), if the physiotherapist deemed this necessary. There was a difference in the uptake of physiotherapy (70% [7/10] uptake in the E group vs. 37% [11/30] uptake in the EWP group). In terms of routine clinical care in the physiotherapy department from which we recruited, 95% of patients on the waiting list typically attend for physiotherapy, and so both groups showed a reduction in uptake and this was much greater in the EWP group.
Sample Size for a Main Trial
We anticipate continuing to use the ODQ as the primary outcome measure in a main trial. There was a moderate between-group effect size in favor of EWP at 6 months (a 6.6% point difference and SD=12). This is smaller than the absolute change recommended for an individual over time using ODQ(16) but is similar to the between-group effect size in another recent trial protocol for this outcome.(28)
DISCUSSION
The main finding of this study was that it was feasible to recruit and retain participants who had been referred to routine physiotherapy, in a trial investigating walking as an adjunct to a single education/advice session. In addition, we observed that: there were numerically larger improvements in functional disability, pain, and perceived PA levels in the EWP; and that there was a reduced uptake of physiotherapy at the end of the intervention period in the EWP. These observations underscore the case for a larger definitive study of this intervention. Both groups demonstrated a shift in their behavior in relation to PA, with over 60% in the action/maintenance phase at 6 months. The EWP group demonstrated an increase in steps/day of 59% and perceived that their PA levels had increased; however, we were unable to explore whether this increase was also captured by the accelerometer device in this trial, due to the high degree of missing data for this outcome. Over 75% of participants in both groups were at least “somewhat satisfied” with their overall care; however, the lower levels of satisfaction in the EWP should be explored further in a main trial. Minor adverse events linked to the walking program were reported by 15% of participants.
Recruitment and Retention Rates
Recruitment rates were similar to other trials by our group, for example, Hunter et al.(29) Dropout in the trial was lower than expected (13% to 18%) for paper-based outcomes, but higher for objective monitoring. Missing data for the ActivPAL devices were mainly explained by lack of adherence to the wearing protocol by participants: either they did not wear the device at all, or it was worn for insufficient days (<3 out of 7 days). In the absence of previously validated methods for process wear time and ultimately making determinations about what constituted a valid monitored day, we felt it was important to establish some rules to avoid the possible confounding effects of including nonwear time in the analysis. However, the approach we necessarily took to cleaning the ActivPAL data could potentially lead to lost data, if there were long protracted periods of nonupright activity while being monitored. To exclude this possibility, we cross-checked our approach against the raw data output from ActivPAL in a subsample of files, and this confirmed that periods defined as nonwear had no obvious activity.
In addition, for the vast majority of records, we observed recordings with either a sufficient number of monitored hours or very few hours with some upright activity (indicating nonwear), and very few days were marginal between these 2 apparent extremes. These data patterns indicate that participants were either adherent with wearing the monitor or did not wear it at all. We therefore believe that the risk of excluding potentially valid days was minimal and likely would not have affected the results from the trial.
In an attempt to monitor and encourage wear time, participants were issued with a wear time diary. This summary data for each day was helpful in the interpretation and cleaning of the data, so we would recommend continued use in main trial. However, these diaries did not include sufficient data on an hour-by-hour basis to precisely establish nonwear time, and so further research is required to identify validated methods of cleaning ActivPAL data files, as has been done for other activity monitors,(30) as well as effective methods to improve adherence with wearing the device.
The device we used has advantages for the measurement of sedentary activity as it is worn on the thigh; however, it is applied with adhesive materials to a bare thigh and is therefore more burdensome to attach than other available devices. It may be that the use of this particular accelerometer in our trial placed too much burden on participants, given the raft of clinical measures that we used. We would recommend therefore that either this outcome is prioritized in clinical trials (along with a very small number of clinical measures) or these devices are used in cohort studies. Alternatively, a waist mounted device, which is less burdensome to apply, could be used given the excellent adherence with wearing the pedometer devices in this trial.
