Abstract
Background
To date, epidemiological studies on degenerative mitral valve disease (DMVD) in dogs have largely reported referral caseloads or been limited to predisposed breeds. Analysis of primary‐care data to identify factors associated with DMVD would help clinicians identify high‐risk individuals and improve understanding.
Objectives
To estimate the prevalence of and identify risk factors for DMVD in dogs attending primary‐care veterinary practices in England.
Animals
Cases were identified within the electronic patient records of 111,967 dogs attending 93 practices. Four hundred and 5 dogs were diagnosed with DMVD (diagnosed cases) and a further 3,557 dogs had a heart murmur (HM) consistent with DMVD (possible cases).
Methods
Retrospective cross‐sectional study design. Prevalence was adjusted for the sampling approach. Mixed effects logistic regression models identified factors associated with DMVD.
Results
Prevalence estimates of diagnosed DMVD and HMs consistent with DMVD (both diagnosed and possible cases) were 0.36% (95% confidence interval [CI]: 0.29–0.45) and 3.54% (95% CI: 3.26–3.84) respectively. In the multivariable analysis, males had higher odds of diagnosed DMVD than did females (odds ratio [OR] 1.40, 95% CI: 1.12–1.74). Insured dogs had increased odds of DMVD compared with noninsured dogs (OR 3.56, 95% CI: 2.79–4.55) and dogs ≥20 kg had approximately half the odds of DMVD diagnosis compared with dogs <20 kg (OR 0.51, 95% CI: 0.36–0.74). Strong associations between a DMVD diagnosis and individual breeds and age were identified.
Conclusions and Clinical Importance
Degenerative mitral valve disease was a common disorder in practice‐attending dogs. Knowledge of identified risk factors for DMVD could improve clinical diagnosis and direct future research.
Keywords: Canine, Cardiac, Epidemiology, Primary‐care practice
Abbreviations
- CKCS
Cavalier King Charles Spaniel
- CI
confidence interval
- DMVD
degenerative mitral valve disease
- EPR
electronic patient record
- HM
heart murmur
- IQR
interquartile range
- OR
odds ratio
Degenerative mitral valve disease (DMVD) is widely accepted to be the most common cardiovascular disorder in dogs. Published estimates of prevalence are currently limited to populations of high‐risk breeds,1, 2, 3, 4 derived from teaching hospitals5 or based on studies conducted several decades ago.6, 7, 8, 9 These estimates range from 3.5% based on electronic patient record (EPR) data5 to over 50% based on the presence of valvular lesions at postmortem examination.8 The prevalence of DMVD in the general population of dogs currently attending primary‐care practices in England might differ from these values. Provision of population‐based estimates would be of much greater relevance to clinicians and researchers and are critical to assessing disease impact. In the clinical setting, prevalence can inform the diagnostic process.10, 11 Knowledge of the likelihood of disease based on prevalence estimates as well as predisposing risk factors, such as breed, can aid the clinician in advising clients on the probability of an animal having DMVD and also help direct veterinarians' recommendations of further tests to those most likely to benefit from them. The design of screening programs might also be guided by prevalence estimates, as this will influence which diagnostic tests are most appropriate in a given situation and which populations should be screened.12
Previous studies have identified several risk factors for DMVD. The disorder predominantly affects small breed dogs,13 although large breeds can also be affected.14 The prevalence of DMVD has been found to increase with age6, 8, 15, 16 and can approach 100% in geriatric populations of high‐risk breeds.8, 15 Males are over‐represented in some epidemiological studies4, 5, 17 but statistically significant differences between the sexes are not consistently reported.3, 6 The current literature lacks multivariable analyses of risk factors for DMVD diagnosis in England that adjust for the effect of other confounding variables. Further evaluation of associations between demographic factors and DMVD might aid clinical diagnosis and generate hypotheses for further research.
The Veterinary Companion Animal Surveillance System (VetCompass)18 collates de‐identified EPR data from primary‐care veterinary practices in the UK. Analysis of this large data resource would allow DMVD prevalence to be estimated and major risk factors for the disease to be identified, as has been demonstrated for other disorders in companion animals.19, 20, 21
It was hypothesized that the breeds most commonly reported to be at increased risk of DMVD in the current literature, Cavalier King Charles Spaniels (CKCS),1, 3, 5, 15, 16, 22 Dachshunds,4, 5, 23, 24 and Poodles,5, 17 would have the highest odds of DMVD among purebred dogs in the VetCompass population. Moreover, small‐breed dogs (<20 kg) and male dogs would have higher odds of DMVD than large‐breed dogs and females respectively.
The aims of this study were to:
Estimate the prevalence of DMVD in a population of practice‐attending dogs in England.
Identify risk factors for DMVD diagnosis in the primary‐care setting.
Materials and Methods
Electronic patient records from veterinary practices sharing data with VetCompass were reviewed retrospectively. The practices were primary‐care companion animal clinics, mainly located in central and southeast England. The denominator population for the overall prevalence estimate consisted of all dogs attending participating practices between 1st January 2010 and 31st December 2011. The study population for the risk factor study was restricted by age to dogs at least 1 year old at the last consultation. Data shared included demographic (date of birth, sex, breed, bodyweight, insurance status, microchip number, partial postcode, veterinary clinic ID) and clinical data (free‐text clinical notes, VeNom diagnostic terms,25 treatments prescribed). Sample size calculations estimated that 246 cases and 61,015 noncases would be required to identify an odds ratio (OR) of 2 for an explanatory variable to which 5% of noncases were exposed at a confidence level of 95% and a power of 80% (Epi Info 7, CDC). The study received ethics approval from the Royal Veterinary College Ethics and Welfare Committee.
