Abstract
India has the second largest number of people with diabetes in the world following China. Evidence indicates that consumption of whole grains can reduce risk of type 2 diabetes. This manuscript describes the study design and methods of a trial in progress evaluating the effects of substituting whole grain brown rice for polished (refined) white rice on biomarkers of diabetes risk (glucose metabolism, dyslipidemia, inflammation). This is a randomized controlled clinical trial with a crossover design conducted in Chennai, India among overweight but otherwise healthy volunteers aged 25–65y with a body mass index ≥23kg/m2 and habitual rice consumption ≥200grams/day. The feasibility and cultural appropriateness of this type of intervention in the local environment will also be examined. If the intervention is efficacious, the findings can be incorporated into national-level policies which could include the provision of brown rice as an option or replacement for white rice in government institutions and food programs. This relatively simple dietary intervention has the potential to substantially diminish the burden of diabetes in Asia and elsewhere.
Keywords: intervention, whole grains, global nutrition
INTRODUCTION
An estimated 382 million people have diabetes worldwide, and an alarming increase to 592 million is expected by the year 2035 (International Diabetes Federation, 2013). Currently, India has the second largest number of people with diabetes and pre-diabetes in the world (62 million and 77 million, respectively) (Anjana et al., 2011). The escalation in diabetes incidence is occurring as global free trade continues to fuel rapid economic and nutrition transitions, especially in urban settings. A predominant feature of these transitions has been a shift in dietary consumption towards more highly refined carbohydrates. Mounting evidence indicates that consumption of refined grains is associated with increased risk of diabetes (Hu et al., 2012), while conversely, pooled analysis of three large observational cohort studies had shown substituting 50 grams/day of brown rice for white rice lowered the risk of type 2 diabetes by 16% (Sun et al., 2010). Randomized control trials have also shown similar results (Hsu et al., 2008; Panlasigui and Thompson, 2006).
The proposed proof-of-concept trial we describe herein will evaluate the feasibility and effects of substituting brown rice, a whole grain, for white rice in Chennai, India, on biomarkers of diabetes risk. We will evaluate the effects of brown rice substitution on fasting biomarker measurements of glucose metabolism (i.e., glucose, insulin, hemoglobin A1c, homeostasis model assessment for insulin resistance), dyslipidemia (i.e., triglycerides, total cholesterol, LDL-cholesterol, and HDL-cholesterol), and inflammation (i.e., C-reactive protein) in a 3-month randomized crossover trial. The overarching goal of this research is to provide data for use in designing a global dietary intervention study aimed at reducing diabetes risk through simple, culturally appropriate, feasible and sustainable dietary changes.
This study may have important implications for policy and help local governments to develop national food based strategies for diabetes prevention, including widespread education campaigns about the health benefits of whole grains, and serving whole grain foods in institutional settings (e.g., worksite cafeterias, school lunch programs). Such policies could also encourage Ministries of agriculture to support production of whole grains, thereby improving accessibility and cost. This work is intended to be part of a larger global initiative to identify local, feasible and sustainable dietary interventions to reduce diabetes risk in countries experiencing epidemiologic transition by improving the carbohydrate quality of staple foods (Mattei et al., 2012). Initiatives have already been launched by our group in China (Zhang et al., 2011), focus group discussions and consumer acceptability of unrefined carbohydrate staple foods have been ascertained via our research efforts in India (Kumar et al., 2011, Sudha et al., 2013), Tanzania (Muhihi et al., 2013), Nigeria, Costa Rica (Monge-Rojas et al., 2014), Puerto Rico, and Kuwait; and we are currently planning studies in countries including Mexico, Brazil, Kenya, Iran and Malaysia.
STUDY DESIGN AND METHODS
Study design
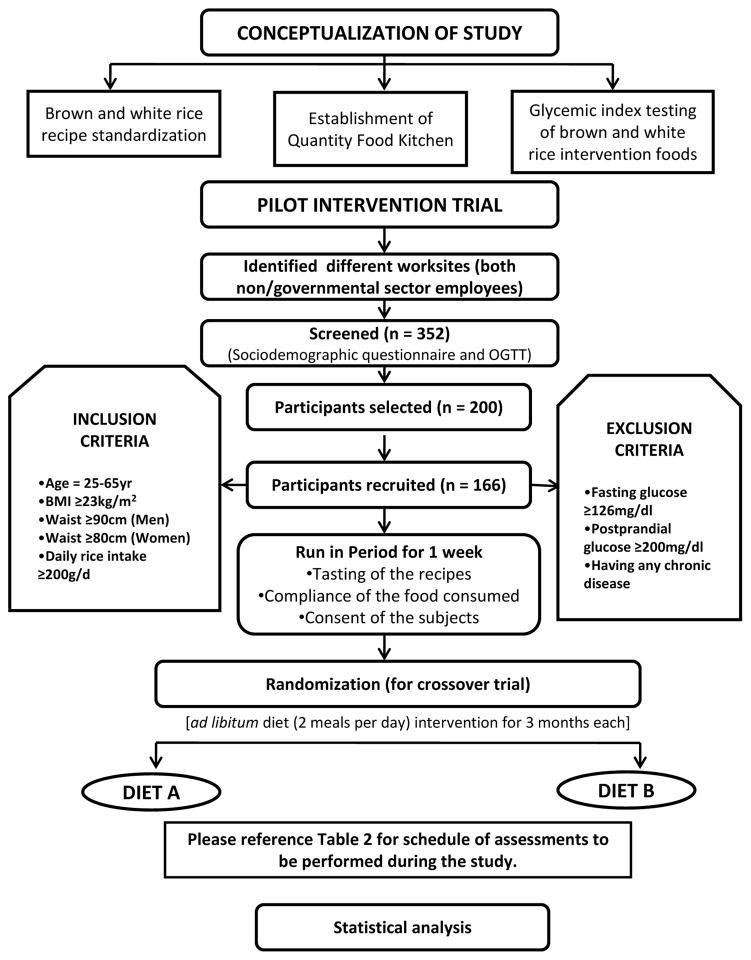
The effects of substituting brown rice for white rice on diabetes risk factors among South Indian adults will be assessed using a randomized non-blinded crossover design in which participants are randomized to one intervention for three months, have a two-week washout period, and then switch to the alternate intervention. All of the recruited participants are given full details about the purpose of the trial, the risks involved, trial benefits, and the importance of the participant’s full cooperation. These details are also included in the consent document, which each participant will read and sign before entering the study. A one-week run-in phase is conducted prior to the start of the randomized intervention to evaluate participant compliance to the study protocol and also to monitor any potential adverse effects. The study protocol was approved by the Institutional Review Boards of both the Harvard School of Public Health and the Madras Diabetes Research Foundation (MDRF) and registered with ClinicalTrials.gov (NCT01814735). The overall study design is shown in Figure 1.