Changes in PA
Pedometer-driven walking has been advocated as a safe and effective means for increasing PA in sedentary adults(4–5) and recent reviews observed an increase of approximately 2500 steps/day as a result of walking interventions. (24,31) A similar increase in steps/day was realized in the current study (mean increase of 2776 steps), which is understandable given that the components of our intervention were similar to those shown to be successful by Bravata et al.(31) A goal was set and a pedometer was used along with the completion of a walking diary. Our approach was informed by the First step program(14,32): specifically, the step goals were selected by each individual (in consultation with their physiotherapist), rather than by a preset absolute increase or percent increase; this notwithstanding, it is possible that the setting of any goal, rather than how it is set, is more important in increasing PA.(33)
In the current study we used the 5As model of behavior change to structure the consultation between the participant and the physiotherapist; in general this worked well as mutually agreed goals were largely met by participants (70% adherence). Compared with data from general population studies, very few participants recruited onto our trial were in the preaction stages (3% in our trial vs. 50% in Laforge et al(34)), and so we were successful in recruiting individuals who more motivated to change. However, we did note a small percentage of participants, who even in collaboration with their physiotherapist, did not change their target steps over the intervention period (n=2), or did not reach their step goals. We would recommend additional training so that the physiotherapist may recognize this resistance to change and has alternative strategies to better assist behavior change. For example, reference to techniques such as brief motivational interviewing were covered in the initial training with the physiotherapists, but debriefing interviews with physiotherapists suggested that more practical examples of how to use such techniques may be required in a main trial. Some of the research team are currently involved in a trial, which is examining the effects of a training package for physiotherapists to enhance their communication skills(35) and will also help inform training in a main trial.
The number of participants reporting AEs (both study unrelated and related to walking) was similar (35%) to that reported by Goodrich et al(25) (31%). The majority of ours, however, were unrelated to the study with only 5% of participants reporting minor musculoskeletal effects that led to a reduction in their participation for a few days, but did not lead to subsequent dropout from the intervention.
Participants in the trial were asked to rate perceived changes in their PA since the start of their current episode of back pain, at the end of the intervention, and at 6-month follow-up. Although both groups perceived that their PA levels were reduced at baseline, there was a numerically larger improvement in perceived PA in those who walked in the EWP versus those who received education/advice alone, which was apparent at both at 9 weeks and 6 months. This was in keeping with the observed increases in steps/day data in the EWP group (substantiated by pedometer data).
CONCLUSIONS
We have shown that it is feasible to conduct a trial investigating walking as an adjunct to an education and advice session in people with CLBP. Participants increased their pedometer-determined steps/day by 59%, reported few AEs, and demonstrated an improvement in functional disability, pain, and perceived PA. Further thought needs to be given to: how best to objectively measure PA (eg, a waist-mounted/wrist-mounted accelerometer) in a main trial; how to recognize and address individuals who do not readily demonstrate a change in their steps/day; and the association between changes in PA and LBP-specific outcomes.
Acknowledgments
Supported by the Physiotherapy Research Foundation (PRF/08/1), Chartered Society of Physiotherapy, London, UK and funding for a PhD studentship from the Department of Employment and Learning, Belfast, Co. Antrim, Northern Ireland.
The authors thank Sharon McCaffrey, LLB HONS Law with Government, School of Law, University of Ulster, Newtownabbey, County Antrim, UK; and David Dodds for their help and support in their roles as service users, and members of the project steering group for this trial; Nadine Bibby, BSc (Hons) Physiotherapy, School of Health Sciences, University of Ulster Newtownabbey, County Antrim, UK; who was the physiotherapist in the Ballymoney Hospital site.
Footnotes
Trial Registration: [ISRCTN67030896].
The authors declare no conflict of interest.
M.A.T. and S.Mc.D. conceived the idea for this study. S.Mc.D., M.A.T., G.D.B., D.A.H., A.D., S.O.N., and I.B. were involved in developing the original idea for funding and were coapplicants on the successful funding proposal. M.A.T., C.T.-L., and S.Mc.D. devised a detailed walking program.
