Abstract
Background
Cervical cancer is one of the leading causes of cancer-related death in females. Human papilloma virus (HPV) is the major risk factor of cervical cancer.
Objectives
The aim of the current study was to explore the frequency and role of 23 different HPVs in patients with cervical cancer.
Materials and Methods
Overall, 117 formalin-fix and paraffin-embedded (FFPE) tissues from cervical cancer patients with squamous cell carcinoma (SCC) or dysplasia were collected from Mirza-Kochakkhan-Jangali hospital, Tehran, Iran during year 2013, to investigate the presence of HPV- HPV- 67, 68, 6, 11, 13, 16, 17, 30, 69, 39, 40, 42, 64, 66 and 51 to 59 genotypes.
Results
The Pap smear report illustrated the presence of malignancy in 71 cases, while 11 cases had no evidence of malignancy. Among the patients, 26 cases had sexually transmitted disease with relative frequency of 0.58. Infection with papilloma virus was observed in 83.6% of SCC patients and 45% of the dysplasia group. The most prevalent HPV genotypes were 18 with 31.62% and 16 with 27.35% of cases. Moreover the relative frequencies of HPV-33, -6, -58, -52, -35 and -51, genotypes were 15.38, 7.69, 5.98, 5.12 and 3.41%, respectively. Among the different genotypes of HPV, 31 had the lowest and 16 had the highest relative frequency.
Conclusions
Our findings demonstrate that HPV-16 and -18 have a higher prevalence in our population than 31 and 51. Further investigations are required to evaluate the role of these genotypes in a larger multicenter setting for establishing their values for early detection of patients, which is useful for screening and vaccination programs of cancerous and precancerous lesions of cervical cancer.
Keywords: Papilloma Virus, Cervical Cancer, HPV-16, HPV-18, Polymerase Chain Reaction
1. Background
Human papilloma viruses (HPVs) are the most important risk factor of cervical cancers, which is the second most prevalent cancer in females (1). The prevalence of invasive cervix cancer is low in some areas and its low incidence could be attributed to Pap smear screening test and HPV DNA test (2). Human papilloma viruses belong to the large and diverse Papillomaviradea family with more than 200 different subtypes, although only 15 types (including 16, 18, 31, 33, 35, 39, 45, 51,52, 56, 58,59, 68, 73 and 82) have been suggested as oncogenes (3). Among these types, high-risk HPV strains such as HPV 16 and 18 cause about 70% of cervical cancers.
Several studies have shown that about 1.3% of 75000 women that died from cervix cancer had a negative Pap smear test (4). Thus identification of HPV and treatment of this infection could prevent precancerous lesions progressing toward cancer (5-7). In vaccination programs it should be documented that immunization against HPV 16 and 18 would be a valuable way to reduce the incidence of cervical cancer, supporting the application of HPV DNA testing (3).
Different methods have been used to detect HPV, such as reverse hybridization and direct sequencing, however application of specific polymerase chain reaction (PCR) seems to be a relatively simple, easy and economical method for the identification of HPV (8-13). On the other hand, there are a few studies on the prevalence of papilloma cases and abundance of high-risk types of HPV in squamous cell carcinoma (SCC) and dysplasia in Iran. However, cervical cancer is still a leading cause of cancer-related death for women in developing countries, including Iran, which is mainly because of the lack of HPV program and HPV DNA test as a part of regular screening.
2. Objectives
Therefore, the present study sought to determine the abundance of high-risk types of papilloma virus, i.e. subtype 16 and 18, in biopsy samples of cervical cancer patients.
3. Materials and Methods
3.1. Populations
Overall, 117 paraffin-embedded cervical tissue samples with dysplastic and cancerous lesions of cervix were collected from Mirza-Kochakkhan-Jangali hospital, Tehran. Tumors were stained for confirmation of cancer tissue and for DNA extraction using haematoxylin and eosin staining. Informed written consent was obtained from all participates, and the research protocol was approved by the ethics committee of Kashan University of Medical Sciences (No. 29/5/1/4222 on 6 Feb 2013).
3.2. DNA Extraction and Human Papilloma Virus Screening
DNA was extracted from all formalin-fix and paraffin-embedded (FFPE) tissue, as described previously (14) and stored at -20°C. Beta globulin primer was used as the control. Positive HPV samples were identified by MY09 and MY11 primers (Table 1) (15). Genome of HPV was determined using the Bio PAP kit (Biotools B & M Labs SA, Madrid, Spain). Presence of 450 bp band indicated that it is generic and its association with the 250 bp band makes it an oncogene (5, 6). Moreover, HPV genotyping was performed using the BioTYPAP kit (Biotools, B & M Labs, SA, Spain) for detection of HPV- 67, 68, 6, 11, 13, 16, 17, 30, 69, 39, 40, 42, 64, 66 and 51 to 59.