Figure 1.
Study design for pilot intervention trial
Participant recruitment and eligibility criteria
Participants from the government, non-governmental sectors, staff and relatives of a large tertiary care centre for diabetes in South India are identified, and formal approval to screen for participants is obtained. Volunteers are selected for the study based on the inclusion and exclusion criteria as shown in Figure 1. A tasting session of intervention foods, an awareness talk with brochures emphasizing the health benefits of whole grains such as brown rice, and an introduction to the intervention are given prior to screening.
Standardization of intervention recipes and establishment of food production kitchen
From our previous studies (Radhika et al., 2011), the most commonly consumed rice preparations in the community were selected and a total of 9 white-rice-based- and 9 corresponding brown-rice-based recipes were prepared and standardized for the trial in our test kitchen (Table 1) and recipes recorded in the in-house database ‘EpiNu’. The glycemic indices (GI) of these rice-based meals were evaluated in the GI testing laboratory of MDRF (manuscript under preparation) using a validated protocol (Henry et al., 2008) adapted from that described by the Food and Agriculture Organization/World Health Organization (FAO/WHO, 1998) and Wolever et al. (Wolever et al., 2003), and was kept as a basis for menu planning for the intervention trial. Amounts of proximate and available carbohydrate were measured for cooking preparations serving various numbers of people (i.e., 5, 10, 25 and 60) to ensure that cooking in bulk would not alter the estimated GI and other sensory attributes of the dishes prepared. The nutritive values of the menu along with the GI and glycemic load (GL) per serving of food were alternatively calculated using ‘EpiNu’. The range of GIs for the intervention brown rice and white rice based dishes were estimated using the published International GI table (Atkinson et al., 2008) and applying the calculation provided by FAO/WHO (1998) for mixed meals. However, the intervention being ad libitum breakfast and lunch meals, the GL is expected to vary between participants. The estimated GI of all white rice based recipes showed high GI category (74 to 84) except sambar rice which showed medium GI category (69.4). Similarly, all of the brown rice based recipes showed medium GI category (56.5 to 59.7) except sambar rice (50.2) and pongal (54.7) which showed low GI category. The estimated GI showed an approximate 25–30% drop in brown rice recipes compared to white rice based recipes (Table 2).
Table 1.
Non-isocaloric rice-based cyclic meal plan
| 1a. Cyclic breakfast menu | ||||
|---|---|---|---|---|
| Menu | White Rice | Brown Rice | Description | Accompaniments |
| DAY 1 | idly-white rice with black gram split legumes | idly-brown rice with black gram split legumes | steamed pancake made from fermented rice and black gram whole/split legume batter (3:1, rice-to-legume ratio) | onion chutney , tomato gravy (dip)/ sambhar |
| DAY 2 | rava upma-white rice | rava upma-brown ricebrown rice | rice grits stir-fried with mustard, bengal gram dhal, pepper and cumin seeds and boiled in water | tomato chutney (dip) brinjal-green gram dhal gravy |
| DAY 3 | oothappam-white rice with black gram split legume | oothappam-with black gram split legume | shallow fried pancake made from fermented rice and with whole/split legume batter (5:1, rice-to-lentil/legume ratio) | mint chutney (dip),vegetable gravy |
| DAY 4 | veg kitchidi-white rice | veg kitchidi-brown rice | vegetables stir-fried with onion and ginger, garlic paste and rice grits and boiled in water | coconutchutney (dip), onion sambar |
| DAY 5 | pongal-white rice with green gram split legume | pongal-brown rice with green gram split legume | rice pressure-cooked with with green gram split/whole legume (4:1, rice-to-legume ratio) and seasoned with ghee (clarified butter), pepper, cumin seeds, grated ginger, asafoetida and curry leaves. | coriander chutney (dip), sambhar |
| DAY 6 | dosa-white rice with black gram split legumes | dosa-brown rice with black gram split legumes | thin crisp pancake made from fermented rice and black gram whole/split legume batter (3:1, rice-to-legume ratio) | potato masala, sambhar |
| 1b. Cyclic lunch menu | ||||
|---|---|---|---|---|
| Menu | Main course | Description | Accompaniments | |
| White Rice | Brown Rice | |||
| DAY 1 | plain cooked white rice | plain cooked brown rice | pressure-cooked white rice in the ratio (rice:water-1:2.5) pressure-cooked brown rice in the ratio (rice:water-1:2) | yogurt, rasam (tamarind soup),sambhar with vegetables, fried potato, papadum (thin crisp cracker) and pickle |
| DAY 2 | white rice vegetable pulao and curd rice | brown rice vegetable pulao and curd rice | pulao: vegetables sauted with spices, onion and ginger - garlic - green chilli paste and stir-fried with rice .curd rice: pressure-cooked rice mixed with curd (yogurt) and milk and seasoned with grated ginger, asafoetida and curry leaves | fried split red gram and bengal gram legume gravy,rasam, yogurt with onion, papadum (thin crisp cracker) and pickle |
| DAY 3 | plain cooked white rice | plain cooked brown rice | pressure cooked white rice in the ratio(rice: water-1:2.5) pressure cooked brown rice in the ratio (rice: water 1:2) |
ash gourd and split chickpea gravy, yogurt, rasam (tamarind soup), poriyal (stir fried vegetables), papadum (thin crisp cracker) and pickle |
| DAY 4 | white sambar rice made with split red gram lentil and curd rice | brown sambar rice made with split red gram lentil and curd rice | sambar rice: rice mixed with sambar (vegetables cooked with pressure-cooked legume) curd rice: pressure-cooked rice mixed with curd (yogurt) and milk and seasoned with grated ginger, asafoetida and curry leaves | yogurt, poriyal (stir fried vegetables), papadum (thin crisp cracker) and pickle |
| DAY 5 | plain cooked white rice | plain cooked brown rice | pressure cooked white rice in the ratio(rice: water 1:2.5) pressure cooked brown rice in the ratio (rice: water 1:2) |
greens and split green gram lentil gravy, yogurt, rasam (tamarind soup), carrot salad, papadum (thin crisp cracker) and pickle |
| DAY 6 | plain cooked white rice | plain cookedbrown rice | pressure cooked white rice in the ratio(rice: water 1:2.5) pressure cooked brown rice in the ratio (rice: water 1:2) |
Egg plant spicy gravy, yogurt, rasam (tamarind soup), poriyal (stir fried vegetables), papadum (thin crisp cracker) and pickle |
Table 2.