References
- 1.Airaksinen O, Brox JI, Cedraschi C, et al. European guidelines for the management of chronic nonspecific low back pain. EurSpine J. 2006;15(suppl 2):s192–s300. doi: 10.1007/s00586-006-1072-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Maniadakis N, Gray A. The economic burden of back pain in the UK. Pain. 2000;84:95–103. doi: 10.1016/S0304-3959(99)00187-6. [DOI] [PubMed] [Google Scholar]
- 3.Savigny P, Watson P, Underwood M. Early management of persistent non-specific low back pain: summary of NICE guidance. BMJ. 2009;338:1441–1442. doi: 10.1136/bmj.b1805. [DOI] [PubMed] [Google Scholar]
- 4.National Institute of Clinical Excellence. Four commonly used methods to increase physical activity. London: NICE; 2006. [Google Scholar]
- 5.Foster C, Hillsdon M, Thorogood M. Interventions for promoting physical activity. Cochrane Database Syst Rev. 2005;25:CD003180. doi: 10.1002/14651858.CD003180.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hendrick P, Te Wake AM, Tikkisetty AS, et al. The effectiveness of walking as an intervention for low back pain:a systematic review. Eur Spine J. 2010;19:1613–1620. doi: 10.1007/s00586-010-1412-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Connor SR, Tully MA, Ryan B, et al. Walking interventions for chronic musculoskeletal pain: systematic review. Phys Ther Rev. 2010;15:123–124. [Google Scholar]
- 8.Hartvigsen J, Morsø L, Bendix T, et al. Supervised and nonsupervised Nordic walking in the treatment of chronic low back pain: a single blind randomized clinical trial. BMC Musculoskelet Disord. 2010;10:11–30. doi: 10.1186/1471-2474-11-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hurley DA, O’Donoghue G, Tully MA, et al. A walking programme and a supervised exercise class versus usual physiotherapy for chronic low back pain: a single-blinded randomised controlled trial. (The Supervised Walking In comparison to Fitness Training for Back Pain (SWIFT) Trial) BMC Musculoskelet Disord. 2009;10:79–89. doi: 10.1186/1471-2474-10-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tully MA, Cupples ME. UNISTEP (university students exercise and physical activity) study: a pilot study of the effects of accumulating 10,000 steps on health and fitness among university students. J Phys Acta Health. 2011;8:663–667. doi: 10.1123/jpah.8.5.663. [DOI] [PubMed] [Google Scholar]
- 11.McDonough SM, Tully MA, O’Connor SR, et al. The Back 2 Activity Trial: education and advice versus education and advice plus a structured walking programme for chronic low back pain. BMC Musculoskelet Disord. 2010;11:163–170. doi: 10.1186/1471-2474-11-163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Roland M, Waddell G, Klaber Moffett J, et al. The Back Book:The Best Way to Deal With Back Pain—Get Back Active. Norwich: The Stationary Office; 2004. [Google Scholar]
- 13.Whitlock EP, Orleans T, Pender N, et al. Evaluating primary care behavioural counseling interventions. an evidence-based approach. Am J Prev Med. 2002;22:267–284. doi: 10.1016/s0749-3797(02)00415-4. [DOI] [PubMed] [Google Scholar]
- 14.Tudor-Locke C, Myers AM, Rodger NW. Formative evaluation of The First Step Program: a practical intervention to increase daily physical activity. Can J Diabetes Care. 2000;24:56–60. [Google Scholar]
- 15.European Medicines Agency. [Accessed November 1, 2012];Guideline on Missing Data in Confirmatory Clinical Trials. 2011 available at: http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2010/09/WC500096793.pdf.