Table 1. Primer Sequences and Polymerase Chain Reaction Product Fragment Sizes for Human Papilloma Virus and Beta Globin.
| Target | Primer Sequences (5’ to 3’) | Primer Name | Nucleotides Number | Product Size, bp |
|---|---|---|---|---|
| HPV-L1 | 450 | |||
| F | GCMCAGGGWCATAAYAATGG | MY09 | 20 | |
| R | CGTCCMAARGGAWACTGATC | MY11 | 20 | |
| Beta globin | 468 | |||
| F | GAAGAGCCAAGGACAGGTAC | PC04 | 20 | |
| R | CAACTTCATCCACGTTCACC | GH20 | 20 |
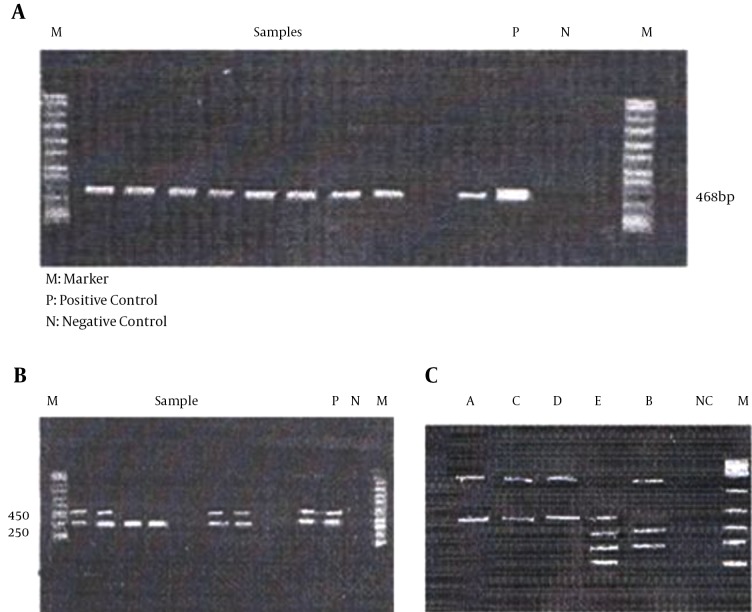
The reaction was done by the enzymatic digestion method (Table 2) and the tests were performed using the RFLP technique. The positive control was a plasmid DNA consisting of some HPV sequences in Tris-HCl-EDTA solution (5, 7). These results showed that 78 samples were positive and 39 samples didn’t have any virus (Figure 1). The GeneRuler™ 100 bp DNA ladder plus weight (Thermo Scientific, Waltham, MA, USA), was used as the standard for detection of fragments (in base pairs).
Table 2. Enzymatic Pattern of 450 bp and 250 bp Bands of Oncogenic Genotypes After Enzymatic Digestion.
| Genotype | Band Pattern, bp | ||||
|---|---|---|---|---|---|
| Digestion D | Digestion C | Digestion B | Digestion A | Digestion E | |
| HPV 18 ~ 450 bp fragment | 85, 72, 38, 135 and 125 | 183 and 269 | 455 | 455 | 455 |
| HPV 18 ~ 250 bp fragment (268 bp) | 172 and 96 | NC | 172 and 96 | NC | NC |
Figure 1. Molecular Tests Results.
A, Beta globin gene (band size: 468 bp); B, 450 bp and 250 bp were detected, suggesting oncogene or generic of the candidate gene; and C, polymerase chain reaction results from BioTAYPAP kit restriction analysis of the patient infected with HPV 16. Lane A, digestion of A pattern; lane B, digestion of B pattern; lane C, digestion of C pattern; lane E, digestion of E pattern; lane D, digestion of D pattern; lane NC, negative control; lane M, molecular marker.
4. Results
4.1. Clinical Characteristic of the Population
The risk factors and demographic characteristics associated with cervical cancer in patients are summarized in Table 3. The results of the Pap smear showed the presence of malignancy in 71 cases with relative frequency of 0.87; 11 cases had an absence of malignancy in their report. Among the patients, 26 cases had sexual transmitted diseases with relative frequency of 0.58 (Table 3).
