Abstract
Background
Exclusive feeding of an iodine‐restricted diet has been proposed as a method for controlling clinical manifestations of hyperthyroidism in hyperthyroid cats.
Objectives
To determine the effect of feeding an iodine‐restricted diet on TT4 concentrations and clinical signs in cats with spontaneous hyperthyroidism.
Animals
Forty‐nine client‐owned cats with spontaneous hyperthyroidism.
Methods
Retrospective case series. Hyperthyroid cats were exclusively fed a commercially available iodine‐restricted diet. Clinical response was assessed by change in weight and heart rate and serum TT4, blood urea nitrogen (BUN), and creatinine concentrations at various times during dietary management (21–60 days, 60–180 days).
Results
Serum TT4 normalized in 20/48 cats (42%) and 39/47 cats (83%) at 21–60 days and 61–180 days, respectively. Cats in which the TT4 concentrations were still above reference range at 21–60 days had a significantly higher starting TT4 than those that normalized their TT4 levels during the same time period (P = .038). Body weight did not significantly increase (P = .34) nor heart rate decrease (P = .64) during the study. There was a significant decrease in serum creatinine (P = .028). Cats in the low reference range for serum TT4 concentrations did not have a significant increase in body weight (P = .41) nor creatinine (P = .54) when compared to those with high reference range.
Conclusions and Clinical Importance
Restricted‐iodine diets were effective at maintaining serum TT4 concentrations within reference ranges for a majority of cats with spontaneous hyperthyroidism over 1 year, although not all clinical signs of hyperthyroidism improved.
Keywords: Endocrine, Feline, Thyroid gland, Thyroid hormone
Abbreviations
- AAFCO
Association of American Feed Control Officials
- BCS
body condition score
- CEIA
chemiluminescent enzyme immunoassay
- EIA
homogenous enzyme immunoassay
- FT4
free thyroxine
- GFR
glomerular filtration rate
- NRC
National Research Council
- PUVTH
Purdue University Veterinary Teaching Hospital
- T3
triiodothyronine
- T4
thyroxine
- TPO
thyroid peroxidase
- TSH
thyroid‐stimulating hormone
- TT4
total thyroxine
- VCA‐WLA
VCA West Los Angeles Referral Center
Hyperthyroidism is a common endocrinopathy of geriatric cats that leads to clinical signs such as weight loss with polyphagia, vomiting, diarrhea, nervousness, unkempt hair coat, and tachycardia.1, 2, 3 Long‐term treatment options for hyperthyroidism in cats include medical management with methimazole, treatment with radioactive iodine (I131), or thyroidectomy.2, 4 Various factors such as client and pet compliance, adverse effects of treatment, financial burden, duration of hospitalization, and availability of treatment options dictate treatment choice. A novel therapeutic approach for management of hyperthyroidism in the past several years has been the use of an iodine‐restricted diet.
Decreasing dietary iodine intake to 0.17 ppm (μg/kg) decreases serum total thyroxine (TT4) concentrations in cats with spontaneous hyperthyroidism.1 Restricting iodine content of the food to 0.28 ppm or less normalizes serum TT4 concentrations in a majority of hyperthyroid cats for up to 17 weeks.1 There are a limited number of studies assessing efficacy of an iodine‐restricted diet for treating spontaneous hyperthyroidism in cats.5
The aim of this study was to assess the efficacy of a commercial iodine‐restricted diet to control the manifestations of hyperthyroidism in cats as measured by changes in thyroid hormone concentrations and objective measures of clinical improvement, as well as to determine the risk of unmasking subclinical renal disease. We hypothesized that the restricted‐iodine diet would be effective in lowering thyroid hormone into reference range by 8 weeks and that once normalized, subsequently body weight would increase, and heart rate would decrease during management. It was also hypothesized that BUN and creatinine concentrations would increase as the glomerular filtration rate (GFR) decreased once the euthyroid state was re‐established.
Materials and Methods
Study Design
Retrospective review of medical records of cats with spontaneous hyperthyroidism that had been fed a commercial iodine‐restricted diet for sole management of thyroid dysfunction.
