Abstract
Background
Feline hypersomatotropism (HST) is a cause of diabetes mellitus in cats. Pasireotide is a novel multireceptor ligand somatostatin analog that improves biochemical control of humans with HST.
Hypothesis/Objectives
Pasireotide improves biochemical control of HST and diabetes mellitus in cats.
Animals
Hypersomatotropism was diagnosed in diabetic cats with serum insulin‐like growth factor‐1 (IGF‐1) concentration >1,000 ng/mL by radioimmunoassay and pituitary enlargement.
Methods
Insulin‐like growth factor 1 was measured and glycemic control assessed using a 12‐hour blood glucose curve on days 1 and 5. On days 2, 3, and 4, cats received 0.03 mg/kg pasireotide SC q12h. IGF‐1, insulin dose, and estimated insulin sensitivity (product of the area under the blood glucose curve [BGC] and insulin dose) were compared pre‐ and post treatment. Paired t‐tests or Wilcoxon signed rank tests were employed for comparison where appropriate; a linear mixed model was created to compare BGC results.
Results
Insulin‐like growth factor 1 decreased in all 12 cats that completed the study (median [range] day 1: 2,000 ng/mL [1,051–2,000] and day 5: 1,105 ng/mL [380–1,727], P = .002, Wilcoxon signed rank test). Insulin dose was lower on day 5 than on day 1 (mean reduction 1.3 [0–2.7] units/kg/injection, P = .003, paired t‐test). The product of insulin dose and area under the BGC was lower on day 5 than day 1 (difference of means: 1,912; SD, 1523; u × mg/dL × hours, P = .001; paired t‐test). No clinically relevant adverse effects were encountered.
Conclusions
Short‐acting pasireotide rapidly decreased IGF‐1 in cats with HST and insulin‐dependent diabetes. The decrease in IGF‐1 was associated with increased insulin sensitivity.
Keywords: Diabetes mellitus, Growth hormone, Inhibition, Pituitary
Abbreviations
- BGC
blood glucose curve
- GH
growth hormone
- HST
hypersomatotropism
- IGF‐1
insulin‐like growth factor 1
- LA
long acting
- RVC
Royal Veterinary College
- SD
standard deviation
- SSTR
somatostatin receptor
- QMHA
Queen Mother Hospital for Animals
Spontaneously occurring feline hypersomatotropism (HST) is caused by a functional growth hormone (GH)‐secreting pituitary somatotrophinoma. Prevalence studies estimate 1 in 3 to 1 in 5 diabetic cats have HST‐induced diabetes mellitus.1 , 1 , 2 The hypersomatropic state induces insulin resistance, arthropathy, general abdominal organomegaly, and cardiovascular disease.2, 3, 4 These conditions impair the health of affected cats. Diabetic cats affected by HST often are difficult to control with exogenous insulin alone, and glycemic control will not manage all aspects of the disease.5
Several different treatment options are available for humans with HST including hypophysectomy, radiotherapy, and medical treatment. Medical treatment of excessive GH secretion consists of use of dopamine agonists, growth hormone receptor antagonists, and somatostatin analogs, with the latter being the drug of choice in most patients.6 Five somatostatin receptors subtypes (SSTR 1 to 5) have been reported in humans, and all subtypes are present in human somatotrophinomas but expression levels vary.7 Members of the somatostatin analog family bind with differing affinity to these receptor subtypes, and binding affinity variability could explain the different growth hormone lowering efficacy of each analog.8
No previous report has documented successful clinical management of HST in cats by medical inhibition of pituitary GH secretion.3 Use of the dopamine agonist, L‐selegiline, which is a selective irreversible monoamine oxidase‐B inhibitor, did not result in clinical improvement.9 Intravenously administered octreotide decreases serum GH concentration in a minority of affected cats, but clinical effects were not reported.10 These results raise questions, such as: do somatotrophinomas in cats express somatostatin receptors consistently or is the configuration of their SSTRs different from those found in somatotrophinomas of humans?
A recent advancement in the medical treatment of HST in humans is the use of a novel somatostatin analog, pasireotide.3 Pasireotide has 30‐, 5‐, and 39‐fold higher binding affinity for SSTR1, 3, and 5, respectively, compared with octreotide.11, 12, 13 Pasireotide decreases GH release in some octreotide‐resistant somatotrophinomas in humans and resulted in better GH control in a clinical trial in humans as compared with octreotide.14
We hypothesized that somatotrophinomas in cats would be amenable to medical inhibition when treated with a somatostatin analog that has high binding affinity at several SSTRs, thereby also providing indirect evidence of STTR expression by these tumors in cats. We specifically aimed to assess if sc administration of a short‐acting pasireotide preparation q12h would improve biochemical control of HST in cats and improve control of their diabetes mellitus. Our secondary goal was to document the tolerability of the short‐acting pasireotide preparation in cats with HST.