Table 3. Risk Factors and Demographic Characteristics Associated With Cervical Cancer in Patients.
| Risk Factors | Number (Relative Frequency) |
|---|---|
| Age | |
| < 28 | 63 (0.67) |
| > 28 | 20 (0.24) |
| Total number | 83 |
| Smoking | |
| Yes | 15 (0.33) |
| No | 30 (0.67) |
| Total number | 45 |
| Sexual partner | |
| Single | 68 (0.78) |
| Multiple | 19 (0.22) |
| Total number | 87 |
| Parity | |
| Less than 3 | 16 (0.28) |
| Less than 3 | 41 (0.72) |
| Total number | 57 |
| History of sexual transmitted disease | |
| Positive | 26 (0.58) |
| Negative | 19 (0.42) |
| Total number | 45 |
| Contraceptive method | |
| OCP | 24 (0.32) |
| Non-OCP | 51 (0.68) |
| Total number | 75 |
| Pap smear report | |
| Absence of malignancy | 11 (0.13) |
| Presence of malignancy | 71 (0.87) |
| Total number | 82 |
| Co-infection | |
| Negative: infection with one type | 10 (0.21) |
| Positive: infection with two or more types | 37 (0.79) |
| Total number | 47 |
4.2. Screening of Patients With Human Papilloma Virus
In order to explore the frequency of high risk HPVs in cervical cancer patients, we determined the presence of more than 20 phenotypes of HPV in patients with squamous cell dysplasia or carcinoma. This analysis showed that 66.66% of samples had oncogene and non-oncogene types of this virus (Figure 1 C and Table 4), while no HPV was detected in 33.33% of cases. Moreover, 23.07% of cases had non-oncogene virus, 31.62% of the subjects had type 18. Moreover, 27.35% of cases had type 16, 15.38 had type 33, 4.27% had type 31, 7.69% had type 58, 5.98% had type 52 and 5.12% of cases had type 35 (Table 4).
Table 4. Human Papilloma Virus Typing of Patients With Dysplasia and Cancer of Cervix .
| HPV Types | No. (%) |
|---|---|
| 16 | 37 (37.62) |
| 18 | 32 (27.35) |
| 33 | 18 (15.38) |
| 6 and 11 genotypes and other oncogenes | 27 (23.07) |
| 58 | 9 (7.69) |
| 52 | 7 (5.98) |
| 35 | 6 (5.12) |
| 51 | 4 (3.41) |
| 31 | 5 (4.27) |
| HPV negative | 39 (33.3) |
| Total | 117 (100) |
5. Discussion
To the best of our knowledge this is the first study evaluating the association of 23 different HPVs (including HPV- 67, 68, 6, 11, 13, 16, 17, 30, 69, 39, 40, 42, 64, 66 and 51 to 59) in Iranian patients with cervical cancer. Our results showed that more than 80% of SCC patients and 45% of the dysplasia group had an infection with HPV. Amongst the different genotypes, the relative frequencies of HPV of 16 and 31 were ranked as the highest and lowest, supporting the HPV DNA test for screening and vaccination programs of cancerous and precancerous lesions of cervical cancer.
Cervix cancer in many countries is considered as an important health issue (16). There is a growing body of evidence showing the impact of particular types of HPV in pathogenesis of cervix dysplasia (17). Human papilloma virus infection has been known as a risk factor of cervical cancer (16), although there are a few data about the frequency of specific types and their correlation with dysplasia in Iran. Therefore, in the present study we investigated the frequency of infection with HPV types in individuals diagnosed with dysplasia and cancer of the cervix. Our data showed that more than 63% of patients with squamous-cell carcinoma were under the age of 18 years old. Moreover, 72% of patients had more than three types of HPV. Human papilloma virus 18, 32, 26 and 33 were found in 37, 32 and 18 cases, respectively. Among the types, the frequency of HPV 16 was high, compared to the other types. In agreement with our observation, Farjadian et al. (18) showed that more than 87% of samples were positive for HPV-16. Several other studies have also shown that 94% of cervix cancers and 88% of dysplasia were positive for HPV16 (19).
Cytological limitations in prediction of cervix cancers have led to the development of more sensitive methods for HPV detection such as PCR (20). In addition, considering the problems of general tests, e.g. Pap smear, for detecting wide spectrum of HPV in lower limited detection (19) and also carcinogenesis of dangerous types of virus, differentiation of oncogene types of virus seems to be necessary (9). Our results reinforce the rationale for HPV testing in combination with, or even instead of, cytology in a population-based screening program (21, 22). A major strength of the study was that it was performed on a large number of HPV types at different stages of the disease; however the main limitation of this study was the modest sample size.