Cat Selection
The cats that were included in this study were referred to Purdue University Teaching Hospital (PUVTH) between June 2011 to May 2013, and VCA West Los Angeles (VCA‐WLA) from June 2011 to July 2013. Seventeen and thirty‐two cats were included from PUVTH and VCA‐WLA, respectively. Inclusion criteria were cats with a plasma serum TT4 concentrations >4.6 μg/dL that had clinical signs of hyperthyroidism (polyphagia, weight loss, polyuria/polydipsia) and had been started on an iodine‐restricted diet for management. Cats previously treated with methimazole were included in the study provided the medication had been discontinued, and subsequent TT4 concentrations were documented to be above the reference range, before starting the diet. Cats with evidence of azotemia (elevated creatinine, increased BUN, or both) were also included in the study. Cats were excluded if there was no baseline body weight, BUN/creatinine, or heart rate collected before starting the diet. The clinical records of each cat were reviewed from the time of the initial visit when the patients were started on the diet until dietary treatment was discontinued. The signalment, medical history, clinical signs, physical examination findings, and laboratory results were recorded. Variables that were evaluated to provide evidence for an objective clinical response to the therapeutic diet included: serum TT4 concentration, body weight, heart rate, BUN, and creatinine. Reference ranges for BUN were 14–36 mg/dL and 15–25 mg/dL for VCA‐WLA and PUVTH, respectively. Reference ranges for creatinine were 0.6–2.4 mg/dL and 0.9–2.3 mg/dL for VCA‐WLA and PUVTH, respectively.
Restricted‐Iodine Diet
The restricted‐iodine diet2 used in this study was a commercially available nutritionally complete diet formulated for management of cats with hyperthyroidism. Iodine content of both the canned and dry form of the diet is 0.2 ppm (μg/kg). Association of American Feed Control Officials (AAFCO) guidelines in 2008 and National Research Council (NRC) guidelines in 2006 have minimum recommendations for iodine content of commercial diets of 0.35 ppm6 and 1.4 ppm,6 respectively. The protein content for both the dry and wet formulations is 8.2 g/100 kcal. The fat contents are 6.2 g/100 kcal and 5.8 g/100 kcal for the canned and dry formulas, respectively. The dry product contains 517 kcal/cup, and the canned product contains 188 kcal/can. The owners were instructed to feed cats only the therapeutic diet, to transition over to the diet through mixing it in increasing proportions with the cats' previous diet over the course of 1 week, and to prevent consumption of any other diets or treats.
Thyroid Hormone Assays
Serum TT4 concentrations were measured by 1 of the 2 laboratories3 , 4 by either of 2 assays.5 , 6 The chemiluminescent enzyme immunoassay (CEIA) (reference range: 2.5–4.6 μg/dL) has been previously validated.7 The homogenous enzyme immunoassay (EIA) (reference range: 0.8–4.0 μg/dL) was validated internally by the laboratory using serum from 20 cats with a wide range of TT4 values. Intra‐assay coefficients of variation (CV) were 4.3% (at mean T4 concentration, 4.21 ug/dL) and 2.3% (at mean T4 concentration, 14.13 μg/dL). Interassay CV was 8% (at mean T4 concentration 3.73 μg/dL). The lower limit of detection (sensitivity) was 0.5 μg/dL. There was excellent correlation of the assay with a previously validated assay for feline TT4 (correlation coefficient of 0.98 and slope intercept at 1.079 with a range of 0.99–1.170). The reference range for the EIA was determined by nonparametric methods in >1,000 healthy cats ranging in age from 1 to 3 years of age.
All cats included in this study had an initial TT4 concentration >4.6 μg/dL. TT4 serum concentrations within the reference range for respective laboratories were then subclassified as low normal (bottom 50% of the reference range) versus high normal (top 50% of the reference range). Low reference ranges for the CEIA and EIA were defined as 2.5–3.5 and 0.8–2.3 μg/dL, respectively. High reference ranges for CEIA and EIA were defined as 3.5–4.6 and 2.3–4.0 μg/dL, respectively.