Materials and Methods
Animals
The clinical trial was approved by the Ethics and Welfare Committee at the RVC (Royal Veterinary College ethical approval reference number: URN 2011 1120). Cats were prospectively enrolled from December 2011 to December 2013. Enrolled cats had a diagnosis of HST based on a history of diabetes mellitus, serum IGF‐1 concentration > 1000 ng/mL and pituitary enlargement diagnosed using computed tomography. Written informed consent was obtained from owners of enrolled cats. All cats had a CBC, serum biochemistry, urinalysis, and abdominal ultrasound examination performed before enrollment. Patients were excluded if they had a disease that was considered to be more critical to the cat's welfare than the consequences of HST.
Serum IGF‐1 concentration was measured at a commercial laboratory4 using a radioimmunoassay validated for cats.15 The upper limit of IGF‐1 concentration quantitation was 2,000 ng/mL, and any result above this value was recorded as 2,000 ng/mL. Pituitary contrast‐enhanced computed tomography was performed as previously described and a pituitary dorsoventral height, measured by a board‐certified radiologist, > 4 mm was considered to be enlargement.16
Treatment Protocol
All treatments were carried out at the Queen Mother Hospital for Animals (QMHA), RVC. Enrolled cats were hospitalized for at least 5 consecutive days. All cats were fed the same food as normally provided by their owners at home. All cats had a period of at least 12 hours of acclimatization to their hospital environment before beginning the study. On day 1, all cats received the same insulin regimen as prescribed before enrollment in the study. A 12‐hours blood glucose curve (BGC) was performed using a glucometer validated for use in cats5 every 2 hours from the time of morning insulin administration to the time of evening insulin administration. Blood was collected for measurement of pretreatment serum IGF‐1 concentration. On days 2, 3, and 4, cats received 0.03 mg/kg of a short‐acting pasireotide preparation SC q12h (the dosage was extrapolated from a dosage proven to be effective and safe in human patients).17 Glycemic control monitoring and insulin dosage on days 2, 3, and 4 were determined by the attending clinician to adjust the insulin dose to the individual cat's need, but the insulin type was not changed. At the minimum, blood glucose concentration was measured each day at the time of insulin injection. Cats did not receive any insulin if the blood glucose concentration at time of injection was < 126 mg/dL. If the blood glucose concentration was > 126 mg/dL, a sliding scale was used to guide the clinician in his or her choice of insulin dose. If the blood glucose concentration was > 250 mg/dL, the original insulin dose given at time of enrollment was used. Incremental decreases in insulin dose were made if blood glucose concentration was between 180 and 250 mg/dL or between 126 and 180 mg/dL. The magnitude of the decrease was left to the discretion of the attending clinician, given the known interindividual variation in insulin sensitivity and exogenous insulin response in diabetic animals. However, clinicians were clearly instructed to avoid hypoglycemia at all times and to decrease the most recently administered insulin dose by 50% should hypoglycemia be identified. On day 5, cats had a second 12‐hour BGC performed and a blood sample was collected for a second measurement of serum IGF‐1 concentration.
Monitoring for adverse drug reactions consisted of several daily physical examinations, inspection of the pasireotide injection site during interactions not associated with physical examination by the nursing staff and clinicians such as blood glucose monitoring, ‘over‐the‐door’ monitoring, grooming and general husbandry during hospitalization. Written records of the amount of food eaten by each cat, urination and defecation behavior including fecal consistency, any unusual patient behavior and intermittent measurements of blood glucose concentration were recorded. The frequency of glycemic monitoring was increased if any cat had a blood glucose concentration < 145 mg/dL. A cat was fed additional quantities of its food and put under more frequent monitoring, if blood glucose concentration was < 72 mg/dL and the cat was asymptomatic for hypoglycemia.
Statistical Analysis
Data were analyzed using a spreadsheet and commercially available statistical software.6 , 7 A P value < 0.05 was considered significant. Data were analyzed for normal distribution visually using histograms and by performing Shapiro–Wilk tests. Nonparametric data are presented as median and range and parametric data as mean and standard deviation (SD). Wilcoxon signed rank tests were performed to compare paired nonparametric data, paired t‐tests for paired parametric data; a Pearson's correlation test was performed when comparing the correlation between the change in IGF‐1 concentration on day 1 and day 5 and pituitary height. A linear mixed model was created to compare BGC determined on days 1 and 5. The area under the BGC was multiplied by the exogenous insulin dose, as a surrogate measure of insulin sensitivity, and to compare the insulin sensitivity on days 1 and 5.
