Abstract
Purpose
Prior to availability of anti-HER2 therapies, HER2-positive metastatic breast cancer (MBC) was associated with a poor prognosis. Prospective randomized trials have demonstrated survival benefit from anti-HER2 treatments. Anecdotal observations have suggested that a small but meaningful fraction of patients with HER2-positive MBC may be “exceptional responders” with long survival. We hypothesized that demographic and/or clinicopathologic characteristics can be identified to distinguish short-term from long-term survivors.
Methods
A retrospective, single institution review of 168 patients with HER2-positive MBC who received treatment with anti-HER2 therapy in the metastatic setting was performed. Cox proportional hazards analysis was used to assess factors associated with long-term survival.
Results
Median overall survival from the time of breast cancer recurrence was 3.9 years (95% CI 3.4–5.2). From the time of diagnosis of MBC, 56 (33%) survived for 5 or more years and 12 (7%) survived more than 10 years. Of the 66 patients diagnosed with central nervous system metastases, 9 (14%) survived more than 5 years following that diagnosis. Younger age at diagnosis, lower stage, hormone receptor positive status, and only having one organ involved at diagnosis were associated with longer survival. Four patients discontinued anti-HER2 therapy and are without evidence of progression of disease after a median 7.4 years (0.2–12.0) since stopping therapy.
Conclusions
In a cohort of patients with HER2-positive MBC treated primarily with trastuzumab and lapatinib, 7% of patients were “exceptional responders”. Combining these clinical factors associated with molecular determinants of prolonged survival with may provide insights for individualizing treatment selection.
Keywords: metastatic breast cancer, HER2 positive, survival, brain metastasis
Introduction
Before the advent of targeted anti-Her2 therapies, HER2-overexpressing (HER2-positive) tumors portended a worse outcome compared to Her2-negative tumors.[1, 2] The development of trastuzumab in the late 1990s revolutionized the management of HER2-positive metastatic breast cancers (MBC), and improved the prognosis of patients with HER2-positive tumors.[3–7] However, HER2-positive MBC is still considered an incurable disease, with the majority of patients ultimately dying within 5 years. The median overall survival (OS) from the time of initiation of anti-HER2 therapy was recently found to be 32–42 months in the metastatic setting.[4, 8] It has been recently reported that 25–30% of patients with HER2-positive MBC survived five or more years following treatment with HER2-targeted therapy.[9, 10]
We hypothesized that demographic and clinicopathologic factors can distinguish short-term from long-term HER2-positive MBC survivors. To investigate this hypothesis, we analyzed data from a retrospective database of patients with HER2-positive MBC treated at a single institution with anti-HER2 therapy as part of their treatment regimen. We report a cohort of “exceptional responders” who have survived ≥10 years after metastatic disease, some of whom have discontinued therapy without clinical evidence of progression.
Methods
Study design
All patients diagnosed with HER2-positive MBC and treated with at least 21 days of trastuzumab or lapatinib in the metastatic setting at the University of Michigan (U-M) Comprehensive Cancer Center between 1991 and 2015 were included. Potentially eligible patients were identified through review of pharmacy records. Patients with distant metastatic disease were included; those with locoregional recurrence (including axillary or supraclavicular nodal disease) or second primary tumors were excluded. Those patients who received only a single 3 week dose or fewer than 4 weekly doses of trastuzumab were excluded (Supplemental Figure 1). Biopsies of suspected metastatic sites were performed at the discretion of the treating physician. HER2 status was determined clinically at the time of primary or metastatic diagnosis using immunohistochemistry and/or fluorescence in situ hybridization (FISH) according to period-appropriate institutional guidelines. This retrospective, single institution study was approved by the Institutional Review Board, which granted a waiver of informed consent.