Data Management and Statistical Analysis
Variables of interest were compared between baseline, 21–60 days and 61–180 days after beginning the diet, by the Wilcoxon sign rank test. The analysis was performed by a commercial software program.7 A fractional polynomial regression line was fitted to the data for graphical display. Although “best fit” regression lines are typically based on linear models, linear or quadratic (curved) lines are often not good fits for biologic data. Fractional polynomials can improve modeling of continuous variables, and these were tested in first (Y = b 0 + b 1 X p1) and second (Y = b 0 + b 1 X p1 + b 2 X p2) degree models using a vector of powers {−2, −1, −0.5, ln, 0.5, 1, 2, 3} as p.8 Statistical significance was set at P < .05.
Results
Signalment, Clinical Signs, and Concurrent Illnesses
Forty‐nine cats (22 female and 27 male) were included in the study. All animals were neutered. Median age was 12.8 years (range of 8.6–18.0 years). The most common clinical signs were weight loss (30/49, 61%), polydipsia/polyuria (28/49, 57%), polyphagia (14/49, 28%), and vomiting, diarrhea, or both vomiting and diarrhea (13/49, 26%). Nine cats (18%) had been treated with methimazole but treatment had been stopped for various reasons (vomiting = 3/9, poor control of TT4 concentrations with methimazole = 3/9, difficulty pilling = 1/9, unknown = 2/9).
Concurrent illnesses included 1 each of nasal cryptococcus, feline asthma, digital squamous cell carcinoma, chronic rhinitis, skin allergies, urinary tract infection, feline immunodeficiency virus infection, infectious upper respiratory disease, inflammatory bowel disease, pulmonary mass, urethral obstruction, and biliary disease.
Physical Examination Findings
At the initial visit, median heart rate was 158 beats per minute (bpm) (range 122–280 bpm), and median body weight was 3.8 kg (range 2.1–5.3 kg). Median heart rate did not significantly decrease when comparing the initial visit to 21–60 days (median 156 bpm; range 118–264 bpm; P = .6) nor the initial visit to 61–180 days (median 154 bpm; range 120–234 bpm; P = .64). Median body weight did not significantly increase from initial visit to 21–60 days (median 3.6 kg; range 1.8–5.9 kg; P = .84) nor from initial visit to 61–180 days (median 3.9 kg; range 1.7–6.0 kg; P = .34).
Biochemistry Findings
At baseline, 22 cats (45%) had isosthenuria (USG <1.020), and 7 (32%) of these cats were azotemic. The median BUN at the initial visit was 29 mg/dL (range 13–75 mg/dL). There was no significant change in BUN from the initial visit to 21–60 days (median 28 mg/dL; range 15–60 mg/dL; P = .22). There was a significant decrease in median BUN from initial visit to 61–180 days, (median 26 mg/dL; range 16–68 mg/dL; P = .028). At the initial visit, the median creatinine was 1.8 mg/dL (range 0.4–4.9 mg/dL). There was no significant change in creatinine from initial visit to 21–60 days (median 1.6 mg/dL; range 0.4–3.4 mg/dL; P = .21), but a significant decrease from the initial visit to 61–180 days (median 1.5 mg/dL; range 0.4–3.3 mg/dL; P = .03).
Serum Thyroxine Concentrations
Median TT4 concentration before feeding the restricted‐iodine diet was 8.5 μg/dL, with a range of 5.0–21.1 μg/dL. All follow‐up TT4 concentrations were grouped into measurements collected between 21 and 60 days, and between 61 and 180 days. The median number of times a cat was tested was 6 times, with a range of 3–10 times. Of 49 cats, 41 (84%) were followed up for at least 180 days, and 16 (33%) were followed up for more than 1 year. Of the 49 cats, 48 (98%) were sampled at least once within the time periods of 21–60 days, and 47 (96%) were sampled within the time periods of 61–180 days.
At 21–60 days and at 61–180 days, 20/48 cats (42%; 95% CI: 28–57%) and 39/47 cats (83%; 95% CI: 69–92%) had thyroid values within their institutional reference ranges, respectively (Fig 1). Median initial T4 was 3.2 μg/dL (range 5.2–21.1 μg/dL) at 21–60 days for the cats within reference range at 21–60 days, whereas for 26 cats (54%) that had initial TT4 concentrations above reference range at 21–60 days, the median TT4 was 4.8 μg/dL (range 4.1–8.3 μg/dL). Expressed as percent above upper limit of the reference range TT4, initial percent above upper limit for cats in the latter group were significantly more likely (P = .038) to have a higher starting percentage (median 118%; range 58–215%) than those that did normalize (median 83%, range 28–359%) (Fig 2). With cats that were followed longer than 180 days, 32/40 cats (80%) had serum TT4 concentrations within their respective reference ranges.