Results
Twenty two cats were excluded from enrollment; owners of 14 cats elected hypophysectomy, 7 cats were not included because owners declined to participate and 1 cat was not included, because it had a temperament unsuited for the required trial procedures. Thirteen cats were enrolled in the study.
Twelve cats completed the 5‐day study period. The cat that did not complete the study was lethargic before inclusion. This cat had experienced 2 episodes of tonic‐clonic seizure activity on day 4, experienced grade 3 International Renal Interest Society acute kidney injury (serum creatinine concentration on day 3 was 1.43 mg/dL and on day 5 was 2.53 mg/dL) and the owners elected for the cat to be euthanized on day 5. The cat subsequently underwent postmortem examination. Pituitary histopathology identified an acidophilic adenoma of the pars distalis, consistent with a somatotrophinoma, which had 5 mitotic figures per 10 high‐powered fields. The hypothalamus and thalamus overlying the pituitary mass were compressed and had focal extensive malacia.
The remaining 12 cats had significantly lower serum IGF‐1 concentrations on day 5 compared with day 1 (median [range] day 1: 2,000 ng/mL [1,051–2,000] and day 5: 1,105 ng/mL [380–1,727], P = .002, related samples Wilcoxon signed rank test); Fig 1A. The mean decrease in serum IGF‐1 concentration was 698 ng/mL (SD, 320) and the decrease in serum IGF‐1 concentration was significantly correlated with pituitary height (r = 0.69, P = .013, Pearson's correlation test). The mean insulin decrease between days 1 and 5 was 1.3 units/kg/injection, Fig 1B. Blood glucose curves performed on days 1 and 5 were not significantly different, having a mean blood glucose concentration on days 1 and 5 of 278 mg/dL (SD, 78 mg/dL) and 260 mg/dL (SD, 78 mg/dL), respectively. The products of the insulin dose and area under BGC for all 12 cats were significantly lower on day 5 than day 1 (difference 1,912; SD, 1,523, u × mg/dL × hours, P = .001, paired t‐test), consistent with increased insulin sensitivity (Fig 1C).
Figure 1.
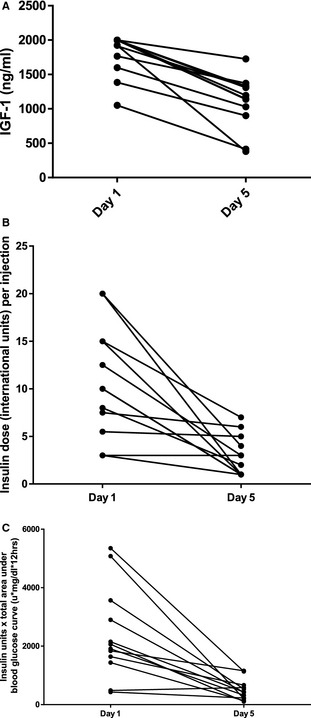
(A) Scatter plot representing the change in serum IGF‐1 concentrations of 12 diabetic acromegalic cats that completed a pasireotide drug trial receiving 0.03 mg/kg q12h of a short‐acting pasireotide compound SC on days 2, 3, and 4. (B) Scatter plot representing the change in insulin dose on days 1 and 5 of 12 diabetic acromegalic cats that completed a pasireotide drug trial receiving 0.03 mg/kg q12h of a short‐acting pasireotide compound SC on days 2, 3, and 4. (C) Scatter plot representing the change in the product of insulin dose per injection and total area under the 12‐hour blood glucose curve (BGC) of 12 diabetic acromegalic cats that completed a pasireotide drug trial receiving 0.03 mg/kg q12h of a short‐acting pasireotide compound SC on days 2, 3, and 4. (u × mg/dL × hours) = insulin units × area under BGC.
Three cats experienced small intestinal diarrhea, having voluminous soft stools that did not resolve during the study period. One of these 3 cats also experienced moderate abdominal enlargement suspected to be caused by gaseous small intestinal distention, which waxed and waned during the study period. Treatments were not administered to manage these clinical signs because appetite and demeanor were not affected. Five cats had nonsymptomatic hypoglycemia (blood glucose concentration < 72 mg/dL), which resolved after provision of additional food and prompted a decrease in insulin dosage at the time of next injection. No cat experienced symptomatic hypoglycemia.
Discussion
Our study is the first to document effective medical treatment for hypersomatotropism in cats. Pasireotide was well tolerated and decreased serum IGF‐1 concentrations in all cats. The decrease in IGF‐1 was likely > 698 ng/mL, because 7/12 cats that completed the study had a serum IGF‐1 concentrations > 2000 ng/mL at enrollment. In addition, insulin sensitivity improved in almost all cats, despite the short treatment time.