Clinicopathologic characteristics of eligible patients were abstracted from the electronic medical record by two reviewers (PM, NLH), including demographics; dates of diagnosis of primary breast cancer, MBC and first progression; tumor characteristics at the time of original diagnosis and at the time of disease recurrence; initial sites of metastatic disease; systemic treatments received in the adjuvant and metastatic settings; date of last follow-up at U-M; and date of death, if applicable. Site(s) of metastatic disease at time of diagnosis of MBC were grouped into four categories based on findings demonstrated on imaging studies: single organ involvement (bone, viscera, and central nervous system (CNS)) and multi-organ involvement.
Statistical methods
The primary endpoint was OS, defined as the time between a patient’s diagnosis of MBC and death from any cause or last follow-up. The secondary endpoint was progression-free survival (PFS), defined as the time from diagnosis of MBC to time of initiation of second line therapy or death from any cause, whichever occurred first. OS and PFS were analyzed using the Kaplan-Meier product-limit method and log rank test. Data from patients with no event recorded were censored at the date of last follow-up.
Cox proportional hazards models were used to evaluate the association between OS and covariates, including age at initial diagnosis, time to development of MBC (defined as the time elapsed between initial diagnosis of breast cancer and diagnosis of MBC), grade of primary tumor, cancer stage at initial diagnosis, hormone receptor (HR) status, and site(s) of metastatic disease at time of disease recurrence. 95% confidence intervals (CI) are presented. P values less than 0.05 were considered statistically significant.
Landmark analysis of CNS metastasis diagnosis at 1 year was used to investigate the relationship between development of CNS metastases and OS. The patients who died before 1 year (N=13, 8%) were excluded from the landmark analysis to provide a conservative estimate of the effect.[11] Women who did not have CNS metastasis at their last follow-up time were censored. We used both Kaplan-Meier analysis and log-rank test and Cox proportional hazards models controlling for age at initial diagnosis, stage, and HR-positive status for the landmark analysis. Additionally, the correlation between time to diagnosis of CNS metastasis and OS was investigated using Kendall’s tau rank correlation for bivariate censored data using the R NADA library.
Results
Baseline patient and tumor characteristics
Two hundred and forty-eight potentially eligible patients were identified (Supplemental Figure S1). Patients with in-breast recurrence (N=11), locoregional recurrence only (N=14), patients who did not receive at least 21 days of trastuzumab or lapatinib therapy after diagnosis of recurrent disease (N=28), and patients who were subsequently determined to not have HER2-positive recurrent disease (N=13) were excluded. In addition, patients with only a single clinic visit at the institution (N=14) were excluded because of lack of data about treatments administered. A total of 168 patients were eligible and included in the analysis.
Demographic factors and primary and metastatic tumor pathological characteristics for all eligible patients are given in Supplemental Table S1. Mean age at initial diagnosis of breast cancer was 47.9 (range 25.3–91.9). At initial diagnosis, 77 (46%) patients had grade 3 disease, 101 (60%) had HR-positive disease, and 49 (29%) had stage 4 disease. Twenty patients (12%) received adjuvant trastuzumab.
Median time to distant metastatic disease was 2.2 years (range 0–19.2 years). All 168 patients had radiographic evidence of distant metastases. Ninety-six (57%) underwent biopsy to confirm distant metastasis. Sixty-six (39%) patients developed CNS metastases during their disease course; it was the initial metastatic site of disease for 7 (4%) patients.
Timing of initiation of HER2-targeting therapy was influenced by the year of diagnosis and duration of survival. In this cohort first line systemic therapy for metastatic disease contained trastuzumab for 71%, lapatinib for 2%, and endocrine therapy or chemotherapy without HER2-targeted therapy for 26.2%. Forty-six patients received treatment with lapatinib (27%), 18 (11%) received ado-trastuzumab emtansine, and 15 (9%) received pertuzumab as part of their second-line or later treatment regimens.