Two case definitions were developed to account for different levels of diagnosis: diagnosed DMVD and possible DMVD cases. Diagnosed DMVD cases were defined as dogs with a diagnosis of DMVD (or synonym) in their clinical notes or VeNom diagnostic terms. Possible DMVD cases were defined as dogs over 1 year old with a documented heart murmur (HM) consistent with DMVD, without a specified cardiac diagnosis. Possible cases were restricted by age to avoid inclusion of dogs with HMs because of congenital disease. Dogs reported to have continuous or diastolic murmurs were excluded as possible cases; as were dogs that had murmurs detected only during pregnancy or clinically important systemic disease (moderate to severe anemia, pyrexia, severe hypovolemia, or dehydration). Dogs with murmurs or mitral valve regurgitation because of other diagnosed cardiac disorders were excluded. The point of maximal intensity, which relates to the thoracic location where the HM is heard most loudly, was not used as an exclusion criterion. Diagnosed and possible DMVD cases were combined to form a population of dogs with HMs consistent with DMVD, hereafter described as HM cases. Where available, the EPRs of all diagnosed cases until May 2014 were examined in detail. The diagnostic tests performed and the types of cardiac medications prescribed were extracted where available.
Data were checked and cleaned in a spreadsheet (Microsoft Office Excel 2010),1 and exported to Stata Version 132 for analysis. Prevalence and 95% confidence intervals (95% CI) were calculated for dogs with diagnosed DMVD and for HM cases. Prevalence was adjusted for clustering at the practice level.26 Descriptive statistics were calculated to characterize the cross‐sectional study population. Breed, sex, insurance status, age at last consultation and maximum recorded bodyweight (kg) were evaluated as explanatory variables in the risk factor study. The expected number of HM cases for each breed was calculated by multiplying the total number of individuals within the breed by the overall prevalence of HM cases. Breeds that had a sum of observed and expected cases >50 dogs were evaluated individually in the analysis. Less common breeds were combined into a “purebred other” category. Some breeds were combined to increase statistical power. For example, Poodles included both miniature and standard varieties. Age at last consultation (years) was categorized into rounded quintiles (1.0 to <4.0, 4.0 to <7.0, 7.0 to <10.0, 10.0 to <13.0 and ≥13.0). Maximum bodyweight was further dichotomized based on the median of the entire population, for statistical efficiency (<20.0 and ≥20.0 kg). Dogs without a documented bodyweight or insurance status were included in “not recorded” categories. Univariable and multivariable logistic regression models were used to identify explanatory variables associated with DMVD. Separate models were created for HM cases and for diagnosed cases only. Variables significant at the 20% level in univariable analyses were taken forward for consideration in mixed effects multivariable models. Manual backward stepwise regression was used to sequentially eliminate variables with a P‐value > .05 in the multivariable model.12 Interactions between explanatory variables were also evaluated. Veterinary clinic was assessed as a random effect to account for clustering at the practice‐level, and the magnitude of the clustering was measured by the intraclass correlation (rho).12 The stability of the quadrature approximation in the model fitting algorithm was assessed.26 The use of the Firth logit method allowed inference of ORs and CIs when complete separation (zero cells) occurred.27 The Hosmer‐Lemeshow test and the area under ROC curves were used to assess model fit and predictive ability respectively.
Results
Prevalence Estimates
The denominator population consisted of 111,967 dogs attending 93 veterinary clinics from 1st January 2010 to 31st December 2011. Four hundred and 5 dogs were identified as having diagnosed DMVD, giving a prevalence, adjusted for the clustering effect of clinic, of 0.36% (95% CI: 0.29–0.45). A further 3,557 dogs were classified as possible cases, having a HM consistent with DMVD recorded within their EPRs. A total of 3,962 dogs were HM cases (possible or diagnosed DMVD), giving a prevalence, adjusted for the clustering effect of clinic, of 3.54% (95% CI: 3.26–3.84).
Descriptive Statistics and Risk Factors for Diagnosed Degenerative Mitral Valve Disease Cases
The mean age at which DMVD was diagnosed or the presence of a HM was first recorded in 116 incident cases (newly diagnosed during the study period) was 9.5 years (standard deviation 3.2 years). Two hundred and fifty‐two (62.2%) dogs with diagnosed DMVD were male and 264 (68.9%) were insured. The median bodyweight was 10.9 kg (interquartile range [IQR] 8.3–15.8 kg). The breeds most frequently diagnosed with DMVD were CKCS (n = 131, 32.4% of dogs with diagnosed DMVD), crossbreds (n = 45, 11.1%), Yorkshire Terriers (n = 25, 6.2%), and Jack Russell Terriers (n = 22, 5.4%). Veterinary surgeons recorded heart rate at least once in 331 (81.7%) dogs diagnosed with DMVD. Heart murmur intensity was graded (I–VI) in 361 (89.1%) dogs. During the period of data collection, echocardiography was the most frequently performed diagnostic procedure after thoracic auscultation, with 62.5% of diagnosed cases being confirmed by echocardiogram (Fig 1). Two hundred and ninety (71.6%) dogs diagnosed with DMVD received at least 1 treatment for their cardiac disease: ACE inhibitor (n = 218, 53.8%), frusemide (n = 216, 53.3%), pimobendan (n = 210, 51.9%), spironolactone (n = 108, 26.7%), amlodipine (n = 18, 4.4%), amiloride with hydrochlorothiazide (n = 11, 2.7%), digoxin (n = 9, 2.2%), beta‐blocker (n = 2, 0.5%), aspirin (n = 1, 0.2%), heparin (n = 1, 0.2%), lignocaine (n = 1, 0.2%), mexiletine (n = 1, 0.2%), nitroglycerin cream (n = 1, 0.2%). The majority (n = 123/156, 78.8%) of dogs starting treatment during the study period had at least 1 clinical sign that could be attributable to DMVD recorded at the time treatment was initiated (Fig 2).