We chose to use IGF‐1 as a biomarker of GH rather than measure GH directly. This decision was made for several reasons. A validated radioimmunoassay for feline GH is described, but was unavailable at the time of the study.18 Serum GH concentrations can vary as a result of pulsatile release from the pituitary gland, and dynamic growth hormone tests have not been validated in the cat. The half‐life of GH in humans is 13.8 to 14.2 minutes, whereas IGF‐1 bound to IGF‐1 binding protein 3 has a half‐life of 15.7 to 21 hours.19 Insulin‐like growth factor 1 therefore represents an average GH concentration over a longer period of time. Finally, serum IGF‐1 concentration has been shown to be a useful marker of GH activity in the cat and in humans with acromegaly.18
Measuring serum IGF‐1 concentrations on days 1 and 5 was considered sufficient to identify a clinically relevant change in average blood GH concentration based on IGF‐1 half‐life in humans, despite a lack of studies to determine the half‐life of feline IGF‐1. A longer study period may have allowed more marked changes in IGF‐1 concentrations to be identified if IGF‐1 in cats has a longer half‐life than IGF‐1 in humans. We performed a serum biochemical profile at entry to the study, because liver and kidney disease can cause increased serum IGF‐1 concentrations in humans and if a similar effect occurs in cats, it could have contributed to a misdiagnosis of HST.20 None of the enrolled cats had evidence of impaired liver or kidney function on these blood tests. We closely monitored food intake during the study because short‐term fasting can decrease serum IGF‐1 concentration in cats.21 Fortunately, none of the cats in the study experienced noticeable decrease in caloric intake during hospitalization.
The area under the BGC was multiplied by the exogenous insulin dose given as a surrogate measure of insulin sensitivity and to compare insulin sensitivity on days 1 and 5. This calculated parameter was used based on the physiological principle that in a diabetic animal with a set insulin sensitivity, a decrease in exogenous insulin would lead to a worsening hyperglycemia (and vice versa). If such worsening hyperglycemia is not observed, the insulin sensitivity must have changed. Although this indicator has not been previously evaluated in the cat, it seems more appropriate than reporting only the exogenous insulin dose or area under the BGC because both are intrinsically related. In addition, the use of this surrogate marker was deemed more appropriate for this study, given that the more commonly accepted measures of insulin sensitivity (e.g., hyperinsulinemic clamp, minimal model analysis of the frequently sampled glucose tolerance test), would have been more invasive and likely would not have provided additional clinically useful information.22, 23
Somatrophinomas in humans predominantly express SSTR1, 2 and 5. These receptors have been pharmacological targets in the management of acromegaly in humans.24, 25 Pasireotide exhibits high affinity binding to SSTR1, 2, 3, and 5 unlike octreotide which preferentially binds to SSTR2.26 Previous attempts to use octreotide in cats to control GH release and improve glycemic control have been unrewarding. Our results suggest that this lack of effect may be because SSRT1 or SSRT5 activation is required to more effectively inhibit GH release in feline somatotrophinomas and to achieve a clinically relevant effect.
Eleven of the 12 cats that completed the trial were given a lower dose of insulin on day 5 than on day 1 in an attempt to avoid hypoglycemia. The lack of blinding may have been a source of bias and led to a greater inclination toward insulin dose reduction, despite the provided guidelines. There is also an inherent weakness of using BGCs as a diagnostic tool to assess diabetic control considering the day‐to‐day variability in results from these curves and altered level of activity of hospitalized cats compared with those in a home environment.27 Nevertheless, the area under these curves did not change significantly from days 1 to 5 despite decreases in the exogenous insulin dose, implying truly increased insulin sensitivity.
Stress hyperglycemia is a commonly recognized phenomenon in cats presented to veterinary practices.28, 29 Hospitalization could either have contributed to progressively increasing levels of stress because of repeated procedures such as blood sampling. Alternatively, hospitalization also could have allowed acclimatization and decreased patient stress. It is impossible to establish the exact influence of these factors in this study, but it seems unlikely that decreased insulin needs encountered in the treated cats were simply a reflection of decreased stress hyperglycemia or coincidence. Diabetic cats with HST have a type of diabetes that usually requires high dosages of insulin.1 In addition, all cats had diabetes mellitus‐related clinical signs (e.g., polyuria, polydipsia) before the start of the study. This finding suggests the insulin dose administered before enrollment actually was still insufficient to control their disease and the blood glucose concentrations recorded on day 1 were truly representative and not a result of stress hyperglycemia alone. Use of appropriate statistical methodology should further have decreased the impact of random interday variability in BGCs on the study's conclusions.