Survival Outcomes
Median follow-up from the time of diagnosis of MBC was 3.2 years (range 0.1–16.3 years). Median OS from the time of MBC diagnosis was 3.9 years (95% CI 3.4–5.2 years; Figure 1A). Notably, 56 (33%) and 12 (7%) patients were alive ≥5 years and ≥10 or more years, respectively, after diagnosis of MBC. Median PFS from the time of diagnosis of MBC to initiation of second line therapy was 1.3 years (95% CI 1.1–1.6; Figure 1B). Of the 96 patients with biopsy-proven distant metastases, 5 (5%) were free of detectable active disease by routine imaging at ≥10 years. There was no difference in survival between those with and without biopsy confirmation of metastatic disease (HR 0.88 [95% CI 0.6–1.3], p=0.49). For the 39% of patients who were diagnosed with CNS metastases at any time during their disease course, median survival from the time of diagnosis of CNS metastasis was 1.5 years (95% CI 1.1–2.6).
Figure 1.
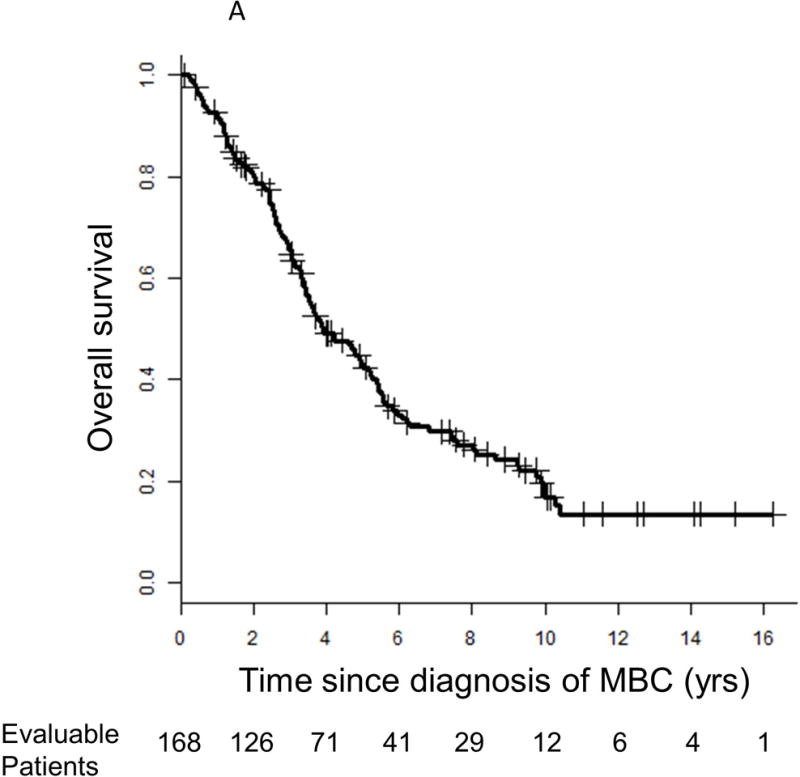
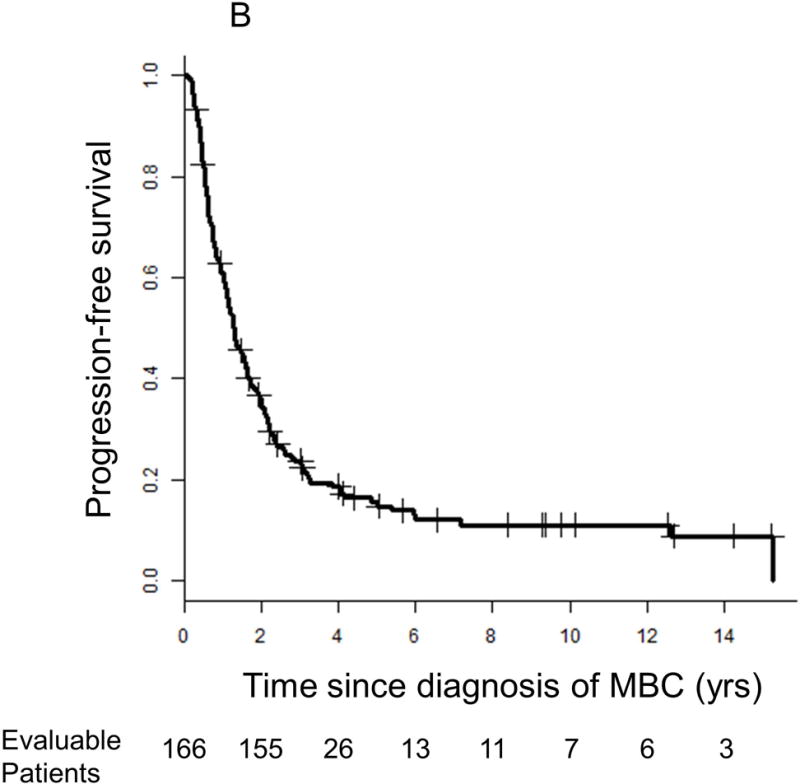
Kaplan-Meier survival curves for (A) overall survival and (B) progression-free survival. Vertical lines represent censored patients at last known follow-up. Two patients’ information about progression-free survival is unknown.