Figure 1.
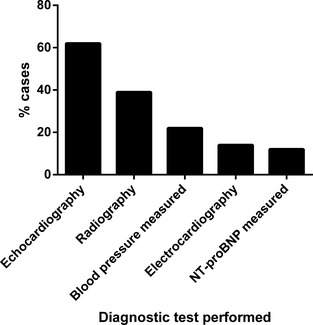
Diagnostic procedures undertaken in 405 dogs diagnosed with degenerative mitral valve disease attending primary‐care practices in England.
Figure 2.
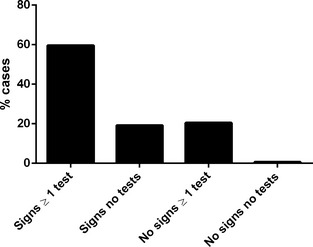
Presence or absence of clinical signs and whether diagnostic testing (excluding physical examination) was undertaken when treatment for DMVD was initiated in 156 dogs attending primary‐care practices in England.
The cross‐sectional study population for diagnosed DMVD cases contained 90,605 dogs. Of these, 15,632 (17.3%) dogs did not have an insurance status recorded and 22,067 (24.4%) dogs did not have a documented bodyweight. Other variables had <0.2% missing data (Table 1). Breed, sex, insurance status, age, and bodyweight were all strongly associated with diagnosed DMVD in the univariable analysis (Table 2). Although Rottweilers were a popular breed (sum of observed and expected cases >50 dogs), none were diagnosed with DMVD, so this breed was incorporated into the “purebred other” category in the logistic regression models. The final multivariable model for factors associated with a diagnosis of DMVD contained observations for 90,464 dogs and included the following explanatory variables: breed, sex, insurance status, age at last consultation, and maximum bodyweight (Table 3). Cavalier King Charles Spaniel, King Charles Spaniels, Chihuahuas, Whippets, Poodles, Shih Tzus, Yorkshire Terriers, and Border Collies had statistically significant increased odds of DMVD diagnosis compared with crossbred dogs (Table 4); whereas Labrador Retrievers had lower odds. Males had higher odds of DMVD than females (OR 1.40, 95% CI: 1.12–1.74, P = .0024). Insured dogs had more than 3 times the odds of DMVD diagnosis compared with noninsured dogs (OR 3.56, 95% CI: 2.79–4.55, P < .001). Dogs weighing 20 kg or more had approximately half the odds of DMVD compared with lighter dogs (OR 0.51, 95% CI: 0.36–0.74, P < .001). A strong, positive association between increasing age and DMVD diagnosis was identified (P < .001). No significant interactions were detected. Veterinary clinic was included as a random effect as clustering was significant (rho = 0.17, 95% CI: 0.11–0.25, P < .001). The choice of quadrature points did not significantly affect the outcome (coefficients did not change more than a relative difference of 0.01%).26 The Hosmer‐Lemeshow test indicated poor model fit (P < .001) but the area under the ROC curve was considered good (AUC = 0.93). Using the Firth logit method, it was estimated that Rottweilers had 0.15 times the odds of DMVD diagnosis compared with crossbred dogs in univariable analysis (OR 0.15, 95% CI: 0.01–2.51) and 0.44 times the odds of DMVD diagnosis compared with crossbred dogs in the multivariable model (OR 0.44, 95% CI: 0.03–7.26).
Table 1.
Descriptive statistics for 405 dogs diagnosed with DMVD within a population of dogs attending primary‐care practices in England
| Variable | Median (IQR) or Number (%) | Number (%) of Dogs with Missing Data | |||
|---|---|---|---|---|---|
| Diagnosed DMVD Cases (n = 405) | Noncases (n = 90,200) | Diagnosed DMVD Cases | Noncases | Total Cross‐sectional Study Population | |
| Breed (crossbred) | 45 (11.11) | 16,242 (18.01) | 0 (0.00) | 28 (0.03) | 28 (0.03) |
| Sex (male) | 252 (62.22) | 46,949 (52.05) | 0 (0.00) | 0 (0.00) | 0 (0.00) |
| Insurance status (insured) | 264 (68.93) | 30,406 (33.71) | 22 (5.43) | 15,610 (17.31) | 15,632 (17.25) |
| Age at last consultation (years) | 10.60 (8.35–12.71) | 5.24 (2.65–9.00) | 0 (0.00) | 121 (0.13) | 121 (0.13) |
| Maximum bodyweight (kg) | 10.90 (8.3–15.75) | 18.70 (9.50–29.30) | 25 (6.17) | 22,042 (24.44) | 22,067 (24.36) |
IQR, interquartile range; DMVD, degenerative mitral valve disease.