Most humans diagnosed with acromegaly are nondiabetic, and use of pasireotide induces mild hyperglycemia in up to 63% of these patients.14, 30 Pasireotide‐induced hyperglycemia in humans is not completely understood. It might be explained by a decrease in the hypoglycemic effect of IGF‐1, and decreased incretin and insulin release.31 In rats, the insulin/glucagon balance was identified as the most relevant mechanism of pasireotide‐induced hyperglycemia.32 The physiology in the cat seems to be different. The insulin resistance associated with excess GH secretion exerts a more marked and dominant effect on insulin sensitivity in cats compared with humans with HST. Therefore, decreasing GH secretion and serum IGF‐1 concentration improves insulin sensitivity in the cat.
In addition to inhibiting pituitary hormone secretion, somatostatin drugs have been shown to inhibit somatotroph growth and induce apoptosis.33 Pasireotide inhibits vascular endothelial growth factor and vascular endothelial proliferation in pituitary cell cultures within 48 hours of exposure, and is more potent than octreotide at inhibiting vascular proliferation and pituitary tumor growth in mice, rats, dogs, and humans.34, 35, 36, 37 Whether pasireotide could induce a decrease in pituitary tumor size in cats with HST remains to be determined, and long‐term studies are indicated to investigate this potential effect.
The cat that did not complete the trial showed progressive neurological deterioration and hypoglycemia after 2 seizure episodes. This cat had the second largest pituitary height in the study, and pituitary histopathology identified a proliferative tumor with 5 mitotic figures per 10 high‐powered fields. Pituitary neoplasms with a high proliferation rate are associated with poorer outcomes, and this cat had clinical signs of altered mentation before enrollment in the study.38 Postmortem examination was consistent with an aggressive pituitary neoplasm causing thalamic and hypothalamic compression. We believe the death of this cat is unlikely to be related to pasireotide treatment but this possibility cannot be excluded. Additional studies using pasireotide to treat large pituitary masses or those that have a high mitotic rate would be needed to determine the safety of pasireotide in this patient subset.
Clinical signs that might be compatible with drug‐induced adverse effects were mild gastrointestinal disturbances in 3/12 cats. These adverse effects were similar to those reported in octreotide and pasireotide trials in human patients with acromegaly.39, 40 Endogenous somatostatin inhibits gastric emptying, gastric acid production, and pancreatic, and biliary secretions. Administration of octreotide induces similar effects and pasireotide also is likely to alter gastrointestinal physiology.41 These effects would affect nutrient digestion and absorption, explaining the encountered small intestinal diarrhea and bloating. Studies performed over a longer period of time using the short‐acting pasireotide or a long‐acting pasireotide formulation are needed to determine the long term clinical tolerability of this drug in cats, whether or not pasireotide increases the risk of gastrointestinal upset, or if the observed signs were coincidental.
A positive correlation was identified between the decrease in serum IGF‐1 concentration and pituitary size. Tumor size and pretreatment serum IGF‐1 concentration were inversely correlated with likelihood of biochemical control in humans diagnosed with acromegaly and treated with somatostatins.42 Surgical debulking has been used to improve biochemical control when using somatostatin treatment, and has proven successful even in patients that were previously poorly responsive to somatostatin treatment.43 A clear explanation for this difference in biochemical behavior in cats is not available, and might lie in the fact that the behavior of somatotrophinomas in cats still needs to be fully elucidated. For example, different subsets of somatotrophinomas in humans (densely granulated versus sparsely granulated) respond differently to somatostatin analog treatment.44
The duration of time a cat had been receiving insulin treatment was not taken into account. A previous study indicating that diabetic cats treated with insulin long term have increased serum IGF‐1 concentrations compared with diabetic cats receiving insulin treatment over shorter periods of time. Theoretically, this could lead to a false increase in IGF‐1 concentration and therefore the false impression of the presence of HST.45 However all included cats underwent intracranial imaging and had convincing evidence of pituitary enlargement to avoid inclusion of cats without HST. The inclusion of cats with pituitary‐dependent hyperadrenocortism, which also could present with diabetes mellitus and have similar computed tomography findings are also unlikely because serum IGF‐1 concentrations are expected to be within normal limits or suppressed in such patients.46 Finally, it would have been preferential if IGF‐1 concentrations > 2,000 ng/mL also had been exactly quantified. Nevertheless, any negative consequences of this omission would only have resulted in a false impression that the drug was not effective in decreasing IGF‐1, which was not the case.
In conclusion, we identified consistently effective medical management of HST in these cats. The novel somatostatin analog pasireotide decreased circulating IGF‐1 and increased insulin sensitivity in confirmed acromegalic cats. These results also suggest that most somatotrophinomas in cats express somatostatin receptors. All owners of cats that completed this short‐acting pasireotide compound trial opted to enroll their cats in a long‐acting pasireotide compound trial, which was started immediately after completion of the short‐acting pasireotide trial. The results will be communicated separately on completion of the second trial.