Predictors of survival with MBC
Using a Cox proportional hazards analysis, younger age at initial diagnosis of breast cancer, lower stage, hormone receptor-positivity of primary tumor, and only having one organ involved with metastasis at diagnosis of MBC were associated with statistically significantly longer OS (Table 1). In addition, there was a borderline longer OS for those with metastatic disease at diagnosis compared to those with stage I–III at initial diagnosis (HR 0.66 [95% CI 0.43–1.02], p=0.059). Time to development of MBC and grade of primary tumor did not statistically significantly impact survival.
Table 1.
Univariable proportional hazards analysis of overall survival (N=168).
| Variable | HR (95% CI) | P value |
|---|---|---|
| Age (continuous variable) | 1.02 (1.01–1.04) | 0.005 |
|
| ||
| Time to development of distant metastatic disease | ||
|
| ||
| Stages 1, 2, and 3 | 1.01 (0.96–1.05) | 0.83 |
|
| ||
| Grade of primary tumor (vs. 3) | 0.91 | |
| 1 | 1.01 (0.40–2.53) | |
| 2 | 1.11 (0.70–1.75) | |
|
| ||
| Stage at initial diagnosis (vs. 1) | 0.039 | |
|
| ||
| 2 | 0.88 (0.51–1.52) | |
|
| ||
| 3 | 1.56 (0.81–2.98) | |
|
| ||
| 4 | 0.66 (0.36–1.20) | |
|
| ||
| Hormone receptor positive | 0.66 (0.45–0.97) | 0.032 |
|
| ||
| Any CNS metastasis | 1.31 (0.91–1.89) | 0.14 |
|
| ||
| Initial metastatic site (vs. bone only) | 0.027 | |
| CNS only | 1.67 (0.58–4.84) | |
| Visceral only | 1.00 (0.56–1.79) | |
| Multi-organ involvement | 1.78 (1.12–2.84) | |
CNS: central nervous system
Patients with prolonged survival
Twelve (7%) of the 168 patients in the cohort survived more than 10 years after diagnosis of MBC (Figure 1A; Table 2). Seven of the 12 patients had no evidence of disease at the time of last follow-up (Table 2). At the time of data analysis, 4 of the 10 patients who were still living had discontinued all anti-cancer therapy after a median of 6.2 years (range 0.4–12.0) of treatment and have remained without evidence of disease progression for a median of 7.4 years (range 0.2–12.0) of follow-up. All 4 had biopsy-proven distant metastases.
Table 2.
Characteristics of patients (pt) who survived at least 10 years (yr) following diagnosis of Her2+ metastatic breast cancer (MBC) (N=12).