- 16.Ostelo RW, Deyo RA, Stratford P, et al. Interpreting change scores for pain and functional status in low back pain: towards international consensus regarding minimal important change. Spine. 2008;33:90–94. doi: 10.1097/BRS.0b013e31815e3a10. [DOI] [PubMed] [Google Scholar]
- 17.Pallant J. SPSS Survival Manual. 3. Berkshire: Open University Press; 2007. p. 208. [Google Scholar]
- 18.Cohen JW. Statistical Power Analysis for the Behavioural Sciences. 2. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. p. 22. [Google Scholar]
- 19.Perry MA, Hendrick PA, Hale L, et al. Utility of the RT3 triaxial accelerometer in free living: an investigation of adherence and data loss. Appl Ergon. 2010;41:469–476. doi: 10.1016/j.apergo.2009.10.001. [DOI] [PubMed] [Google Scholar]
- 20.Tully M, Bleakley C, O’Connor S, et al. Functional management of ankle sprains: what volume and intensity of walking is undertaken in the first week post injury. Br J Sports Med. 2012b doi: 10.1136/bjsports-2011-090692. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 21.Bassett DR, Jr, Mahar MT, Rowe DA, et al. Walking and measurement. Med Sci Sports Exerc. 2008;40(suppl):s529–s536. doi: 10.1249/MSS.0b013e31817c699c. [DOI] [PubMed] [Google Scholar]
- 22.Dahlgren G, Carlsson D, Moorhead A, et al. Test-retest reliability of the activPALTM in common daily activities in healthy participants. Gait Posture. 2010;32:386–390. doi: 10.1016/j.gaitpost.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 23.Tudor-Locke C, Sisson SB, Collova T, et al. Pedometer determined step count guidelines for classifying walking intensity in a young ostensibly healthy population. Can J Appl Physiol. 2005;30:666–676. doi: 10.1139/h05-147. [DOI] [PubMed] [Google Scholar]
- 24.Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/ day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8:80–98. doi: 10.1186/1479-5868-8-80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goodrich DE, Larkin AR, Lowery JC, et al. Adverse events among high-risk participants in a home-based walking study: a descriptive study. Int J Behav Nutr Phys Act. 2007;4:20–31. doi: 10.1186/1479-5868-4-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. [Accessed November 1, 2012];Guidelines for Data Processing and Analysis of the International Physical Activity Questionnaire (IPAQ) Available at: http://www.ipaq.ki.se/scoring.pdf.
- 27.Tudor-Locke C, Bassett DR. How many steps/day are enough? Preliminary pedometer indices for public health. Sports Med. 2004;34:1–8. doi: 10.2165/00007256-200434010-00001. [DOI] [PubMed] [Google Scholar]
- 28.Fritz JM, Thackeray A, Childs JD, et al. A randomized clinical trial of the effectiveness of mechanical traction for sub-groups of patients with low back pain: study methods and rationale. BMC Musculoskelet Disord. 2010;11:1–10. doi: 10.1186/1471-2474-11-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hunter RF, McDonough SM, Bradbury I, et al. Exercise and auricular acupuncture for chronic low-back pain: a feasibility randomized-controlled trial. Clin J Pain. 2012 doi: 10.1097/AJP.0b013e3182274018. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 30.Matthews CE, Chen KY, Freedson PS, et al. Amount of time spent in sedentary behaviors in the United States, 2003–2004. Am J Epidemiol. 2008;167:875–881. doi: 10.1093/aje/kwm390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bravata DM, Smith-Spangler C, Sundaram V, et al. Using pedometers to increase physical activity and improve health: a systematic review. JAMA. 2007;298:2296–2304. doi: 10.1001/jama.298.19.2296. [DOI] [PubMed] [Google Scholar]
- 32.Tudor Locke C. Promoting lifestyle physical activity: experiences with the First Step Program. Am J Lifestyle Med. 2009;3(suppl 5):s0–s54. doi: 10.1177/1559827609331710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tudor-Locke C, Lutes L. Why do pedometers work?: a reflection upon the factors related to successfully increasing physical activity. Sports Med. 2009;39:981–993. doi: 10.2165/11319600-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 34.Laforge RG, Velicer WF, Richmond RL, et al. Stage distributions for five health behaviours in the United States and Australia. Prev Med. 1999;28:61–74. doi: 10.1006/pmed.1998.0384. [DOI] [PubMed] [Google Scholar]
- 35.Lonsdale C, Hall AM, Williams GC, et al. Communication style and exercise compliance in physiotherapy (CONNECT). A cluster randomized controlled trial to test a theory-based intervention to increase chronic low back pain patients’ adherence to physiotherapists’ recommendations: study rationale, design, and methods. BMC Musculoskelet Disord. 2012;15:104–118. doi: 10.1186/1471-2474-13-104. [DOI] [PMC free article] [PubMed] [Google Scholar]