Acknowledgments
We thank Kristine Jensen and Sophie Keyte for their work in the Feline Acromegalic Cat Clinic, Yu‐Mei Chang for her help with the statistical analysis of the data, the Clinical Investigation Centre team at the RVC, participating cat owners, their cats, and the veterinary surgeons who referred the cats to the RVC for this study.
Conflict of Interest Declaration: Dr H.A. Schmid is employed by Novartis Pharma AG, Basel who manufacture and market pasireotide for the treatment of hyperadrenocorticism and hypersomtotropism in humans.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Meeting, if any, at which the paper was presented: Presented in part as an abstract at the annual congress of the American College of Veterinary Internal Medicine in Seattle (2013).
Footnotes
Niessen SJM, Forcada Y, Jensen K, Glanemann B, Church DB. Routine Screening of Diabetic Cats for Acromegaly: Overdue or Overkill? Proceedings of 21st ECVIM‐CA Congress 2011 Sevilla, Spain. J Vet Int Med 2011;25:1470–1509
Schäfer S, Kooistra HS, Künzle A, et al. Evaluation of Insulin Like Growth Factor 1 (IGF‐1), Total Thyroxine (TT4), Feline Pancreatic Lipase Immunoreactivity (FPLI) and Urinary Corticoid Creatinine Ratio (UCCR) in Cats with Diabetes Mellitus in Switzerland and the Netherlands. Proceeding 23rd ECVIM‐CA Congress Liverpool, UK. J Vet Int Med 2014;28:711–744
Signifor, Novartis Pharma AG, Basel, Switzerland
Dechra Specialist Laboratories, now trading as Nationwide Specialist Laboratories, Cambridge, UK
AlphaTrak2, Abbott Laboratories, Chicago, IL
Excel 2010, Microsoft Corp, Redmond, WA
SPSS Statistics 21.0, SPSS Inc., Chicago, IL
References
- 1. Niessen SJM, Petrie G, Gaudiano F, et al. Feline acromegaly: An underdiagnosed endocrinopathy? J Vet Intern Med 2007;21:899–905. [DOI] [PubMed] [Google Scholar]
- 2. Meij BP, Auriemma E, Grinwis G, et al. Successful treatment of acromegaly in a diabetic cat with transsphenoidal hypophysectomy. J Feline Med Surg 2010;12:406–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Niessen S. Feline Acromegaly: An essential differential diagnosis for the difficult diabetic. J Feline Med Surg 2010;12:15–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Myers JA, Lunn KF, Bright JM. Echocardiographic Findings in 11 Cats with Acromegaly. J Vet Intern Med 2014;28:1235–1238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Niessen SJM, Church DB, Forcada Y. Hypersomatotropism, acromegaly, and hyperadrenocorticism and feline diabetes mellitus. Vet Clin North Am Small Anim Pract 2013;43:319–350. [DOI] [PubMed] [Google Scholar]
- 6. Katznelson L, Laws ER, Melmed S, et al. Acromegaly: An endocrine society clinical practice guideline. J Clin Endocrinol Metab 2014;99:3933–3951. [DOI] [PubMed] [Google Scholar]
- 7. Taboada GF, Luque RM, Bastos W, et al. Quantitative analysis of somatostatin receptor subtype (SSTR1‐5) gene expression levels in somatotropinomas and non‐functioning pituitary adenomas. Eur J Endocrinol 2007;156:65–74. [DOI] [PubMed] [Google Scholar]
- 8. Cuevas‐Ramos D, Fleseriu M. Somatostatin receptor ligands and resistance to treatment in pituitary adenomas. J Mol Endocrinol 2014;52:R223–R240. [DOI] [PubMed] [Google Scholar]
- 9. Abraham L, Helmond S, Mitten RW, et al. Treatment of an acromegalic cat with the dopamine agonist L‐deprenyl. Aust Vet J 2002;80:479–483. [DOI] [PubMed] [Google Scholar]
- 10. Slingerland LI, Voorhout G, Rijnberk A, Kooistra HS. Growth hormone excess and the effect of octreotide in cats with diabetes mellitus. Domest Anim Endocrinol 2008;35:352–361. [DOI] [PubMed] [Google Scholar]
- 11. Schmid HA, Schoeffter P. Functional activity of the multiligand analog SOM230 at human recombinant somatostatin receptor subtypes supports its usefulness in neuroendocrine tumors. Neuroendocrinology 2004;80(Suppl 1):47–50. [DOI] [PubMed] [Google Scholar]