| Pt | Age at initial diagnosis | Stage at initial diagnosis | HR status | Time to MBC (yr) | CNS mets | Initial metastatic sitê | Biopsy proven mets | Time to 2nd-line therapy (years) | OS* | Duration of anti-Her2 therapy for MBC (yr) | Anti-Her2 therapy | NED at last follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 61 | 36 | II | + | 3.6 | Yes | Multiple | Yes | 1.7 | >10.1 | continues | T, L, A, P | No |
| 97 | 48 | II | − | 2.9 | No | Visceral | Yes | NA | >10.1 | 9.9 | T | Yes |
| 47 | 40 | III | + | 2.5 | No | Multiple | Yes | 1.3 | 10.3 | 1.2 | T, L | No |
| 76 | 46 | II | + | 2.0 | Yes | Bone | No | 5.4 | 10.4 | intermittent | T, L | No |
| 220 | 41 | I | + | 2.3 | No | Multiple | Yes | 0.2 | >11.6 | continues | T, A, P | No |
| 83 | 34 | I | + | 5.2 | No | Multiple | No | 2.9 | >11.6 | continues | T | No |
| 187 | 55 | II | − | 2.2 | No | Bone | Yes | NA | >12.5 | 0.4 | T | Yes |
| 96 | 50 | 1 | + | 3.0 | No | Visceral | Yes | NA | >12.7 | continues | T | Yes |
| 176 | 42 | II | − | 1.9 | Yes | Multiple | Yes | 1.7 | >14.1 | >9.3 | T | Yes |
| 59 | 66 | IV | + | At diagnosis | No | Visceral | Yes | NA | >14.2 | 2.5 | T | Yes |
| 41 | 37 | II | + | 1.5 | No | Visceral | Yes | NA | >15.2 | 12.0 | T | Yes |
| 93 | 26 | IV | + | At diagnosis | No | Bone | No | 1.4 | >16.3 | continues | T | Yes |
Abbreviations: A: ado-trastuzumab emtansine; CNS: central nervous system; HR: hormone receptor; L: lapatinib; met: metastasis; NA: no progression to date; NED: no evidence of disease; OS: overall survival; P: pertuzumab; T: trastuzumab.
Survival for patients still alive at time of data cutoff is censored.
Patients with CNS metastases
Of the 66 patients diagnosed with CNS metastases, 9 (14%) survived at least 5 years following diagnosis of CNS metastasis (Table 3). There was a trend towards younger age and a greater likelihood of undergoing surgical resection of their CNS tumors in this group compared to the remainder of the CNS metastasis cohort.
Table 3.
Characteristics of patients (pt) diagnosed with Her2+ metastatic breast cancer (MBC) who survived at least 5 years (yr) following diagnosis of central nervous system (CNS) metastasis (N=9).
| Pt | Age at initial diagnosis | Stage | HR status | Time initial dx to MBC (yr) | Time MBC to CNS met (yr) | Biopsy-proven met | Radiation therapy | Surgical resection | Anti-Her2 therapy after CNS met | Overall survival since CNS met diagnosis (yrs)* |
|---|---|---|---|---|---|---|---|---|---|---|
| 76 | 46 | II | + | 2.0 | 5.4 | Yes | WBRT | No | Yes | 5.0 |
| 74 | 36 | II | + | 3.7 | At diagnosis | Yes | SRS | Yes | Yes | 5.4 |
| 189 | 37 | II | − | 2.2 | 1.3 | Yes | SRS, WBRT | No | Yes | 5.6 |
| 160 | 40 | I | + | 9.2 | 3.8 | Yes | WBRT | Yes | Yes | 6.0 |
| 108 | 52 | IV | + | At diagnosis | 2.1 | Yes | WBRT | Yes | Yes | >6.0 |
| 63 | 40 | IV | − | At diagnosis | 1.0 | Yes | WBRT | Yes | Yes | >7.9 |
| 61 | 36 | II | + | 3.6 | 1.7 | Yes | WBRT | Yes | Yes | >8.3 |
| 85 | 38 | I | + | 2.5 | At diagnosis | Yes | WBRT | Yes | Yes | >8.4 |
| 176 | 42 | II | − | 1.9 | 0.8 | Yes | WBRT, SRS | Yes | Yes | >13.3 |
Abbreviations: HR: hormone receptor; met: metastasis; SRS: stereotactic radiosurgery; WBRT: whole brain radiation therapy.
Survival for patients still alive at time of data cutoff is censored.