Estimated glycemic index of brown rice and white rice based recipes
| Recipes | * Estimated GI of BR-based recipes | * Estimated GI of WR-based recipes | % difference in the estimated GI |
|---|---|---|---|
| Idly (steamed pancake) | 57.5 | 77.0 | 25 |
| Pongal (rice pressure-cooked with with green gram split/whole legume) | 54.7 | 74.4 | 27 |
| Rice rava upma (stir-fried rice grits) | 58.8 | 80.9 | 27 |
| Oothappam (shallow fried pancake) | 58.6 | 79.4 | 26 |
| Rice vegetable kitchdi (rice boiled with stir fried vegetables) | 59.7 | 81.8 | 27 |
| Dosa (thin crisp pancake) | 58.6 | 79.4 | 26 |
| Plain rice pressure cooked | 58.8 | 84.0 | 30 |
| Vegetable pulav (sauted vegetables stir fried with boiled rice) | 58.5 | 79.8 | 27 |
| Sambar rice (rice mixed with sambar (vegetables cooked with pressure-cooked legume) | 50.2 | 69.4 | 28 |
GI=glycemic index; BR=brown rice; WR=white rice.
Estimated GI was calculated using FAO/WHO 1998.
A cyclic menu with variation in accompaniments only was developed based on GI testing and typical dietary patterns of the participants (Table 1). All menu items were standardized by trained dietitians at the in-house test kitchen. A cook was hired and trained for bulk preparation of intervention foods in the food production kitchen according to Hazard Analysis and Critical Control Point (HACCP) regulations. The kitchen area specially designed for this study includes cooking, storage and dining sections. Dietitians will regularly monitor the hygiene, sanitation, cooking, preparations, storage and procurement of raw ingredients and dispatch of food.
Intervention
Recruited participants are randomized to start first either a brown or white rice dietary regimen in a crossover design. Randomization is performed using computer generated random numbers. A stratification factor for gender is included in the randomization scheme to avoid differences in the proportion of men and women between the order of intervention administration. The study is designed to replace white rice in 2 meals (breakfast and lunch), 6 days a week for a period of 3 months. The brown rice for this intervention is produced under the supervision of a food technologist from MDRF by a local rice miller (Ramesh Modern Rice Mill, Redhills, Chennai, India), as authentic brown rice is not presently available in the Indian market (Kumar et al., 2011). Participants are served either cooked rice grain or processed rice grit dishes for breakfast and cooked rice grain (for lunch) keeping with the usual local cultural preferences. To monitor whether the participants are receiving the correct white or brown rice meal items according to randomization, they were given photo identification badges with colored ribbon (blue for white rice and brown for brown rice) when they enter the study dining area. Participants are requested to sign the register kept at the dining area before they pick up their meal, and this is used to count the number of participatory days. Preparations of both brown and white rice based recipes were previously standardized (Mohan et al., 2014). Prior to the beginning of the randomized intervention trial, a 1-week run-in phase is conducted to evaluate participant’s motivation, and willingness to adhere to the diet throughout the study period.
Monitoring and follow-up
Participants are contacted directly by study staff every month to motivate continuation in the study. Participants who discontinue the study will be contacted and their reasons for discontinuation recorded. To assess compliance, participants are directly monitored to note their daily participation, number of meals consumed, and serving sizes of foods consumed. Participants are informed about possible adverse effects due to consumption of brown rice such as diarrhea, flatulence, nausea, and/or bloating early in the trial period. If any serious side effects of the interventions are detected, participants will be withdrawn from the study.
The study parameters and timeline of the assessments are shown in Table 3.
Table 3.
Schedule of assessments
| Timepoint | ||||
|---|---|---|---|---|
|
| ||||
| Measures | Baseline | 1 month | 2 month | 3 month |
|
| ||||
| Sociodemographic measures: | ||||
| Screening questionnaire | • | |||
| Anthropometric measures: | ||||
| (Height, weight, waist circumference) | • | • | • | • |
| Biochemical measures: | ||||
| Glucose tolerance test | • | |||
| Fasting plasma glucose | • | • | ||
| Glycosylated haemoglobin | • | • | ||
| Triglycerides | • | • | ||
| Total cholesterol | • | • | ||
| HDL cholesterol | • | • | ||
| LDL cholesterol | • | • | ||
| Fasting insulin | • | • | ||
| Inflammatory markers (hs-CRP) | • | • | ||
| Clinical measures: | ||||
| Blood pressure | • | • | • | |
| Diet and physical activity measures: | ||||
| 24 hour recall (2 times at each time point) | • | • | • | |
| Food frequency questionnaire | • | |||
| Satiety | • | • | ||
| Adverse events | • | • | • | • |
| Physical activity | • | |||
Oral glucose tolerance test (OGTT)
An OGTT is performed at the screening phase to exclude diabetes. Fasting blood samples are obtained from participants by collecting finger-prick capillary blood samples. An 82.5 g oral glucose load (equivalent to 75 g of anhydrous glucose) is administered, and the 2-hour post load capillary blood glucose estimated. Capillary blood glucose is measured using an automatic lancet device (Accu-Chek ® Sensor, Roche Diagnostics GmbH, Mannheim, Germany), which is calibrated daily using the control solution and the Hemocue Glucose 201 + analyzer (Hemocue® Ltd, Angelholm, Sweden). Participants with fasting capillary blood glucose ≥126 mg/dl or 2-hour post capillary blood glucose ≥220 mg/dl (Deepa et al., 2009) will be deemed ineligible and not further screened for the study.