- 12. Bruns C, Lewis I, Briner U, et al. SOM230: A novel somatostatin peptidomimetic with broad somatotropin release inhibiting factor (SRIF) receptor binding and a unique antisecretory profile. Eur J Endocrinol 2002;146:707–716. [DOI] [PubMed] [Google Scholar]
- 13. Hofland LJ, van der Hoek J, van Koetsveld PM, et al. The novel somatostatin analog SOM230 is a potent inhibitor of hormone release by growth hormone‐ and prolactin‐secreting pituitary adenomas in vitro. J Clin Endocrinol Metab 2004;89:1577–1585. [DOI] [PubMed] [Google Scholar]
- 14. Colao A, Bronstein MD, Freda P, et al. Pasireotide versus octreotide in acromegaly: A head‐to‐head superiority study. J Clin Endocrinol Metab 2014;99:791–799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Church DB, Watson AD, Emslie DR, et al. Effects of proligestone and megestrol on plasma adrenocorticotrophic hormone, insulin and insulin‐like growth factor‐1 concentrations in cats. Res Vet Sci 1994;56:175–178. [DOI] [PubMed] [Google Scholar]
- 16. Lamb CR, Ciasca TC, Mantis P, et al. Computed tomographic signs of acromegaly in 68 diabetic cats with hypersomatotropism. J Feline Med Surg 2014;16:99–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Golor G, Hu K, Ruffin M, et al. A first‐in‐man study to evaluate the safety, tolerability, and pharmacokinetics of pasireotide (SOM230), a multireceptor‐targeted somatostatin analog, in healthy volunteers. Drug Des Devel Ther 2012;6:71–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Niessen SJM, Khalid M, Petrie G, Church DB. Validation and application of a radioimmunoassay for ovine growth hormone in the diagnosis of acromegaly in cats. Vet Rec 2007;160:902–907. [DOI] [PubMed] [Google Scholar]
- 19. Sohmiya M, Kato Y. Renal clearance, metabolic clearance rate, and half‐life of human growth hormone in young and aged subjects. J Clin Endocrinol Metab 1992;75:1487–1490. [DOI] [PubMed] [Google Scholar]
- 20. Juul A. Serum levels of insulin‐like growth factor I and its binding proteins in health and disease. Growth Hormon IGF Res 2003;13:113–170. [DOI] [PubMed] [Google Scholar]
- 21. Maxwell A, Butterwick R, Batt RM, Camacho‐Hübner C. Serum insulin‐like growth factor (IGF)‐I concentrations are reduced by short‐term dietary restriction and restored by refeeding in domestic cats (Felis catus). J Nutr 1999;129:1879–1884. [DOI] [PubMed] [Google Scholar]
- 22. Petrus DJ, Jackson MW, Kemnitz JW, et al. Assessing insulin sensitivity in the cat: Evaluation of the hyperinsulinemic euglycemic clamp and the minimal model analysis. Res Vet Sci 1998;65:179–181. [DOI] [PubMed] [Google Scholar]
- 23. Appleton DJ, Rand JS, Priest J, Sunvold GD. Determination of reference values for glucose tolerance, insulin tolerance, and insulin sensitivity tests in clinically normal cats. Am J Vet Res 2001;62:630–636. [DOI] [PubMed] [Google Scholar]
- 24. Miller GM, Alexander JM, Bikkal HA, et al. Somatostatin receptor subtype gene expression in pituitary adenomas. J Clin Endocrinol Metab 1995;80:1386–1392. [DOI] [PubMed] [Google Scholar]
- 25. Polska E, Journal P, Pisarek H, et al. Expression of somatostatin receptor subtypes in human pituitary adenomas – immunohistochemical studies. Endokrynol Pol 2009;60:240–251. [PubMed] [Google Scholar]
- 26. Murray RD, Kim K, Ren S‐G, et al. The novel somatostatin ligand (SOM230) regulates human and rat anterior pituitary hormone secretion. J Clin Endocrinol Metab 2004;89:3027–3032. [DOI] [PubMed] [Google Scholar]
- 27. Alt N, Kley S, Haessig M, Reusch CE. Day‐to‐day variability of blood glucose concentration curves generated at home in cats with diabetes mellitus. J Am Vet Med Assoc 2007;230:1011–1017. [DOI] [PubMed] [Google Scholar]
- 28. Ray CC, Callahan‐Clark J, Beckel NF, Walters PC. The prevalence and significance of hyperglycemia in hospitalized cats. J Vet Emerg Crit Care 2009;19:347–351. [DOI] [PubMed] [Google Scholar]
- 29. Rand JS, Kinnaird E, Baglioni A, et al. Acute stress hyperglycemia in cats is associated with struggling and increased concentrations of lactate and norepinephrine. J Vet Intern Med 2002;16:123–132. [DOI] [PubMed] [Google Scholar]