Figure 1.

Median serum TT4 (μg/dL) during feeding of an iodine‐restricted diet (fractional polynomial regression line (red) fitted to the data).
Figure 2.
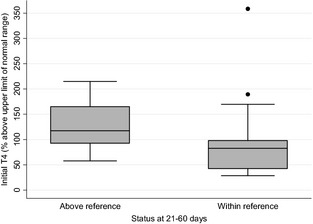
A box plot representation of initial serum TT4 for cats that had not normalized TT4 at 21–60 days (median 118%; range 58–215%) versus cats that had normalized TT4 at 21–60 days (median 83%; range 28–359%) expressed as percent above upper limit of reference range. The whiskers show the upper and lower values of the ranges for their respective groups within 1.5× the interquartile range (IQR) and values more than 1.5IQR are shown as outliers/dots. Percent above upper limit of reference range TT4 for the above reference group was significantly higher than the within reference group (P = .038).
Of the 8 cats with TT4 concentrations out of reference range from days 61–180, 5 cats were below the reference range and 3 were above reference range. Of those 3 cats, 1 had TT4 concentrations within reference range between 21 and 60 days but escaped control during days 61–180 days. Two cats had persistently increased TT4 concentrations which never came down into the laboratory reference range during the study. These cats were fed the iodine‐restricted diet for 117 and 135 days, respectively.
During days 21–60, 3/48 (6.2%) cats had serum TT4 concentrations below the reference range. At days 61–180, 5/47 (10.6%) cats had serum TT4 concentrations below reference range. None of these cats showed any overt clinical evidence of hypothyroidism.
Of the cats with serum TT4 concentrations in reference range at days 21–60, there were 8 (40%) in the low normal group (bottom 50% of institutional reference values) versus 12 (60%) in the high normal group (upper 50% of institutional reference values). Of the cats with serum TT4 concentrations in reference range at days 61–180, there were 20 cats (51%) in the low normal group and 19 cats (49%) in the high normal group. At days 61–180, the cats with serum TT4 concentrations within the low normal values were not significantly more likely to gain body weight (P = .413) nor have an increase in creatinine (P = .538) when compared to those in the high normal group.
Discussion
This retrospective study documents that exclusive feeding of a restricted‐iodine diet is effective in normalizing and maintaining normal serum TT4 concentrations in the majority of cats with spontaneous hyperthyroidism by 61–180 days after starting the diet; however, in these cats as a group, there was no improvement in objective clinical signs such as body weight or tachycardia. This suggests a lack of clinical control of the disease despite the serum TT4 concentration being within the reference range. The restricted‐iodine diet used in this study was developed based on studies showing that restriction of dietary iodine is effective in reducing serum thyroxine concentrations in newly diagnosed hyperthyroid cats in a cattery setting.1 , 8 , 9 Decreasing dietary iodine concentration to 0.17 ppm was effective at reducing serum TT4 concentrations into the reference range within 8 weeks in all 9 of the spontaneous hyperthyroid cats fed the diet. Studies evaluating the clinical efficacy of this diet in client‐owned cats are limited. In the only previous study of this diet in client‐owned hyperthyroid cats, the iodine‐restricted diet was effective in significantly decreasing circulating serum TT4 concentrations and improving clinical signs in 75% of 68 cats.5 However, there were some limitations to the study. Serum TT4 concentrations were monitored for only 8 weeks, clinical changes in weight were assessed subjectively via body condition score (BCS) rather than measurement of actual body weights, and values for biochemical findings and TT4 concentrations were obtained from multiple laboratories.