In conclusion, we demonstrated the significant association of high risk HPVs in cervical cancer patients and showed that patients carrying HPV 16/18 had the highest relative risk, compared to 51, 31 and 33 genotypes, supporting further studies evaluating the role of these types in a larger multi center setting for early detection of high risk types, by using molecular methods at earlier stages. Evaluation of the results showed that quality of obtained products of extracted DNA from paraffin block cuts was sufficient for the PCR test. Considering the efficacy of this protocol, we can routinely perform this test in pathology labs using simple and cheap facilities. In cervix cancer, due to the availability of the tissue, the effects of prevention, early diagnosis and immediate treatment on reduction of mortality rate is evident more than any other cancer. This test, in addition to be used for vaccination after HPV determination can also help with the screening of the population with subclinical infection of the virus.
We concluded that the use of a sensitive PCR method in the detection of cervix cancer and determination of genome presence is helpful, plus with this technique we can detect different types of virus in patients and even apparently healthy individuals and this system can also make a major contribution to epidemiology studies of HPV.
Acknowledgments
We would like to thank the manager and personnel of the Mirza-Kochakkhan-Jangali hospital for their technical support and sample collection.
Footnotes
Authors’ Contribution:Study concept and design: Mohammad Niakan; data acquisition: Seyed Mostafa Mostafavi Zadeh, Zahra Eftekhar, Amir Avan and Mostafa Manian; data analysis and interpretation: Ahmad Piroozmand and Mohammad Niakan; drafting of the manuscript: Reza Nedaeinia and Azita Madani; critical revision of the manuscript for im-portant intellectual content: Amir Avan, Mohammad Moradi and Mohammad Niakan; statistical analysis: Mohammad Niakan; administrative, technical, and material support: Mohammad Niakan, Ahmad Piroozmand, Reza Soleimani and Reza Nedaeinia; and study supervision: Ahmad Piroozmand.
Funding/Support:This study was supported by the microbiology department of Shahed University of Medical Sciences, Tehran, IR Iran.
References
- 1.Lie AK, Skjeldestad FE, Hagen B, Johannessen E, Skarsvag S, Haugen OA. Comparison of light microscopy, in situ hybridization and polymerase chain reaction for detection of human papillomavirus in histological tissue of cervical intraepithelial neoplasia. APMIS. 1997;105(2):115–20. doi: 10.1111/j.1699-0463.1997.tb00549.x. [DOI] [PubMed] [Google Scholar]
- 2.Berner A, Franzen S, Holm R. HPV 16 infection in a patient with two primary squamous cell carcinomas: of the uterine cervix and the anal mucosa. APMIS. 1997;105(3):207–12. doi: 10.1111/j.1699-0463.1997.tb00560.x. [DOI] [PubMed] [Google Scholar]
- 3.Esmaeili M, Bonyadi M, Dastranj A, Alizadeh M, Melli MS, Shobeiri MJ. HPV typing in women with cervical precancerous and cancerous lesions in northwestern Iran. Gynecol Obstet Invest. 2008;66(1):68–72. doi: 10.1159/000134917. [DOI] [PubMed] [Google Scholar]
- 4.Kojic EM, Cu-Uvin S, Conley L, Bush T, Onyekwuluje J, Swan DC, et al. Human papillomavirus infection and cytologic abnormalities of the anus and cervix among HIV-infected women in the study to understand the natural history of HIV/AIDS in the era of effective therapy (the SUN study). Sex Transm Dis. 2011;38(4):253–9. doi: 10.1097/OLQ.0b013e3181f70253. [DOI] [PubMed] [Google Scholar]
- 5.Bosch FX, Lorincz A, Munoz N, Meijer CJ, Shah KV. The causal relation between human papillomavirus and cervical cancer. J Clin Pathol. 2002;55(4):244–65. doi: 10.1136/jcp.55.4.244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sasieni P, Cuzick J. Could HPV testing become the sole primary cervical screening test? J Med Screen. 2002;9(2):49–51. doi: 10.1136/jms.9.2.49. [DOI] [PubMed] [Google Scholar]