Screening questionnaire
An interviewer-administered screening questionnaire is administered to all participants during recruitment to assess their usual rice intake, medical history, demographics, and lifestyle factors such as physical activity, alcohol consumption, and smoking status. Upon completion of the questionnaire, a checklist of inclusion and exclusion criteria is used to qualify participants.
Anthropometrics
Body weight (kg), height (cm) and waist circumference (measurement taken at the end of the expiration) are measured according to standardized procedures detailed previously (Deepa et al., 2003). Body mass index (BMI) is calculated as the weight (kg) divided by height (m2) (meters squared). Overweight and obese are defined as those having a BMI ≥23 kg/m2 and ≥25 kg/m2, respectively, according to the Asia Pacific Classification (World Health Organization, 2000).
Diet
A validated interviewer-administered food frequency questionnaire (FFQ) (Sudha et al., 2006) is used to assess the dietary habits of participants prior to randomization to evaluate their baseline dietary history. Compliance to the study protocol is assessed using multiple 24-hour recalls throughout the study. The food and nutrients including the GI and GL of both brown and white rice meals were estimated with the in-house database ‘EpiNu’. Each participant is administered a weekday and weekend 24-hour dietary recall every month (a total of 6 times) during the intervention period. Satiety and adverse effects of the intervention meals are also ascertained monthly.
Laboratory
The baseline biochemical parameters assessed during the study trial are described in Table 3. Blood samples will be obtained at baseline and at the end of each intervention (after 3 months) and analyzed at the National Accreditation Board for Testing and Calibration Laboratories (NABL) and the College of American Pathologists (CAP) accredited central laboratory at Dr. Mohan’s Diabetes Specialities Centre in Chennai. Fasting blood (after 10–12 hours of fasting) will be obtained by an experienced phlebotomist. The samples are immediately centrifuged, aliquoted, transported on dry ice and stored at −80°C. All assays will be performed at the end of the study to control for assay variation. Laboratory technicians are blinded to the participant’s study group. Plasma glucose (glucose oxidase peroxidase method), serum cholesterol (cholesterol oxidase-peroxidase-4-aminophenazone method), serum triglycerides (glycerol phosphate oxidase-peroxidase-4-aminophenazone method), and HDL cholesterol (direct method with polyethylene glycol pretreated enzymes) will be measured using a Hitachi 912 Autoanalyzer (Roche Diagnostics, GmbH, Mannheim, Germany) and utilizing kits supplied by Boehringer Mannheim (Mannheim, Germany). LDL cholesterol will be calculated using the Friedewald formula in subjects with triglycerides ≤ 400 mg/dl (Friedewald et al., 1972). Serum insulin concentration will be estimated using Dako kits (Dako, Glostrup, Denmark); hemoglobin A1c (HbA1c) will be measured using a Variant machine (Biorad, Hercules, CA). Plasma concentrations of hs-CRP will be measured by a highly sensitive nephelometric assay using a monoclonal antibody to CRP coated on polystyrene beads (Dade Behring, Marburg, Germany). Insulin resistance will be estimated from the homeostasis model assessment (HOMA-IR) by the following formula: (fasting insulin (μU/ml)) × fasting glucose (mmol/l))/22.5) (Matthews et al., 1985).
Statistical analysis
A sample size of 166 participants were randomized for the present study to provide a power of 80%, allowing for 15% drop-out, with a Type 1 error rate of 0.05 and an assumed intra-class correlation of 0.85. Data is stored electronically using in-house software. This unique in-house software makes it possible to maintain a systematic data record of anthropometric, dietary and biochemical parameters and to estimate the nutrient content of the dietary records of the participants at baseline, mid-study and at the end of the study. Participant confidentiality is assured through a computer-based data coding system that de-identifies the data before analysis. Adequacy of the randomization process will be assessed by comparison of baseline characteristics. Differences in changes from baseline between the brown and white rice intervention arms in biomarkers, body measurements, and dietary intake, will be calculated using the optimally efficient SLAIN test (Frison and Pocock, 1997). The natural logarithm or other transformation will be used for any markers that are not normally distributed. If a suitable transformation cannot be found to normalize the distribution of the paired changes in the two groups, the non-parametric Wilcoxon signed-rank test will be used to assess the statistical significance of the deviance of the difference between the paired changes in the two groups. A two-tailed p-value < 0.05 will be considered statistically significant. To calculate the point and interval estimate of effect, mean ± standard deviation (SD) baseline values of each marker will be presented in the control and intervention arms, along with their respective percent changes (SD) from baseline. If groups appear unbalanced at baseline with respect to any determinants of the markers under study, in secondary analysis a generalized linear models approach will be use to adjust the groups for any difference at baseline and calculate the relative changes in each group after adjusting for any group differences.
Participant characteristics at study start of Period 1
A total of 352 participants were screened, of whom 200 expressed their initial interest to participate. These participants were to be tested for undetected diabetes by OGTT and a total of 181 participants were willing to participate further. An interactive session with participants was conducted to address queries or concerns regarding consumption of brown rice and the intervention study. Post-session, 10 people who had initially shown their willingness to participate could not continue because of a work relocation. The remaining 171 volunteers were asked to undergo an OGTT to ensure that participants with known or newly detected diabetes were not included in the study. Out of these 171 volunteers, 5 were newly diagnosed with diabetes by OGTT and were excluded from the study. Hence, the study included 166 participants.
Participants were randomized to start with either the brown or white rice arms (Baseline – Period 1). Participants were aged 25–62 years and 45% were female. Table 4 shows the clinical characteristics according to randomized treatment at the baseline of period 1 prior to crossover. The mean ± SD for BMI was 28.1 ± 3.4 kg/m2 and average waist measured 94.4 ± 7.9 centimeters among men and 89.2 ± 8.8 centimeters among women. The average systolic/diastolic blood pressures were 123.1 ± 14.8 mmHg and 79.9 ± 12.4 mmHg, respectively and fasting blood glucose was 86.6±11.3 mg/dl. There were no statistically significant differences in clinical characteristics between those randomized to the brown or white rice diet at study start.