Table 2.
Univariable logistic regression analysis results for risk factors associated with diagnosed DMVD (405 cases) within a population of dogs attending primary‐care practices in England.
| Variable | Cases n (%) | Noncases n (%) | OR | 95% CI | P‐value |
|---|---|---|---|---|---|
| Breeda | |||||
| Cavalier King Charles Spaniel | 131 (32.35) | 1,645 (1.82) | 28.74 | 20.41–40.48 | <.001 |
| King Charles Spaniel | 18 (4.44) | 316 (0.35) | 20.56 | 11.80–35.92 | |
| Whippet | 6 (1.48) | 410 (0.45) | 5.28 | 2.24–12.45 | |
| Poodle | 9 (2.22) | 824 (0.91) | 3.94 | 1.92–8.09 | |
| Yorkshire Terrier | 25 (6.17) | 2,963 (3.29) | 3.05 | 1.86–4.97 | |
| Shih Tzu | 12 (2.96) | 1,486 (1.65) | 2.91 | 1.54–5.52 | |
| Chihuahua | 9 (2.22) | 1,251 (1.39) | 2.60 | 1.27–5.32 | |
| Miniature Schnauzer | 5 (1.23) | 714 (0.79) | 2.53 | 1.00–6.39 | |
| Dachshund | 6 (1.48) | 985 (1.09) | 2.20 | 0.94–5.17 | |
| Border Collie | 16 (3.95) | 2,739 (3.04) | 2.11 | 1.19–3.74 | |
| Lhasa Apso | 4 (0.99) | 794 (0.88) | 1.82 | 0.65–5.07 | |
| Cocker Spaniel | 13 (3.21) | 3,523 (3.91) | 1.33 | 0.72–2.47 | |
| West Highland White Terrier | 10 (2.47) | 2,838 (3.15) | 1.27 | 0.64–2.53 | |
| Jack Russell Terrier | 22 (5.43) | 6,391 (7.09) | 1.24 | 0.75–2.07 | |
| Crossbred | 45 (11.11) | 16,242 (18.01) | Baseline | – | |
| Bichon Frisé | 3 (0.74) | 1,088 (1.21) | 1.00 | 0.31–3.21 | |
| Border Terrier | 3 (0.74) | 1,095 (1.21) | 1.00 | 0.31–3.19 | |
| Lurcher | 2 (0.49) | 811 (0.90) | 0.89 | 0.22–3.68 | |
| Purebred other | 43 (10.62) | 17,727 (19.66) | 0.88 | 0.58–1.33 | |
| Golden Retriever | 4 (0.99) | 2,096 (2.32) | 0.69 | 0.25–1.92 | |
| Greyhound | 2 (0.49) | 1,057 (1.17) | 0.68 | 0.17–2.82 | |
| Boxer | 2 (0.49) | 1,347 (1.49) | 0.54 | 0.13–2.21 | |
| Labrador Retriever | 8 (1.98) | 9,031 (10.02) | 0.32 | 0.15–0.68 | |
| Staffordshire Bull Terrier | 5 (1.23) | 7,079 (7.85) | 0.25 | 0.10–0.64 | |
| English Springer Spaniel | 1 (0.25) | 2,229 (2.47) | 0.16 | 0.02–1.18 | |
| German Shepherd | 1 (0.25) | 3,491 (3.87) | 0.10 | 0.01–0.75 | |
| Sex | |||||
| Female | 153 (37.78) | 43,251 (47.95) | Baseline | – | .0016 |
| Male | 252 (62.22) | 46,949 (52.05) | 1.52 | 1.24–1.86 | |
| Insurance status | |||||
| Not insured | 119 (29.38) | 44,184 (48.98) | Baseline | – | <.001 |
| Insured | 264 (65.19) | 30,406 (33.71) | 3.22 | 2.60–4.00 | |
| Not recorded | 22 (5.43) | 15,610 (17.31) | 0.52 | 0.33–0.82 | |
| Age at last consultation (years) | |||||
| 1.0 to <4.0 | 11 (2.72) | 34,352 (38.14) | Baseline | – | <.001 |
| 4.0 to <7.0 | 45 (11.11) | 22,095 (24.53) | 6.36 | 3.29–12.30 | |
| 7.0 to <10.0 | 114 (28.15) | 15,363 (17.06) | 23.17 | 12.48–43.04 | |
| 10.0 to <13.0 | 141 (34.81) | 11,107 (12.33) | 39.64 | 21.46–73.25 | |
| ≥13.0 | 94 (23.21) | 7,162 (7.95) | 40.99 | 21.94–76.58 | |
| Maximum bodyweight (kg) | |||||
| <20.00 | 316 (78.02) | 36,103 (40.03) | Baseline | – | <.001 |
| ≥20.00 | 64 (15.80) | 32,055 (35.54) | 0.23 | 0.17–0.30 | |
| Not recorded | 25 (6.17) | 22,042 (24.44) | 0.13 | 0.09–0.19 | |
OR, odds ratio; CI; confidence intervals; DMVD, degenerative mitral valve disease.
Breeds with statistically significant associations with DMVD diagnosis (P < .05) are shown in bold.
Table 3.