- 7.Snijders PJ, van den Brule AJ, Meijer CJ. The clinical relevance of human papillomavirus testing: relationship between analytical and clinical sensitivity. J Pathol. 2003;201(1):1–6. doi: 10.1002/path.1433. [DOI] [PubMed] [Google Scholar]
- 8.Baay MF, Quint WG, Koudstaal J, Hollema H, Duk JM, Burger MP, et al. Comprehensive study of several general and type-specific primer pairs for detection of human papillomavirus DNA by PCR in paraffin-embedded cervical carcinomas. J Clin Microbiol. 1996;34(3):745–7. doi: 10.1128/jcm.34.3.745-747.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Karlsen F, Kalantari M, Jenkins A, Pettersen E, Kristensen G, Holm R, et al. Use of multiple PCR primer sets for optimal detection of human papillomavirus. J Clin Microbiol. 1996;34(9):2095–100. doi: 10.1128/jcm.34.9.2095-2100.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Molijn A, Kleter B, Quint W, van Doorn LJ. Molecular diagnosis of human papillomavirus (HPV) infections. J Clin Virol. 2005;32 Suppl 1:S43–51. doi: 10.1016/j.jcv.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 11.Nobbenhuis MA, Meijer CJ, van den Brule AJ, Rozendaal L, Voorhorst FJ, Risse EK, et al. Addition of high-risk HPV testing improves the current guidelines on follow-up after treatment for cervical intraepithelial neoplasia. Br J Cancer. 2001;84(6):796–801. doi: 10.1054/bjoc.2000.1689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sotlar K, Diemer D, Dethleffs A, Hack Y, Stubner A, Vollmer N, et al. Detection and typing of human papillomavirus by e6 nested multiplex PCR. J Clin Microbiol. 2004;42(7):3176–84. doi: 10.1128/JCM.42.7.3176-3184.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Walboomers JM, Jacobs MV, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189(1):12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 14.Cao W, Hashibe M, Rao JY, Morgenstern H, Zhang ZF. Comparison of methods for DNA extraction from paraffin-embedded tissues and buccal cells. Cancer Detect Prev. 2003;27(5):397–404. doi: 10.1016/s0361-090x(03)00103-x. [DOI] [PubMed] [Google Scholar]
- 15.Qu W, Jiang G, Cruz Y, Chang CJ, Ho GY, Klein RS, et al. PCR detection of human papillomavirus: comparison between MY09/MY11 and GP5+/GP6+ primer systems. J Clin Microbiol. 1997;35(6):1304–10. doi: 10.1128/jcm.35.6.1304-1310.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Melo A, Montenegro S, Hooper T, Capurro I, Roa JC, Roa I. [Human papilloma virus (HPV) typing in preneoplastic and neoplastic lesions of the uterine cervix in the IX region-Chile]. Rev Med Chil. 2003;131(12):1382–90. [PubMed] [Google Scholar]
- 17.Gonzalez-Losa Mdel R, Rosado-Lopez I, Valdez-Gonzalez N, Puerto-Solis M. High prevalence of human papillomavirus type 58 in Mexican colposcopy patients. J Clin Virol. 2004;29(3):202–5. doi: 10.1016/S1386-6532(03)00138-0. [DOI] [PubMed] [Google Scholar]
- 18.Farjadian S, Asadi E, Doroudchi M, Dehaghani AS, Tabei SZ, Kumar VP, et al. High risk HPV types in southern Iranian patients with cervical cancer. Pathol Oncol Res. 2003;9(2):121–5. doi: 10.1007/BF03033756. [DOI] [PubMed] [Google Scholar]
- 19.Kay P, Soeters R, Nevin J, Denny L, Dehaeck CM, Williamson AL. High prevalence of HPV 16 in South African women with cancer of the cervix and cervical intraepithelial neoplasia. J Med Virol. 2003;71(2):265–73. doi: 10.1002/jmv.10479. [DOI] [PubMed] [Google Scholar]
- 20.Mobius G. Cytological early detection of cervical carcinoma: possibilities and limitations. Analysis of failures. J Cancer Res Clin Oncol. 1993;119(9):513–21. doi: 10.1007/BF01686460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Giles M, Garland S. Human papillomavirus infection: an old disease, a new vaccine. Aust N Z J Obstet Gynaecol. 2006;46(3):180–5. doi: 10.1111/j.1479-828X.2006.00580.x. [DOI] [PubMed] [Google Scholar]
- 22.McGlennen RC. Human papillomavirus oncogenesis. Clin Lab Med. 2000;20(2):383–406. [PubMed] [Google Scholar]