Table 4.
Participant characteristics according to randomized treatment at start of study (Baseline of Period 1 before crossover)
| Characteristics | Diet A (n= 85) | Diet B (n= 81) | p-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Female [n (%)] * | 39 | 45.9 | 35 | 43.2 | 0.76 |
| Age (years) | 37.6 | 9.3 | 36.5 | 10.0 | 0.36 |
| Height (cm) | 160.9 | 8.5 | 161.6 | 8.8 | 0.65 |
| Weight (kg) | 72.7 | 11.0 | 73.4 | 10.2 | 0.54 |
| Body mass index (kg/m2) | 28.1 | 3.7 | 28.1 | 3.2 | 0.99 |
| Waist circumference (cm) | 92.0 | 8.3 | 92.2 | 9.1 | 0.71 |
| Male | 93.0 | 8.3 | 95.8 | 7.3 | 0.09 |
| Female | 90.8 | 8.4 | 87.5 | 9.1 | 0.11 |
| Systolic blood pressure (mmHg) | 123 | 14 | 123.0 | 12.4 | 0.99 |
| Diastolic blood pressure (mmHg) | 81.1 | 15.7 | 79.0 | 12.4 | 0.23 |
| Fasting blood sugar (mg/dl) | 85.4 | 10.9 | 87.8 | 11.7 | 0.30 |
| Cholesterol (mg/dl) | 181.1 | 34.0 | 173.3 | 34.1 | 0.12 |
| Triglyceride (mg/dl) | 120.6 | 54.6 | 133.3 | 97.3 | 0.30 |
| HDL cholesterol (mg/dl) | 39.0 | 6.5 | 37.9 | 7.6 | 0.31 |
| LDL cholesterol (mg/dl) ** | 118.0 | 30.1 | 111.4 | 30.8 | 0.25 |
| Fasting insulin ( μIU/ ml) | 12.8 | 9.4 | 12.9 | 6.8 | 0.92 |
| HOMA-IR | 2.7 | 2.2 | 2.9 | 1.7 | 0.71 |
| HbA1c (%) | 5.6 | 0.5 | 5.6 | 0.5 | 0.99 |
| C-reactive protein (mg/l) | 4.2 | 2.9 | 4.0 | 2.7 | 0.74 |
Data are presented as mean and standard deviation (SD), and p-values obtained by independent t-test unless otherwise noted.
Data presented as n (%), and p-value obtained by chi-square test.
Two participants had LDL measures below detectable limits of the assay.
Table 5 shows the dietary profile of participants at the start of the study according to randomized treatment arm. Five people did not complete the FFQ and discontinued the study because of either job transfer or job discontinuation. Mean energy intake was 2831 ± 823 kilocalories with the majority of calories consumed from carbohydrates (59% of total energy intake). The average energy intake from saturated fat consumed was 9% of total energy intake. Whole or whole milled cereals were rarely consumed (1.2 g and 45.4 g, respectively) with the majority of cereal consumed as refined (330.1 g), particularly white rice (255.7 g).
Table 5.
Dietary profile of overweight participants according to randomized treatment at study commencement (Baseline of Period 1 before crossover)
| Nutrients | Diet A (n= 81) | Diet B (n= 80) | p-value | ||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | ||
| Energy (kcal) | 2814.4 | 871.9 | 2847.5 | 774.3 | 0.59 |
| Carbohydrates (g) | 409.6 | 117.2 | 419.8 | 113.9 | 0.46 |
| Carbohydrates (% of total energy) | 58.8 | 5.8 | 59.2 | 4.6 | 0.98 |
| Protein (g) | 78.2 | 24.8 | 81.1 | 23.9 | 0.40 |
| Protein (% of total energy) | 11.2 | 1.5 | 11.4 | 1.2 | 0.27 |
| Fat (g) | 88.6 | 31.0 | 93.7 | 30.1 | 0.20 |
| Fat (% of total energy) | 12.6 | 1.8 | 13.1 | 1.8 | 0.04 |
| Saturated fat (g) | 27.6 | 10.3 | 29.3 | 9.8 | 0.21 |
| Saturated fat (% of total energy) | 8.8 | 1.8 | 9.2 | 1.5 | 0.14 |
| Monounsaturated fat (g) | 24.5 | 8.9 | 26.0 | 9.0 | 0.18 |
| Monounsaturated fat (% of total energy) | 7.8 | 1.3 | 8.2 | 1.4 | 0.11 |
| Polyunsaturated fat (g) | 31.7 | 11.3 | 33.1 | 11.1 | 0.32 |
| Polyunsaturated fat (% of total energy) | 10.2 | 1.8 | 10.5 | 1.9 | 0.37 |
| Dietary fiber (g) | 37.3 | 12.8 | 37.6 | 10.9 | 0.70 |
| Glycemic load | 219.9 | 64.9 | 228.2 | 66.4 | 0.41 |
| Weighted glycemic index | 60.9 | 3.2 | 61.5 | 2.6 | 0.16 |
| Refined grains (g) | 250.0 | 106.4 | 261.5 | 98.8 | 0.33 |
| Whole grains (g)* | 1.5 | 3.7 | 0.9 | 0.6 | 0.19 |
| Whole milled grains (g)* | 42.4 | 39.0 | 48.4 | 40.2 | 0.34 |
| Millets (g) | 2.3 | 2.9 | 6.3 | 12.0 | 0.59 |
| Pulses and legumes (g)* | 82.3 | 37.6 | 76.0 | 30.9 | 0.25 |
| Milk and its products (g)* | 287.0 | 146.0 | 291.9 | 165.8 | 0.84 |
| Fruits and vegetables (g) | 330.9 | 128.8 | 341.2 | 132.9 | 0.607 |
| Tubers (g)* | 33.4 | 22.6 | 34.5 | 24.8 | 0.777 |
| Fats and edible oil (g) * | 52.9 | 20.0 | 55.0 | 19.3 | 0.296 |
| Meat and poultry (g) | 30.7 | 27.1 | 42.7 | 45.1 | 0.065 |
| Egg (g) | 15.7 | 15.1 | 20.4 | 27.8 | 0.334 |
| Fish and seafoods (g) | 20.5 | 26.5 | 23.6 | 25.3 | 0.074 |
| Added salt (g) | 11.0 | 4.4 | 11.1 | 3.7 | 0.387 |
| Added sugar (g) | 35.8 | 22.4 | 38.4 | 27.1 | 0.858 |
Data are presented as mean and standard deviation (SD), and p-values obtained by independent t-test unless otherwise noted
DISCUSSION
The present study is funded by the National Institute of Health (NIH) as a joint collaboration between the Harvard School of Public Health and Madras Diabetes Research Foundation (MDRF), India. The study is a randomized trial to evaluate the effect of substituting brown rice for white rice for 3 months on several biomarkers of diabetes risk among adults in Chennai, India who are at high risk for developing diabetes. The present paper provides the rationale and methodology for this proof-of-concept study.