In this study, 42% and 83% of cats had serum TT4 concentrations within reference range after exclusive feeding of the restricted‐iodine diet by 60 days and 180 days, respectively. Furthermore, the cats in which it took a longer period of time for the TT4 to return to the reference range were more likely to have a higher initial TT4 concentration compared to those in which the TT4 was within range by day 60. These findings indicate that it might take longer to normalize the TT4 in cats with higher initial TT4 concentrations. A potential explanation for this phenomenon is that thyroid hormone is stored within thyroglobulin in sufficient quantities to supply the body with normal requirements of thyroid hormones for 2–3 months; therefore, even though synthesis of thyroid hormone has decreased, the physiologic effects of this decrease are not observed until later in dietary management.9
The serum TT4 concentration did not completely fall into reference ranges in 2 cats during their entire time in the study despite exclusive consumption of the diet. The records indicated potential owner compliance issues with 1 cat. This finding highlights the need for strict dietary adherence, especially making sure the pet has no additional treats or supplemental food. It is also important that the diet be palatable to ensure long‐term compliance. One cat in which the TT4 had normalized during the 21–60 day escaped control in the 61–180 daytime period. In addition to problems with compliance, it is possible that these cats might have undergone alterations in iodine processing. In people with a history of chronic iodine deficiency, there is increased iodine trapping and an increased ratio of T3 to TT4 synthesis10; these cats might have become more efficient at utilizing iodine leading to an increase in serum TT4 concentrations. In our study, we did not measure T3 concentrations so we were unable to evaluate T3 to TT4 ratios.
Normalization of serum TT4 concentration by feeding an iodine‐restricted diet did not lead to significant improvement in objective physical examination variables; median heart rate did not decrease and median body weight did not increase. Changes in heart rate have not been thoroughly explored with other treatments for hyperthyroidism, although a decreased heart rate has been documented in methimazole treated cats.11 Lack of decrease in heart rate in our study could simply be a product of variability in environment and each individual cat's demeanor. Treatment with both I131 and thyroid peroxidase inhibitors (methimazole, carbimazole) increased body weight in treated cats.12, 13, 14, 15 As weight loss is thought to occur in hyperthyroidism because of increased basal metabolic rate from excess thyroid hormone circulation,16 normalization of TT4 concentration in the cats of this study should have led to an increase in body weight. There are a couple potential explanations for this lack of change. The first is that despite normalization of serum TT4 concentrations, some of the cats in this study might have still been physiologically hyperthyroid. In this retrospective study, TSH and fT4 were not measured in most cats. Unfortunately, current assays for measurement of TSH in cats are not adequately sensitive to distinguish between normal and low TSH concentrations. Although in humans, documentation of a low TSH in the face of a TT4 within reference range supports physiologic hyperthyroidism, this is not true using currently available assays for TSH in cats because of the poor sensitivity of the assay. Another possibility is that the many concurrent illnesses present in this population of geriatric cats might have also influenced their body weight. This theory is supported by the fact that although many cats did not gain weight, the body weights stabilized and did not continue to decrease after starting the diet.
Hyperthyroidism in cats leads to increased GFR most likely through elevated cardiac output.17, 18 Treatment with methimazole, 131I, and thyroidectomy has been shown to decrease GFR and sometimes result in renal azotemia and overt clinical signs of renal failure.12, 19, 20 Worsening of azotemia was not documented in this study population despite normalization of TT4 concentrations. In our study, it was not feasible to determine how many cats had primary renal azotemia, because polyuria because of hyperthyroidism might cause a decrease in urine specific gravity and other prerenal and postrenal causes of azotemia could not be ruled out. One explanation for the lack of change in creatinine could be that the cats were still physiologically hyperthyroid which would also explain the lack change in body weight and heart rate. Other potential explanations are that increased omega‐3 fatty acids in the iodine‐restricted diet might have a protective effect on the kidneys, and that small decreases in GFR might have been missed because measurement of serum creatinine concentration is an insensitive indicator of GFR.5 In addition, dietary protein intake might also influence serum BUN, creatinine, or both values, and the iodine‐restricted diet is moderately protein restricted at 8.2 g/100 kcal.
Current recommendations for pharmacologic management of hyperthyroidism include aiming to bring the serum TT4 concentrations into the lower half of the reference range, though evidence to support this recommendation is limited.21 In this study, cats with a TT4 that fell in the low reference range were not significantly more likely to gain weight or have increases in creatinine when compared to the cats with TT4 concentrations in the high reference range. Further studies to determine the ideal posttreatment range for serum TT4 concentrations are warranted.