Multivariable logistic regression model for risk factors associated with diagnosed DMVD in dogs.
| Variable | OR | 95% CI | P‐value |
|---|---|---|---|
| Breeda | |||
| Cavalier King Charles Spaniel | 47.37 | 31.56–71.09 | <.001 |
| King Charles Spaniel | 36.49 | 18.90–70.47 | |
| Chihuahua | 6.16 | 2.85–13.30 | |
| Whippet | 4.73 | 1.88–11.87 | |
| Poodle | 2.92 | 1.38–6.17 | |
| Shih Tzu | 2.89 | 1.47–5.67 | |
| Miniature Schnauzer | 2.27 | 0.86–5.95 | |
| Yorkshire Terrier | 2.15 | 1.28–3.61 | |
| Border Collie | 2.02 | 1.12–3.63 | |
| Dachshund | 1.53 | 0.63–3.72 | |
| Lhasa Apso | 1.19 | 0.41–3.41 | |
| Jack Russell Terrier | 1.15 | 0.67–1.97 | |
| Purebred other | 1.09 | 0.71–1.67 | |
| Cocker Spaniel | 1.05 | 0.55–1.99 | |
| Crossbred | Baseline | – | |
| Greyhound | 0.98 | 0.23–4.16 | |
| Border Terrier | 0.90 | 0.27–2.95 | |
| Lurcher | 0.89 | 0.21–3.74 | |
| Boxer | 0.88 | 0.21–3.73 | |
| Bichon Frisé | 0.73 | 0.22–2.40 | |
| West Highland White Terrier | 0.65 | 0.32–1.33 | |
| Golden Retriever | 0.55 | 0.19–1.59 | |
| Staffordshire Bull Terrier | 0.42 | 0.16–1.06 | |
| Labrador Retriever | 0.40 | 0.18–0.87 | |
| English Springer Spaniel | 0.17 | 0.02–1.22 | |
| German Shepherd | 0.15 | 0.02–1.11 | |
| Sex | |||
| Female | Baseline | – | .0024 |
| Male | 1.40 | 1.12–1.74 | |
| Insurance status | |||
| Not insured | Baseline | – | <.001 |
| Insured | 3.56 | 2.79–4.55 | |
| Not recorded | 0.53 | 0.32–0.88 | |
| Age at last consultation (years) | |||
| 1.0 to <4.0 | Baseline | <.001 | |
| 4.0 to <7.0 | 7.03 | 3.60–13.72 | |
| 7.0 to <10.0 | 38.24 | 20.29–72.08 | |
| 10.0 to <13.0 | 101.61 | 53.79–191.94 | |
| ≥13.0 | 150.76 | 78.11–290.96 | |
| Maximum bodyweight (kg) | |||
| <20.00 | Baseline | – | .001 |
| ≥20.00 | 0.51 | 0.36–0.74 | |
| Not recorded | 0.20 | 0.13–0.31 | |
| Veterinary clinic (included as a random effect) | |||
| Rho | 0.17 | 0.11–0.25 | <.001 |
OR, odds ratio; CI, confidence intervals; DMVD, degenerative mitral valve disease.
Breeds with statistically significant associations with DMVD diagnosis (P < .05) are shown in bold.
Table 4.
Breeds with the highest odds of diagnosed DMVD and HMs consistent with DMVD in multivariable logistic regression analyses
| Diagnosed DMVD Cases | HM Cases | ||||
|---|---|---|---|---|---|
| Breed | ORa | 95% CI | Breed | OR | 95% CI |
| Cavalier King Charles Spaniel | 47.37 | 31.56–71.09 | Cavalier King Charles Spaniel | 18.72 | 16.13–21.73 |
| King Charles Spaniel | 36.49 | 18.90–70.47 | King Charles Spaniel | 16.14 | 12.09–21.55 |
| Chihuahua | 6.16 | 2.85–13.30 | Chihuahua | 4.17 | 3.22–5.40 |
| Whippet | 4.73 | 1.88–11.87 | Boxer | 4.12 | 3.29–5.16 |
| Poodle | 2.92 | 1.38–6.17 | Whippet | 2.56 | 1.74–3.76 |
| Shih Tzu | 2.89 | 1.47–5.67 | Miniature Schnauzer | 2.15 | 1.54–3.01 |
| Miniature Schnauzer | 2.27 | 0.86–5.95 | Poodle | 1.87 | 1.43–2.45 |
| Yorkshire Terrier | 2.15 | 1.28–3.61 | Shih Tzu | 1.82 | 1.43–2.32 |
| Border Collie | 2.02 | 1.12–3.63 | Greyhound | 1.78 | 1.32–2.41 |
| Dachshund | 1.53 | 0.63–3.72 | Lurcher | 1.68 | 1.22–2.31 |
DMVD, degenerative mitral valve disease; HM, heart murmur cases (dogs with heart murmurs consistent with DMVD); OR, odds ratio; CI, confidence intervals a the baseline group for odds ratios is crossbred dogs.
Descriptive Statistics and Risk Factors for Heart Murmur Cases
Heart murmur cases consisted of 405 (10.2%) dogs with diagnosed and 3,557 (89.8%) dogs with possible DMVD. Males accounted for 2,166 (54.7%) HM cases and 1,515 (40.4%) of these dogs were insured. The median bodyweight was 11.6 kg (IQR 8.1–20.0 kg). The most frequently diagnosed breeds were crossbred dogs (n = 677, 17.1%), CKCS (n = 657, 16.6%), Jack Russell Terriers (n = 322, 8.1%), and Yorkshire Terriers (n = 215, 5.4%).