Overall, the development of CNS metastases at any time did not negatively impact survival (Table 1). However, for those patients who development CNS metastases within 1 year of diagnosis of MBC (N=27), the median OS was 2.8 years versus 5.2 years for women without CNS metastasis at one year (log rank test, P=0.0003) (Supplemental Figure S2). This time period was chosen because median time to CNS metastasis for those with metastasis to the brain was 1.1 years. For women with CNS metastasis within 1 year compared to those without CNS metastasis at one year, the hazard ratio for death adjusted for age at diagnosis, stage, and HER2-positive status was 2.4 (95% CI 1.4–4.2, P=0.002). The Kendall’s tau correlation between time to CNS metastasis and OS was 0.14 (P=0.005), which suggests a weak but statistically significant dependence. Of those who survived more than 10 years after diagnosis of MBC, 25% had CNS metastases, compared to 40% of those who survived less than 10 years.
Discussion
This report of 168 patients with HER2-positive MBC treated at a single institution demonstrated that OS was 33% at 5 years and 7% at 10 years. In particular, we describe in detail a cohort of 12 patients who have survived more than 10 years since the diagnosis of MBC, and a cohort of 9 patients who have survived more than 5 years since the diagnosis of CNS metastases.
In our cohort, 4 patients with MBC discontinued trastuzumab, and as of last follow-up had been off all cancer-directed therapy for a median of 7.4 years (range 0.2–12.0). Two patients discontinued treatment after 0.4 and 2.5 years because of possible allergic reactions, whereas the other 2 discontinued because they were without evidence of disease for a prolonged time (9.9 and 12 years). These observational data support the concept that it might be reasonable to safely discontinue anti-HER2 therapy for a subset of patients whose survival beyond a specific time point reflects cure of disease, in a manner similar to that for Hodgkin and non-Hodgkin lymphomas and metastatic testicular cancer. However, prolonged survival could simply occur in the setting of continued dependence of tumor growth on HER2 signaling, and therefore some patients may achieve long-term suppression of disease progression only through continued systemic anti-HER2 therapy.
There has been a recent recognition of the existence of “exceptional responders” in multiple tumor types, and the National Cancer Institute recently launched the NCI Exceptional Responders Pilot Trial specifically to investigate these patients (Clinicaltrials.gov NCT02243592). In the setting of HER2-positive MBC, an understanding of why some patients are able to achieve prolonged survival could yield multiple benefits, including providing information useful for decision-making regarding duration of anti-HER2 therapy or need for single versus dual anti-HER2 directed therapies.
Approximately one third of our cohort had CNS metastases, consistent with the previously reported higher incidence of CNS metastases in HER2-positive compared to HER2-negative MBC.[12, 13] In MBC, regardless of HER2 status, the development of CNS metastases has strongly portended a poor prognosis.[14, 15] In our study of HER2-positive MBC, the development of CNS metastases at any point during the course of the disease did not significantly negatively impact survival (HR 1.3, P=0.14). However, based on the landmark analysis, developing CNS metastasis within 1 year of diagnosis of MBC did negatively impact survival compared to not having developed a CNS metastasis within 1 year, with or without other metastatic disease. Our finding is similar to a previously reported study on CNS metastases in HER2-positive MBC, in which the median OS from time of initial metastatic diagnosis was shorter in patients whose CNS metastases were noted at initial diagnosis than in those whose CNS metastases developed subsequently.[16] This suggests that development of CNS metastasis in the setting of HER2-positive disease does not necessarily translate into a poor outcome, although early development of CNS metastases may herald shorter survival.
Our data must be placed in historical context. The majority of patients in this analysis were treated only with trastuzumab, with or without lapatinib. More recently, multiple anti-HER2 therapies, including other antibodies and antibody-drug conjugates have become available for routine clinical use.[17, 18] It remains to be seen whether the number of long-term survivors with HER2-positive MBC will be even greater. Nonetheless, as noted, our data strongly support that some patients may live for a decade or more without these newer agents (Table 2).