There is a crucial need for innovative prevention strategies to abate the rapid rise in the prevalence and incidence of diabetes in India. Furthermore, Asian Indians have an increased susceptibility to diabetes as they have increased insulin resistance and visceral adiposity, despite a lower prevalence of obesity (Chan et al., 2009). Rice is a staple food for Asians and almost half of daily calories come from refined grains, especially white rice (Radhika et al., 2009). The commonly consumed Indian white rice varieties tend to have high GI values (Shobana et al., 2010), and have been significantly associated with a higher prevalence of metabolic syndrome in the Indian population (Radhika et al., 2009, Mohan et al., 2009). Meta-analyses from observational studies (Barclay et al., 2008, Bhupathiraju et al., 2014) and randomized controlled trials (Brand-Miller et al., 2003) found that a low-GI diet was associated with a reduced incidence of diabetes and low HbA1c among diabetic adults. Using the most recent comprehensive list of GI and glucose load values for international foods (Atkinson et al., 2008), brown rice (with most of the germ and bran intact) generally has a lower GI than its polished counterparts. Findings from large prospective cohort studies have shown a beneficial role of brown rice and whole grains on prevention and management of diabetes (Sun et al., 2010, Fung et al., 2002, McKeown et al., 2002, Ley et al., 2014).
Randomized controlled trials are the necessary next step to provide evidence-based recommendations for brown rice substitution among Asian populations with traditionally high levels of white rice consumption. To our knowledge, there is little evidence available from interventions on the efficacy of whole grains or brown rice substitution for white rice in improving diabetes and cardiovascular disease (CVD) risk markers. A randomized controlled dietary intervention study (WHOLEheart study) was conducted among obese adults (N=316) aged 18–65 years among 3 groups; control (no dietary change), intervention 1 (60 g whole grains/day for 16 weeks) and intervention 2 (60 g whole grains/day for first 8 weeks followed by 120 g whole grains/day for another 8 weeks) (Brownlee et al., 2010). Increasing whole grain consumption did not improve any of the cardiovascular biomarkers, including measures of glucose or insulin over the 16-week study period. Another parallel, randomized dietary intervention trial among middle-aged Chinese adults (N=202) with diabetes or at risk for diabetes was carried out for 16 weeks (Zhang et al., 2011). The study substituted white rice with brown rice (225 g servings) in 2 meals (lunch and dinner for 6 days). Weekday lunches were served in the cafeteria, while dinners and Saturday meals were provided as packed food. Metabolic risk markers such as BMI, waist circumference, serum lipid, glucose and insulin concentrations were measured. With the exception of LDL, and only among the subgroup with diabetes, no significant treatment effects were observed. The WHOLEheart study used a limited number of commercially available cooked/processed whole grain products in the market for the intervention, while the Shanghai study used brown rice prepared by the study staff. It is plausible that the choice of whole grains foods consumed in an intervention could have unexpectedly impacted the glycemic response. Additionally in the aforementioned studies, all or the majority of meals were provided as pre-packaged to be eaten away from the study site possibly challenging treatment adherence. In the Shanghai study, the improvement in blood pressure and lipids was observed only in a subgroup of diabetic patients. In Indian culture and traditions, people consume food together, presenting additional challenges for such intervention studies to be conducted in India. For this reason, the present study provides brown rice milled under the supervision of food technologists, as authentic brown rice is not readily available in Indian markets. Standardized breakfast and lunch are prepared and served at the new dining facility created for this study at MDRF. Participants are directly observed consuming the meals under the supervision of research dietitians for the period of 12 weeks (6 days) for each diet to closely monitor compliance.
The present study has a few limitations. Although participants are educated to adhere to their usual food consumption patterns and not over-consume meals in the evening, we recognize that the study is being carried out in free-living participants, precluding our ability to provide all meals under completely controlled dietary conditions. In addition, for feasibility reasons, the intervention is ad libitum and provides 2 meals a day for 6 days per week excluding Sundays. We are collecting multiple 24-hour dietary recalls throughout the study to help monitor compliance. Second, the study is a 3-month crossover dietary intervention trial where both the control and intervention arms are interchanged after a washout period of 2 weeks. There may be dropouts due to a lengthy study period of approximately 8 months, due to various reasons such as migration, job loss, pregnancy, or illness. Participants were made aware of the study design and duration prior to enrolment and were screened to enhance availability for the full study duration.
A major strength of the present study is the establishment of the food production kitchen, which includes cooking, storage and dining facilities. A cook was hired and trained for the bulk preparations in the quantity food production kitchen according to HACCP regulations. Trained dietitians regularly monitor the hygiene, sanitation, cooking, preparations, storage and procurement of raw ingredients and dispatch of food at the dining section. Monitoring of the food served, attendance, adherence to the assigned intervention and side effects if any are regularly assessed. The perceptions of participants consuming brown rice will be surveyed at the end of the trial which may provide useful data about whether individuals plan to consume brown rice (200 g) in the long-term. Our study efforts will provide GI values of typical rice-based meal preparations made from both brown rice and white rice varieties in India, which may be very useful for future nutritional studies to combat diabetes in developing countries as no such information is currently available. At a minimum, the present study will train Junior and Senior Research Officers in the design, methodology and statistical analysis of nutritional intervention studies; and the research team will be primed to participate in a future larger multi-national intervention study evaluating diabetes incidence endpoints.