The final multivariable model for factors associated with HM cases contained observations for 94,018 dogs and contained the following explanatory variables: breed, sex, insurance status, age at last consultation, and maximum bodyweight. Cavalier King Charles Spaniel, King Charles Spaniels, Chihuahuas, Boxers, Whippets, Miniature Schnauzers, Poodles, Shih Tzus, Greyhounds, Lurchers, Bichon Frisés, Dachshunds, and Yorkshire Terriers had increased odds of being a HM case compared with crossbred dogs. Border Terriers, Golden Retrievers, German Shepherd Dogs, Rotweillers, West Highland White Terriers, Staffordshire Bull Terriers, and Labrador Retrievers had decreased odds compared with crossbred dogs. Males had slightly higher odds of being a HM case than females (OR 1.15, 95% CI: 1.08–1.24, P < .001). Dogs that were insured were more likely to be recorded as having a HM consistent with DMVD than noninsured dogs (OR 1.25, 95% CI: 1.15–1.35, P < .001). An interaction was found between age group and bodyweight (P < .001). The odds of being a HM case were slightly higher in young (<4 years) heavier dogs (≥20 kg) compared with young dogs that were lighter; whereas the odds of being a HM case were higher in older dogs (>7 years) that were lighter (<20 kg) compared with older dogs that were heavier.
Veterinary clinic was included as a random effect as clustering was significant (rho = 0.05, 95% CI: 0.03–0.06, P < .001). The Hosmer‐Lemeshow test indicated poor model fit (P < .001), but the area under the ROC curve was considered good (AUC = 0.86).
Discussion
This study identified a high prevalence of HMs consistent with DMVD in a large cohort of dogs attending primary‐care practices in England. Several demographic risk factors, including breed, sex, age, and bodyweight were independently associated with DMVD, as was whether a dog was insured or not. Clustering at the clinic level was observed, suggesting variation in the diagnosis of this condition across practices.
The prevalence of HMs consistent with DMVD in the current study (3.54%, 95% CI: 3.26–3.84) is similar to that based on clinical record data from a teaching hospital (3.5%),5 but lower than figures derived from postmortem examinations (34.4–69.7%).7, 8, 9 These discrepancies might be because of under‐reporting of HMs within EPRs, geographical, or temporal variation, different methods of case detection and case definitions as well as different denominator populations. Dogs included in postmortem studies are likely to be older and this will have a marked effect on the observed prevalence of disease. Although case detection in the current study might have lacked sensitivity, the apparent prevalence of HMs consistent with DMVD was still considerable, reflecting a significant veterinary concern. Whilst HMs were frequently recorded, the minority of these cases had a specific diagnosis of DMVD. A study which utilized insurance data to explore cardiac‐related mortality also reported that many cardiac diagnoses were nonspecific.22 These findings suggest that the majority of dogs with HMs do not undergo further investigations to confirm the underlying cardiac diagnosis and veterinarians frequently do not make a presumptive diagnosis based on the presence of a murmur alone.
The data support our hypothesis that CKCS and Poodles have among the highest odds of DMVD. Exploratory analysis of separate Poodle varieties suggested similar odds of DMVD diagnosis for the different sizes and hence these were combined into a single Poodle category to improve statistical power. Previous studies4, 5, 23, 24 have also identified Dachshunds as being predisposed to DMVD. Dachshunds had significantly higher odds of being HM cases than crossbreds in the current study (OR 1.42, 95% CI: 1.06–1.90). Although the magnitude of effect for this breed was similar in the diagnosed DMVD model, this association was not statistically significant (OR 1.53, 95% CI: 0.63–3.72), perhaps because the latter model had insufficient power to detect such a modest difference. In agreement with previous studies, the models for both diagnosed DMVD and HM cases identified that CKCS,1, 3, 5, 15, 16, 22 King Charles Spaniels,5 Chihuahuas,5, 17 Whippets,5 Poodles,5, 17 and Shih Tzus4 are predisposed to DMVD. Yorkshire Terriers also had higher odds of both diagnosed DMVD and HM detection than crossbred dogs in the current study. The latter breed association has not, to the authors' knowledge, been reported although a study has found that 8.5% of 165 adult Yorkshire Terriers in France had left apical systolic murmurs.4 Border Terriers, Golden Retrievers, German Shepherd Dogs, Rotweillers, West Highland White Terriers, Staffordshire Bull Terriers, and Labrador Retrievers all had significantly lower odds of being HM cases than crossbred dogs. With the exception of Labrador Retrievers, these associations were not statistically significant in the diagnosed DMVD model. However, many of these breeds were among those with the lowest odds of diagnosed DMVD, probably reflecting a type II error because of lower power in the latter model. In agreement, analysis of clinical records from a teaching hospital in Scotland5 also identified German Shepherd Dogs, West Highland White Terriers, and Labrador Retrievers as having a reduced risk of DMVD. There were some notable differences between the logistic regression models evaluating risk factors for diagnosed DMVD and HM cases. Boxers, Miniature Schnauzers, Greyhounds, Lurchers, Bichon Frisés, and Dachshunds had significantly increased odds of being HM cases, but not being diagnosed with DMVD. These differences might be because of misclassification eg, Boxers are reported to be predisposed to aortic stenosis28 and sight hounds are reported to frequently have physiological murmurs.29, 30 Alternatively, the diagnosed DMVD model might have had limited power to detect associations because of the smaller number of DMVD cases recorded for certain breeds. Conversely, Border Collies had significantly higher odds of having diagnosed DMVD, but not being a HM case. Veterinary practitioners might be more or less likely to attribute a diagnosis of DMVD to an individual based on preconceived knowledge of breed associations and might be more or less likely to consider a HM based on whether a given breed has been reported to suffer from cardiac conditions. For example, CKCS had over 40 times the odds of being diagnosed with DMVD and less than 20 times the odds of being a HM case compared with crossbred dogs. This is likely to reflect widespread awareness of this breed predisposition among practitioners and consequently a confidence to record a diagnosis of DMVD in a dog of this breed presenting with a murmur consistent with this condition. An alternative approach to categorization would be to combine breeds into UK Kennel club breed groups.31 Whilst increasing the statistical power of each category, this level of classification does not represent sufficiently biologically homogenous groups. Loss of specific breed associations also limits the application of the results when assessing an individual's risk of DMVD in practice and when designing breed‐specific screening programs.