The long‐term consequence of a restricted‐iodine diet in hyperthyroid cats is unknown. The iodine concentration of this restricted diet (0.2 ppm) is lower than the iodine requirement of euthyroid adult cats (0.46 ppm),6 though signs of iodine deficiency were not seen in cats fed an even more iodine‐restricted diet (0.17 ppm) for 1 year.6 Physiologic effects of iodine other than thyroid hormone synthesis have not been completely elucidated in humans or animals. Iodine is thought to have moderate anti‐inflammatory and antioxidant effects,22, 23 as well as playing a role in the development of mammary tissue.24 Dietary iodine restriction in people with hyperthyroidism is only utilized in a short‐term setting to increase efficacy of radioiodide ablation postthyroidectomy for thyroid carcinoma.25 Few adverse effects have been reported in this setting.26
Whether long‐term iodine restriction might promote thyroid malignancy in hyperthyroid cats has not been explored. Histologically, adenomatous goiter in cats closely resembles human toxic nodular goiter,27, 28 a condition leading to thyroid autonomy because of mutations in genes coding for TSH receptors.28, 29 Antithyroid drugs such as thyroid peroxidase inhibitors are not considered primary treatments for this condition in humans30, 31 because their use does not address the underlying defects leading to thyroid autonomy. Reports of hyperthyroid cats treated with long‐term methimazole developing thyroid carcinoma seem to support this notion.32 In a sense, the end result of the restricted‐iodine diet is similar to that of thyroid peroxidase inhibitors, normalization of thyroid hormone production without addressing the underlying defects leading to thyroid autonomy.33 Furthermore, iodine deficiency, if indeed a consequence of chronic iodine restriction, can potentially act as a growth promoter in thyroid tissue leading to somatic mutations.34 For these reasons, long‐term use of iodine restriction for management of feline hyperthyroidism warrants further evaluation.
Limitations to this study include the retrospective design of the study and the lack of a gold standard for establishment of euthyroidism. Measurement of free T4 and TSH concentrations in these cats would have allowed a better assessment of thyroid status. Unfortunately, a sensitive assay for feline TSH that differentiates between euthyroidism and a physiologically hyperthyroid state despite normal serum TT4 concentrations is currently not available. Owner opinion on the clinical signs and quality of life of the cats in this study would also have strengthened the study. There were frequently notations regarding clinical improvement in the medical records, but consistent information from owners using a questionnaire was not obtained.
In conclusion, an iodine‐restricted diet was effective at normalizing and maintaining normal serum TT4 concentrations in the majority of spontaneously hyperthyroid cats for 180 days though clinical improvement in body weight and heart rate were not observed. These results could suggest the cats were in a persistently physiologically hyperthyroid state despite improvements in serum TT4 concentrations. However, stabilization of the body weight during the period of the study could mean that the clinical signs did not worsen. Many of the cats in this study had other serious concurrent illnesses that might have contributed to lack of weight gain in this cohort of cats. Further prospective studies comparing response to diet, methimazole, and radioactive iodine treatment, as well as investigation into the long‐term health effects of iodine deficiency in cats are necessary before the merits of this novel approach to management of feline hyperthyroidism can be completely assessed.
Acknowledgments
Conflict of Interest Declaration: Dr. Moore is a consulting editor for experimental design and statistics with the Journal of Veterinary Internal Medicine.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The work was performed at Purdue University Veterinary Teaching Hospital and VCA‐West Los Angeles Animal Hospital.
Some of the diet used in this study was provided to the owners at no cost by Hill's Pet Nutrition.
The paper has not been presented at any meeting.
Footnotes
Melendez L, Yamka R, Forrester S, Burris P. Titration of Dietary Iodine For Reducing Serum Thyroxine Concentrations in Newly Diagnosed Hyperthyroid Cats. J Vet Intern Med 2011; 16:683 (abstract)
Hill's® Prescription Diet® – y/d® Feline Thyroid Health
Purdue University Endocrinology Laboratory
Antech Diagnostics Laboratory
DRI® Thyroxine (T4) Assay, Microgenic Corp, Freemont, CA.