Our study has several limitations. It is a retrospective, single institution study, with limited power to detect prognostic factors with smaller effect sizes. The study encompassed a long period of time during which patients were treated, and the varying treatment patterns over time may confound the data. Finally, pathologic evaluation of HER2 evolved during the study period, as analytical methodology and definitions of Her2 positivity have changed.[19] We relied on clinical determination of HR and HER2 positivity at the time of initial diagnosis, and therefore some tumors considered to be HER2-positive at the time of diagnosis may not be considered so by contemporary standards. We were not able to perform central pathologic assessment of receptor status because of inability to locate many of the primary tissue specimens.
In summary, we have defined a distinct subset of patients with HER2-positive MBC, treated with trastuzumab-containing regimens in the metastatic setting, who survived far longer than expected, some more than 10 years. Additional research is needed to determine the impact of duration and choice of anti-Her2 therapy on outcomes in different patient subsets, and to identify molecular determinants that distinguish these “exceptional responders” from those who progressed and died rapidly. Ultimately, defining clinical and pathologic predictors of prolonged survival in HER2-positive MBC could guide treatment decision-making and may provide insights on mechanisms of drug resistance.
Supplementary Material
Acknowledgments
Funding Information:
This work was supported by the National Cancer Institute at the National Institute of Health (supplement to grant number 3-P30-CA04592 to MSW); the Fashion Footwear Charitable Foundation of New York/QVC Presents Shoes on Sale™ (DFH); and the Breast Cancer Research Foundation (DFH, SDM, MSW). The funding sources played no role in the content of the manuscript.
Footnotes
Conflicts of Interest
MSW reports consultant/advisory role with Verastem, MedImmune, Paganini Biopharma, Dompe Pharma, Cerulean Pharma, stock ownership in OncoMed, and research funding from MedImmune, Paganini Biopharma, and Dompe Pharma. All other authors have declared no conflicts of interest related to this manuscript.
References
- 1.Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, Peintinger F, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27(34):5700–5706. doi: 10.1200/JCO.2009.23.2025. doi: JCO.2009.23.2025 [pii] 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 3.Cobleigh MA, Vogel CL, Tripathy D, Robert NJ, Scholl S, Fehrenbacher L, Wolter JM, et al. Multinational study of the efficacy and safety of humanized anti-HER2 monoclonal antibody in women who have HER2-overexpressing metastatic breast cancer that has progressed after chemotherapy for metastatic disease. J Clin Oncol. 1999;17(9):2639–2648. doi: 10.1200/JCO.1999.17.9.2639. [DOI] [PubMed] [Google Scholar]
- 4.Dawood S, Broglio K, Buzdar AU, Hortobagyi GN, Giordano SH. Prognosis of women with metastatic breast cancer by HER2 status and trastuzumab treatment: an institutional-based review. J Clin Oncol. 2009;28(1):92–98. doi: 10.1200/JCO.2008.19.9844. doi: JCO.2008.19.9844 [pii] 10.1200/JCO.2008.19.9844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 6.Romond EH, Perez EA, Bryant J, Suman VJ, Geyer CE, Jr, Davidson NE, Tan-Chiu E, et al. Trastuzumab plus adjuvant chemotherapy for operable HER2-positive breast cancer. N Engl J Med. 2005;353(16):1673–1684. doi: 10.1056/NEJMoa052122. [DOI] [PubMed] [Google Scholar]
- 7.Slamon DJ, Leyland-Jones B, Shak S, Fuchs H, Paton V, Bajamonde A, Fleming T, et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N Engl J Med. 2001;344(11):783–792. doi: 10.1056/NEJM200103153441101. [DOI] [PubMed] [Google Scholar]