The association between bodyweight and DMVD identified in the diagnosed DMVD cases and reported in the literature13 was not found in the younger age strata in the current model for HM cases. It is possible that the interaction between age and bodyweight identified in this model reveals that younger dogs with HMs might be more likely to have alternative cardiac disorders and have been misclassified as possible DMVD cases.
Males had significantly higher odds compared with females for both diagnosed DMVD (1.40, 95% CI: 1.12–1.74, P = .0024) and being HM cases (OR 1.15, 95% CI: 1.08–1.24, P < .001), which concurs with previous studies.4, 5, 17 Whilst the magnitude of this difference is unlikely to be clinically significant, it raises questions on the influence of sex on the etiology, age of onset, and pathophysiology of the disorder and warrants further research.
Being insured was associated with being a HM case (OR 1.25, 95% CI: 1.15–1.35, P < .001). An even stronger association was identified between insurance status and diagnosed DMVD (OR 3.56, 95% CI: 2.79–4.55, P < .001). This suggests that insured dogs might be more likely to undergo examinations and diagnostic procedures. However, noncases had a higher proportion of missing insurance data compared with cases. If these values were missing systematically, this might bias the associations between insurance status and DMVD. This might have occurred if insured animals with an abnormality such as a HM were more likely to have their insurance status recorded than insured healthy animals, as dogs with abnormalities might require costly diagnostics or treatments necessitating an insurance claim.
Including veterinary clinic in the logistic regression models improved model fit, suggesting that practice‐level factors influenced the outcome independently of the other explanatory variables included in the model. The intraclass correlation coefficient (rho) was higher in the diagnosed DMVD model (rho = 0.17, 95% CI: 0.11–0.25), than the HM case model (rho = 0.05, 95% CI: 0.03–0.06), indicating that the veterinary practice attended had more impact on whether a dog was diagnosed with DMVD than whether a HM consistent with DMVD was recorded in the EPRs.
There were some limitations to this study. Data were not originally recorded for research purposes and were analysed retrospectively. If a practitioner did not perform thoracic auscultation or transcribe the DMVD diagnosis or the presence of a HM into the EPR, an affected dog would fail to be included as a case. Equally, some dogs classified as cases could have had alternative causes for the HM. Echocardiography, which provides a definitive diagnosis of DMVD,32 was performed on 62.5% of diagnosed DMVD cases. However, the presence of a left apical systolic HM in a dog of typical signalment alone is highly suggestive of DMVD.33 Furthermore, logistic regression models only including echocardiogram‐confirmed cases yielded the same conclusions as the diagnosed DMVD model (data not shown). The specificity of the diagnosed DMVD case definition is therefore likely to be high. The case definition for possible DMVD cases was more general, suggesting lower specificity, and included any dog more than 1 year old with a documented HM, that was not diastolic or continuous, in the absence of evidence of an alternative diagnosis. The age and bodyweight distributions were generally similar between diagnosed and possible DMVD cases, suggesting that most of the possible but unconfirmed cases were likely to have DMVD (unless there is another highly prevalent cause of murmurs in this population which occurs with similar age and bodyweight distribution to DMVD). In addition, 8 specific breed associations were identified in both the diagnosed and HM models. However, the interaction between age and bodyweight, and the breed associations observed in dogs previously reported to be predisposed to alternative cardiac disorders in the HM model, indicate that there might have been some misclassification of possible cases. The breed associations identified uniquely in the HM model should therefore be interpreted with caution. The differences in breed and weight distribution in the models might highlight the type of dog in which veterinarians should more actively consider causes of murmurs other than DMVD to be most likely, therefore in sight‐hounds, boxers and larger, younger dogs alternative explanations for an audible murmur should be sought. There might also have been misclassification of explanatory variables. However, when the EPRs of approximately 3,000 dogs within the VetCompass database were cross‐linked to the UK Kennel Club pedigree database using microchip numbers, there was >99% agreement for breed and sex and 97% agreement for the date of birth within 90 days (D.G. O'Neill, unpublished results). The validity of these variables was therefore suggested to be very good. Moreover, any misclassification of these risk factors was likely to be nondifferential and unrelated to the disease diagnosed given all these factors would have been recorded within the EPRs prior to the detection of heart disease, thus at worst biasing ORs toward the null and reducing study power. Although the predictive ability of the model was good, the model fit was poor suggesting important variables not captured by our data might have improved the model explanation of the data if available. Neuter status at the time of diagnosis would have been useful to consider but these data were not available at the time of data analysis. Finally, a convenience sample of corporate and independently owned, companion animal veterinary clinics was studied. Whilst charity or mixed‐species practices might differ from our study population, data from a large number of practices were analysed, so the main conclusions are likely to be generalizable to the practice‐attending dog population in England.