Immulite® Total T4 Assay, Diagnostics Products Corp, Los Angeles, CA.
STATA SE, v. 12.1, StataCorp, College Station, TX
Melendez L, Yamka R, Burris P. Titration of Dietery Iodine for Maintaining Normal Serum Thyroxine Concentrations in Hyperthyroid Cats. J Vet Intern Med 2011; 17:683 (abstract)
Yu, S, Wedekind K, Burris P, Forrester D, Locniskar M. Controlled Level of Dietary Iodine Normalizes Serum Total Thyroxine in Cats with Naturally Occurring Hyperthyroidism. J Vet Intern Med 2011; 18:683–684 (abstract)
References
- 1. Edinboro CH, Scott‐Moncrieff JC, Glickman LT. Feline hyperthyroidism: Potential relationship with iodine supplement requirements of commercial cat foods. J Feline Med Surg 2010;12:672–679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feldman EC, Nelson RW. Canine and Feline Endocrinology and Reproduction, 3rd ed St. Louis: Elsevier; 2004:152–218. [Google Scholar]
- 3. Thoday KL, Mooney CT. Historical, clinical and laboratory features of 126 hyperthyroid cats. Vet Rec 1992;131:257–264. [DOI] [PubMed] [Google Scholar]
- 4. Scott‐Moncrieff JC. Thyroid disorders in the geriatric veterinary patient. Vet Clin North Am Small Anim Pract 2012;42:707–725, vi–vii. [DOI] [PubMed] [Google Scholar]
- 5. Van der Kooij M, Becvárová I, Meyer HP, et al. Effects of an iodine‐restricted food on client‐owned cats with hyperthyroidism. J Feline Med Surg 2014;16:491–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wedekind KJ, Blumer ME, Huntington CE, et al. The feline iodine requirement is lower than the 2006 NRC recommended allowance. J Anim Physiol Anim Nutr (Berl) 2010;94:527–539. [DOI] [PubMed] [Google Scholar]
- 7. Edinboro CH, Scott‐Moncrieff JC, Janovitz E, et al. Epidemiologic study of relationships between consumption of commercial canned food and risk of hyperthyroidism in cats. J Am Vet Med Assoc 2004;224:879–886. [DOI] [PubMed] [Google Scholar]
- 8. Royston P, Altman D. Regression using fractional polynomials of continuous covariates: Parsimonious parametric modeling. Appl Stat 1994;43:429–467. [Google Scholar]
- 9. Hall JE, Guyton AC. Guyton and Hall Textbook of Medical Physiology. Twelfth. (Gruliow R, ed.). Philadelphia: Elsevier Saunders; 2011:4385–4389. [Google Scholar]
- 10. Delange F. The disorders induced by iodine deficiency. Thyroid 1994;4:107–128. [DOI] [PubMed] [Google Scholar]
- 11. Trepanier L, Hoffman SB, Kroll M, et al. Efficacy and safety of once versus twice daily administration of methimazole in cats with hyperthyroidism. J Am Vet Med Assoc 2003;222:954–958. [DOI] [PubMed] [Google Scholar]
- 12. Boag AK, Neiger R, Slater L, et al. Changes in the glomerular filtration rate of 27 cats with hyperthyroidism after treatment with radioactive iodine. Vet Rec 2007;161:711–715. [DOI] [PubMed] [Google Scholar]
- 13. Campos M, van Hoek I, Peremans K, Daminet S. Recombinant human thyrotropin in veterinary medicine: Current use and future perspectives. J Vet Intern Med 2012;26:853–862. [DOI] [PubMed] [Google Scholar]
- 14. Boretti FS, Sieber‐Ruckstuhl NS, Schäfer S, et al. Transdermal application of methimazole in hyperthyroid cats: A long‐term follow‐up study. J Feline Med Surg 2013;16:453–459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Van Hoek I, Lefebvre HP, Kooistra HS, et al. Plasma clearance of exogenous creatinine, exo‐iohexol, and endo‐iohexol in hyperthyroid cats before and after treatment with radioiodine. J Vet Intern Med 2008;22:879–885. [DOI] [PubMed] [Google Scholar]