CONCLUSIONS
The concept of brown rice replacement for white rice is a novel step towards prevention of diabetes, and the present study model can be considered as a prototype for developing a large multi-national trial studying the effects of brown rice substitution (or other low-GI carbohydrates) on diabetes risk. If the invention is found to be efficacious, the findings can be incorporated into national-level policies. Such policies could include the provision of brown rice as an option or replacement for white rice in institutions and food programs. This relatively simple dietary intervention has the potential to substantially diminish the burden of diabetes in Asia and elsewhere.
Acknowledgments
We would like to thank our collaborators in the Global Nutrition and Epidemiologic Transition (GNET) Initiative at the Harvard School of Public Health for their support on this project.
Footnotes
Trial registration: ClinicalTrials.gov Identifier: NCT01814735
DECLARATION OF INTEREST
This work was funded by the Fogarty International Center at the National Institutes of Health (R03TW008726). On behalf of Dr. Mohan and colleagues, we disclose that brown rice and a new high fiber white rice is currently being marketed at Dr. Mohan’s Diabetes Specialities Centre.
References
- 1.Anjana RM, Pradeepa R, Deepa M, Datta M, Sudha V, Unnikrishnan R, et al. Prevalence of diabetes and prediabetes (impaired fasting glucose and/or impaired glucose tolerance) in urban and rural India: phase I results of the Indian Council of Medical Research-INdia DIABetes (ICMR-INDIAB) study. Diabetol. 2011;54:3022–7. doi: 10.1007/s00125-011-2291-5. [DOI] [PubMed] [Google Scholar]
- 2.Atkinson FS, Foster-Powell K, Brand-Miller JC. International tables of glycemic index and glycemic load values: 2008. Diab Care. 2008;31:2281–3. doi: 10.2337/dc08-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barclay AW, Petocz P, McMillan-Price J, Flood VM, Prvan T, Mitchell P, et al. Glycemic index, glycemic load, and chronic disease risk--a meta-analysis of observational studies. Am J Clin Nutr. 2008;87:627–37. doi: 10.1093/ajcn/87.3.627. [DOI] [PubMed] [Google Scholar]
- 4.Bhupathiraju SN, Tobias DK, Malik VS, Pan A, Hruby A, Manson JE, et al. Glycemic index, glycemic load, and risk of type 2 diabetes: results from 3 large US cohorts and an updated meta-analysis. Am J Clin Nutr. 2014;100:218–232. doi: 10.3945/ajcn.113.079533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brand-Miller J, Hayne S, Petocz P, Colagiuri S. Low-glycemic index diets in the management of diabetes: a meta-analysis of randomized controlled trials. Diab Care. 2003;26:2261–7. doi: 10.2337/diacare.26.8.2261. [DOI] [PubMed] [Google Scholar]
- 6.Brownlee IA, Moore C, Chatfield M, Richardson DP, Ashby P, Kuznesof SA, et al. Markers of cardiovascular risk are not changed by increased whole-grain intake: the WHOLEheart study, a randomised, controlled dietary intervention. Br J Nutr. 2010;104:125–34. doi: 10.1017/S0007114510000644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan JC, Malik V, Jia W, Kadowaki T, Yajnik CS, Yoon KH. Diabetes in Asia: epidemiology, risk factors, and pathophysiology. JAMA. 2009;301:2129–40. doi: 10.1001/jama.2009.726. [DOI] [PubMed] [Google Scholar]
- 8.Deepa M, Farooq S, Deepa R, Manjula D, Mohan V. Prevalence and significance of generalized and central body obesity in an urban Asian Indian population in Chennai, India (CURES: 47) Eur J Clin Nutr. 2009;63:259–67. doi: 10.1038/sj.ejcn.1602920. [DOI] [PubMed] [Google Scholar]
- 9.Deepa M, Pradeepa R, Rema M, Mohan A, Deepa R, Shanthirani S, Mohan V. The Chennai Urban Rural Epidemiology Study (CURES)--study design and methodology (urban component) (CURES-I) J Assoc Physicians India. 2003;51:863–70. [PubMed] [Google Scholar]
- 10.Food and Agriculture Organization/World Health Organization. Report of a Joint FAO/WHO Expert Consultation. Rome: FAO; 1998. Carbohydrates in Human Nutrition. [Google Scholar]
- 11.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 12.Frison LJ, Pocock SJ. Linearly divergent treatment effects in clinical trials with repeated measures: efficient analysis using summary statistics. Stat Med. 1997;16:2855–72. doi: 10.1002/(sici)1097-0258(19971230)16:24<2855::aid-sim749>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Fung TT, Hu FB, Pereira MA, Liu S, Stampfer MJ, Colditz GA, et al. Whole-grain intake and the risk of type 2 diabetes: a prospective study in men. Am J Clin Nutr. 2002;76:535–40. doi: 10.1093/ajcn/76.3.535. [DOI] [PubMed] [Google Scholar]
- 14.Henry CJ, Lightowler HJ, Newens K, Sudha V, Radhika G, Sathya RM, et al. Glycaemic index of common foods tested in the UK and India. Br J Nutr. 2008;99:840–5. doi: 10.1017/S0007114507831801. [DOI] [PubMed] [Google Scholar]
- 15.Hsu TF, Kise M, Wang MF, Ito Y, Yang MD, Aoto H, et al. Effects of pre-germinated brown rice on blood glucose and lipid levels in free-living patients with impaired fasting glucose or type 2 diabetes. J Nutr Sci Vitaminol. 2008;54(2):163–168. doi: 10.3177/jnsv.54.163. [DOI] [PubMed] [Google Scholar]
- 16.Hu EA, Pan A, Malik V, Sun Q. White rice consumption and risk of type 2 diabetes: meta-aanlysis and systematic review. BMJ. 2012;344:e1454. doi: 10.1136/bmj.e1454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.International Diabetes Federation. IDF Diabetes Atlas. 6. Brussels, Belgium: International Diabetes Federation; 2013. [Google Scholar]