- 30. Sheppard M, Bronstein MD, Freda P, et al. Pasireotide LAR maintains inhibition of GH and IGF‐1 in patients with acromegaly for up to 25 months: Results from the blinded extension phase of a randomized, double‐blind, multicenter, Phase III study. Pituitary 2014. doi: 10.1007/s11102‐014‐0585‐6 [e‐pub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Henry RR, Ciaraldi TP, Armstrong D, et al. Hyperglycemia associated with pasireotide: Results from a mechanistic study in healthy volunteers. J Clin Endocrinol Metab 2013;98:3446–3453. [DOI] [PubMed] [Google Scholar]
- 32. Schmid HA, Brueggen J. Effects of somatostatin analogs on glucose homeostasis in rats. J Endocrinol 2012;212:49–60. [DOI] [PubMed] [Google Scholar]
- 33. Zou YI, Xiao X, Li Y, Zhou T. Regulation of DNA damage response and cell cycle in radiation‐resistant HL60 myeloid leukemia cells. Oncol Rep 2009;28:55. [DOI] [PubMed] [Google Scholar]
- 34. Zatelli MC, Piccin D, Vignali C, et al. Pasireotide, a multiple somatostatin receptor subtypes ligand, reduces cell viability in non‐functioning pituitary adenomas by inhibiting vascular endothelial growth factor secretion. Endocr Relat Cancer 2007;14:91–102. [DOI] [PubMed] [Google Scholar]
- 35. Adams RL, Adams IP, Lindow SW, Atkin SL. Inhibition of endothelial proliferation by the somatostatin analogue SOM230. Clin Endocrinol 2004;61:431–436. [DOI] [PubMed] [Google Scholar]
- 36. Fedele M, De Martino I, Pivonello R, et al. SOM230, a new somatostatin analogue, is highly effective in the therapy of growth hormone/prolactin‐secreting pituitary adenomas. Clin Cancer Res 2007;13:2738–2744. [DOI] [PubMed] [Google Scholar]
- 37. Castillo V, Theodoropoulou M, Stalla J, et al. Effect of SOM230 (pasireotide) on corticotropic cells: Action in dogs with Cushing's disease. Neuroendocrinology 2011;94:124–136. [DOI] [PubMed] [Google Scholar]
- 38. Fernandez‐Rodriguez E, Casanueva FF, Bernabeu I. Update on prognostic factors in acromegaly: Is a risk score possible? Pituitary 2014. doi: 10.1007/s11102‐014‐0574‐9 [e‐pub ahead of print]. [DOI] [PubMed] [Google Scholar]
- 39. Kvols LK, Oberg KE, O'Dorisio TM, et al. Pasireotide (SOM230) shows efficacy and tolerability in the treatment of patients with advanced neuroendocrine tumors refractory or resistant to octreotide LAR: Results from a phase II study. Endocr Relat Cancer 2012;19:657–666. [DOI] [PubMed] [Google Scholar]
- 40. Lamberts SWJ, van der Lely AJ, de Herder WW, Hofland LJ. Octreotide. N Engl J Med 1996;25:246–254. [DOI] [PubMed] [Google Scholar]
- 41. Stolk MF, van Erpecum KJ, Koppeschaar HP, et al. Effect of octreotide on fasting gall bladder emptying, antroduodenal motility, and motilin release in acromegaly. Gut 1995;36:755–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bhayana S, Booth GL, Asa SL, et al. The implication of somatotroph adenoma phenotype to somatostatin analog responsiveness in acromegaly. J Clin Endocrinol Metab 2005;90:6290–6295. [DOI] [PubMed] [Google Scholar]
- 43. Colao A, Attanasio R, Pivonello R, et al. Partial surgical removal of growth hormone‐secreting pituitary tumors enhances the response to somatostatin analogs in acromegaly. J Clin Endocrinol Metab 2006;91:85–92. [DOI] [PubMed] [Google Scholar]
- 44. Fougner SL, Casar‐Borota O, Heck A, et al. Adenoma granulation pattern correlates with clinical variables and effect of somatostatin analogue treatment in a large series of patients with acromegaly. Clin Endocrinol 2012;76:96–102. [DOI] [PubMed] [Google Scholar]
- 45. Starkey SR, Tan K, Church DB. Investigation of serum IGF‐I levels amongst diabetic and non‐diabetic cats. J Feline Med Surg 2004;6:149–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Wajchenberg BL, Liberman B, Neto G, et al. Growth Hormone Axis in Cushing's Syndrome. Horm Res 1996;45:99–107. [DOI] [PubMed] [Google Scholar]