- 8.Olson EM, Najita JS, Sohl J, Arnaout A, Burstein HJ, Winer EP, Lin NU. Clinical outcomes and treatment practice patterns of patients with HER2-positive metastatic breast cancer in the post-trastuzumab era. Breast. 2013 doi: 10.1016/j.breast.2012.12.006. doi: S0960-9776(12)00250-0 [pii] 10.1016/j.breast.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yardley DA, Tripathy D, Brufsky AM, Rugo HS, Kaufman PA, Mayer M, Magidson J, et al. Long-term survivor characteristics in HER2-positive metastatic breast cancer from registHER. Br J Cancer. 2014;110(11):2756–2764. doi: 10.1038/bjc.2014.174. doi: 10.1038/bjc.2014.174 bjc2014174 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yeo B, Kotsori K, Mohammed K, Walsh G, Smith IE. Long-term outcome of HER2 positive metastatic breast cancer patients treated with first-line trastuzumab. Breast. 2015;24(6):751–757. doi: 10.1016/j.breast.2015.09.008. doi: 10.1016/j.breast.2015.09.008 S0960-9776(15)00209-X [pii] [DOI] [PubMed] [Google Scholar]
- 11.Halabi S, Vogelzang NJ, Ou SS, Owzar K, Archer L, Small EJ. Progression-free survival as a predictor of overall survival in men with castrate-resistant prostate cancer. J Clin Oncol. 2009;27(17):2766–2771. doi: 10.1200/JCO.2008.18.9159. doi: 10.1200/JCO.2008.18.9159 JCO.2008.18.9159 [pii] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Leyland-Jones B. Human epidermal growth factor receptor 2-positive breast cancer and central nervous system metastases. J Clin Oncol. 2009;27(31):5278–5286. doi: 10.1200/JCO.2008.19.8481. doi: JCO.2008.19.8481 [pii] 10.1200/JCO.2008.19.8481. [DOI] [PubMed] [Google Scholar]
- 13.Park IH, Kwon Y, Ro JY, Lee KS, Ro J. Concordant HER2 status between metastatic breast cancer cells in CSF and primary breast cancer tissue. Breast Cancer Res Treat. 2010;123(1):125–128. doi: 10.1007/s10549-009-0627-3. [DOI] [PubMed] [Google Scholar]
- 14.Hall WA, Djalilian HR, Nussbaum ES, Cho KH. Long-term survival with metastatic cancer to the brain. Med Oncol. 2000;17(4):279–286. doi: 10.1007/BF02782192. [DOI] [PubMed] [Google Scholar]
- 15.Pienkowski T, Zielinski CC. Trastuzumab treatment in patients with breast cancer and metastatic CNS disease. Ann Oncol. 2010;21(5):917–924. doi: 10.1093/annonc/mdp353. doi: 10.1093/annonc/mdp353 mdp353 [pii] [DOI] [PubMed] [Google Scholar]
- 16.Brufsky AM, Mayer M, Rugo HS, Kaufman PA, Tan-Chiu E, Tripathy D, Tudor IC, et al. Central nervous system metastases in patients with HER2-positive metastatic breast cancer: incidence, treatment, and survival in patients from registHER. Clin Cancer Res. 2011;17(14):4834–4843. doi: 10.1158/1078-0432.CCR-10-2962. doi: 10.1158/1078-0432.CCR-10-2962 17/14/4834 [pii] [DOI] [PubMed] [Google Scholar]
- 17.Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, et al. Pertuzumab plus trastuzumab plus docetaxel for metastatic breast cancer. N Engl J Med. 2012;366(2):109–119. doi: 10.1056/NEJMoa1113216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Swain SM, Baselga J, Kim SB, Ro J, Semiglazov V, Campone M, Ciruelos E, et al. Pertuzumab, trastuzumab, and docetaxel in HER2-positive metastatic breast cancer. N Engl J Med. 2015;372(8):724–734. doi: 10.1056/NEJMoa1413513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wolff AC, Hammond ME, Hicks DG, Dowsett M, McShane LM, Allison KH, Allred DC, et al. Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists clinical practice guideline update. J Clin Oncol. 2013;31(31):3997–4013. doi: 10.1200/JCO.2013.50.9984. doi: 10.1200/JCO.2013.50.9984 JCO.2013.50.9984 [pii] [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