- 18.Kumar S, Mohanraj R, Sudha V, Wedick NM, Malik V, Hu FB, et al. Perceptions about varieties of brown rice: a qualitative study from Southern India. J Am Diet Assoc. 2011;111:1517–22. doi: 10.1016/j.jada.2011.07.002. [DOI] [PubMed] [Google Scholar]
- 19.Ley SH, Hamdy O, Mohan V, Hu FB. Prevention and management of type 2 diabetes: dietary components and nutritional strategies. Lancet. 2014;383:1999–2007. doi: 10.1016/S0140-6736(14)60613-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mattei J, Malik V, Wedick NM, Campos H, Spiegelman D, Willett W. A symposium and workshop report from the Global Nutrition and Epidemiologic Transition Initiative: nutrition transition and the global burden of type 2 diabetes. Br J Nutr. 2012;108:1325–35. doi: 10.1017/S0007114512003200. [DOI] [PubMed] [Google Scholar]
- 21.Matthews DR, Hosker JP, Rudenski AS, Naylor BA, Treacher DF, Turner RC. Homeostasis model assessment: insulin resistance and beta-cell function from fasting plasma glucose and insulin concentrations in man. Diabetol. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 22.McKeown NM, Meigs JB, Liu S, Wilson PW, Jacques PF. Whole-grain intake is favorably associated with metabolic risk factors for type 2 diabetes and cardiovascular disease in the Framingham Offspring Study. Am J Clin Nutr. 2002;76:390–8. doi: 10.1093/ajcn/76.2.390. [DOI] [PubMed] [Google Scholar]
- 23.Mohan V, Radhika G, Sathya RM, Tamil SR, Ganesan A, Sudha V. Dietary carbohydrates, glycaemic load, food groups and newly detected type 2 diabetes among urban Asian Indian population in Chennai, India (Chennai Urban Rural Epidemiology Study 59) Br J Nutr. 2009;102:1498–506. doi: 10.1017/S0007114509990468. [DOI] [PubMed] [Google Scholar]
- 24.Mohan V, Spiegelman D, Sudha V, Gayathri R, Hong B, Praseena K, et al. Effect of brown rice, white rice, and brown rice with legumes on blood glucose and insulin responses in overweight Asian Indians: a randomized controlled trial. Diabetes Technol Ther. 2014;16:317–25. doi: 10.1089/dia.2013.0259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Monge-Rojas R, Mattei J, Fuster T, Willett W, Campos H. Influence of sensory and cultural perceptions of white rice, brown rice and beans by Costa Rican adults in their dietary choices. Appetite. 2014;81C:200–208. doi: 10.1016/j.appet.2014.06.028. [DOI] [PubMed] [Google Scholar]
- 26.Muhihi A, Gimbi D, Njelekela M, Shemaghembe E, Mwambene K, Chiwanga F. Consumption and acceptability of whole grain staples for lowering markers of diabetes risk among overweight and obese Tanzanian adults. Global Health. 2013;9:26. doi: 10.1186/1744-8603-9-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panlasigui LN, Thompson LU. Blood glucose lowering effects of brown rice in normal and diabetic subjects. Int J Food Sci Nutr. 2006;57:151–158. doi: 10.1080/09637480500410879. [DOI] [PubMed] [Google Scholar]
- 28.Radhika G, Sathya RM, Ganesan A, Saroja R, Vijayalakshmi P, Sudha V, et al. Dietary profile of urban adult population in South India in the context of chronic disease epidemiology (CURES-68) Public Health Nutr. 2011;14:591–8. doi: 10.1017/S136898001000203X. [DOI] [PubMed] [Google Scholar]
- 29.Radhika G, Van Dam RM, Sudha V, Ganesan A, Mohan V. Refined grain consumption and the metabolic syndrome in urban Asian Indians (Chennai Urban Rural Epidemiology Study 57) Metabolism. 2009;58:675–81. doi: 10.1016/j.metabol.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 30.Shobana S, Harsha MR, Platel K, Srinivasan K, Malleshi NG. Amelioration of hyperglycaemia and its associated complications by finger millet (Eleusine coracana L.) seed coat matter in streptozotocin-induced diabetic rats. Br J Nutr. 2010;104:1787–95. doi: 10.1017/S0007114510002977. [DOI] [PubMed] [Google Scholar]
- 31.Sudha V, Radhika G, Sathya RM, Ganesan A, Mohan V. Reproducibility and validity of an interviewer-administered semi-quantitative food frequency questionnaire to assess dietary intake of urban adults in southern India. Int J Food Sci Nutr. 2006;57:481–93. doi: 10.1080/09637480600969220. [DOI] [PubMed] [Google Scholar]
- 32.Sudha V, Spiegelman D, Hong B, Malik V, Jones C, Wedick NM. Consumer Acceptance and Preference Study (CAPS) on brown and undermilled Indian rice varieties in Chennai, India. J Am Coll Nutr. 2013;32:50–7. doi: 10.1080/07315724.2013.767672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sun Q, Spiegelman D, Van Dam RM, Holmes MD, Malik VS, Willett WC. White rice, brown rice, and risk of type 2 diabetes in US men and women. Arch Intern Med. 2010;170:961–9. doi: 10.1001/archinternmed.2010.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wolever TM, Vorster HH, Bjorck I, Brand-Miller J, Brighenti F, Mann JI, et al. Determination of the glycaemic index of foods: interlaboratory study. Eur J Clin Nutr. 2003;57:475–82. doi: 10.1038/sj.ejcn.1601551. [DOI] [PubMed] [Google Scholar]
- 35.World Health Organization. Redefining Obesity and Its Treatment. Melbourne: International Diabetes Institute for the International Association for the Study of Obesity and International Obesity Task Force; 2000. The Asia Pacific Perspective. [Google Scholar]
- 36.Zhang G, Pan A, Zong G, Yu Z, Wu H, Chen X, et al. Substituting white rice with brown rice for 16 weeks does not substantially affect metabolic risk factors in middle-aged Chinese men and women with diabetes or a high risk for diabetes. J Nutr. 2011;141:1685–90. doi: 10.3945/jn.111.142224. [DOI] [PubMed] [Google Scholar]