- 16. Dale J, Daykin J, Holder R, et al. Weight gain following treatment of hyperthyroidism. Clin Endocrinol (Oxf) 2001;55:233–239. [DOI] [PubMed] [Google Scholar]
- 17. Adams WH, Daniel GB, Legendre M. Investigation of the effects of hyperthyroidism on renal function in the cat. Can J Vet Res 1997;61:53–56. [PMC free article] [PubMed] [Google Scholar]
- 18. Van Hoek I, Daminet S. Interactions between thyroid and kidney function in pathological conditions of these organ systems: A review. Gen Comp Endocrinol 2009;160:205–215. [DOI] [PubMed] [Google Scholar]
- 19. Becker TJ, Graves TK, Kruger JM, et al. Effects of methimazole on renal function in cats with hyperthyroidism. J Am Anim Hosp Assoc 2000;36:215–223. [DOI] [PubMed] [Google Scholar]
- 20. Graves TK, Olivier NB, Nachreiner RF, et al. Changes in renal function associated with treatment of hyperthyroidism in cats. Am J Vet Res 1994;55:1745–1749. [PubMed] [Google Scholar]
- 21. Daminet S, Kooistra HS, Fracassi F, et al. Best practice for the pharmacological management of hyperthyroid cats with antithyroid drugs. J Small Anim Pract 2014;55:4–13. [DOI] [PubMed] [Google Scholar]
- 22. Soriguer F, Gutiérrez‐Repiso C, Rubio‐Martin E, et al. Iodine intakes of 100–300 μg/d do not modify thyroid function and have modest anti‐inflammatory effects. Br J Nutr 2011;105:1783–1790. [DOI] [PubMed] [Google Scholar]
- 23. Aceves C, Anguiano B, Delgado G. The extrathyronine actions of iodine as antioxidant, apoptotic, and differentiation factor in various tissues. Thyroid 2013;23:938–946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patrick L. Iodine: Deficiency and therapeutic considerations. Altern Med Rev 2008;13:116–127. [PubMed] [Google Scholar]
- 25. Pluijmen MJHM, Eustatia‐Rutten C, Goslings BM, et al. Effects of low‐iodide diet on postsurgical radioiodide ablation therapy in patients with differentiated thyroid carcinoma. Clin Endocrinol (Oxf) 2003;58:428–435. [DOI] [PubMed] [Google Scholar]
- 26. Al Nozha OM, Vautour L, How J. Life‐threatening hyponatremia following a low‐iodine diet: A case report and review of all reported cases. Endocr Pract 2011;17:e113–e117. [DOI] [PubMed] [Google Scholar]
- 27. Peterson ME, Ward CR. Etiopathologic findings of hyperthyroidism in cats. Vet Clin North Am Small Anim Pract 2007;37:633–645. [DOI] [PubMed] [Google Scholar]
- 28. Watson SG, Radford AD, Kipar A, et al. Somatic mutations of the thyroid‐stimulating hormone receptor gene in feline hyperthyroidism: Parallels with human hyperthyroidism. J Endocrinol 2005;186:523–537. [DOI] [PubMed] [Google Scholar]
- 29. Vitti P, Rago T, Tonacchera M, Pinchera A. Toxic multinodular goiter in the elderly. J Endocrinol Invest 2002;25(10 Suppl):16–18. [PubMed] [Google Scholar]
- 30. Cooper DS. Antithyroid drugs. N Engl J Med 2005;352:905–917. [DOI] [PubMed] [Google Scholar]
- 31. Porterfield JR, Thompson GB, Farley DR, et al. Evidence‐based management of toxic multinodular goiter (Plummer's Disease). World J Surg 2008;32:1278–1284. [DOI] [PubMed] [Google Scholar]
- 32. Peterson M. Hyperthyroidism in cats: What's causing this epidemic of thyroid disease and can we prevent it? J Feline Med Surg 2012;14:804–818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Siegel RD, Lee SL. Toxic nodular goiter. Toxic adenoma and toxic multinodular goiter. Endocrinol Metab Clin North Am 1998;27:151–168. [DOI] [PubMed] [Google Scholar]
- 34. Zimmermann MB. Iodine deficiency. Endocr Rev 2009;30:376–408. [DOI] [PubMed] [Google Scholar]