In summary, this study estimated a high prevalence of HMs consistent with DMVD and highlighted that geriatric, small‐ to medium‐sized dogs were most likely to receive a diagnosis. Individual breeds, sex, insurance status, and veterinary practice attended were also associated with DMVD. These results could aid practitioners and provide insight into factors influencing DMVD diagnosis in the primary‐care setting.
Acknowlegments
Funding for this research was provided by Petplan Charitable Trust. The authors are grateful to the Medivet Veterinary Partnership and other veterinary practices and their clients who participated in this project. We thank Peter Dron, Noel Kennedy, and James Hoontrakul (Royal Veterinary College) for database development and management and Professors Dirk Pfeiffer and Virginia Luis Fuentes (Royal Veterinary College) for their advice on the study design. This manuscript has been approved by the Royal Veterinary College's publications approval system, to comply with Good Research Practice Policy on Publications (manuscript number PPH_00845).
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at the Royal Veterinary College, London, UK.
Footnotes
Microsoft Corp, Redmond, WA
Stata Corporation, TX College Station, Texas
References
- 1. Darke PG. Valvular incompetence in cavalier King Charles spaniels. Vet Rec 1987;120:365–366. [DOI] [PubMed] [Google Scholar]
- 2. Pedersen HD, Lorentzen KA, Kristensen BO. Echocardiographic mitral valve prolapse in cavalier King Charles spaniels: Epidemiology and prognostic significance for regurgitation. Vet Rec 1999;144:315–320. [DOI] [PubMed] [Google Scholar]
- 3. Lundin T, Kvart C. Evaluation of the Swedish breeding program for cavalier King Charles spaniels. Acta Vet Scand 2010;52:54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Serfass P, Chetboul V, Sampedrano CC, et al. Retrospective study of 942 small‐sized dogs: Prevalence of left apical systolic heart murmur and left‐sided heart failure, critical effects of breed and sex. J Vet Cardiol 2006;8:11–18. [DOI] [PubMed] [Google Scholar]
- 5. Thrusfield MV, Aitken CGG, Darker PGG. Observations on breed and sex in relation to canine heart valve incompetence. J Small Anim Pract 1985;26:709–717. [Google Scholar]
- 6. Detweiler DK, Patterson DF. The prevalence and types of cardiovascular disease in dogs. Ann N Y Acad Sci 1965;127:481–516. [DOI] [PubMed] [Google Scholar]
- 7. Das KM, Tashjian RJ. Chronic mitral valve disease in the dog. Vet Med Small Anim Clin 1965;60:1209–1216. [PubMed] [Google Scholar]
- 8. Whitney JC. Observations on the effect of age on the severity of heart valve lesions in the dog. J Small Anim Pract 1974;15:511–522. [DOI] [PubMed] [Google Scholar]
- 9. Jones TC, Zook BC. Aging changes in the vascular system of animals. Ann N Y Acad Sci 1965;127:671–684. [DOI] [PubMed] [Google Scholar]
- 10. Slater MR. Veterinary Epidemiology. Amsterdam and London: Butterworth‐Heinemann; 2003. [Google Scholar]
- 11. Pfeiffer D. Veterinary Epidemiology: An Introduction. Oxford: Wiley‐Blackwell; 2010. [Google Scholar]
- 12. Dohoo IR, Martin SW, Stryhn H. Veterinary Epidemiologic Research, 2nd ed Charlotte, P.E.I: VER, Inc; 2009. [Google Scholar]
- 13. Parker HG, Kilroy‐Glynn P. Myxomatous mitral valve disease in dogs: Does size matter? J Vet Cardiol 2012;14:19–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Borgarelli M, Zini E, D'Agnolo G, et al. Comparison of primary mitral valve disease in German Shepherd dogs and in small breeds. J Vet Cardiol 2004;6:27–34. [DOI] [PubMed] [Google Scholar]
- 15. Chetboul V, Tissier R, Villaret F, et al. [Epidemiological, clinical, echo‐doppler characteristics of mitral valve endocardiosis in Cavalier King Charles in France: A retrospective study of 451 cases (1995 to 2003)]. Can Vet J 2004;45:1012–1015. [PMC free article] [PubMed] [Google Scholar]
- 16. Haggstrom J, Hansson K, Kvart C, et al. Chronic valvular disease in the cavalier King Charles spaniel in Sweden. Vet Rec 1992;131:549–553. [PubMed] [Google Scholar]
- 17. Buchanan JW. Chronic valvular disease (endocardiosis) in dogs. Adv Vet Sci Comp Med 1977;21:75–106. [PubMed] [Google Scholar]
- 18. VetCompass . VetCompass: Health surveillance for UK companion animals. RVC ElectronicMedia Unit; 2013. Available at: http://www.rvc.ac.uk/VetCompass. Accessed August 20, 2013.
- 19. Kearsley‐Fleet L, O'Neill DG, Volk HA, et al. Prevalence and risk factors for canine epilepsy of unknown origin in the UK. Vet Rec 2013;172:338. [DOI] [PubMed] [Google Scholar]
- 20. O'Neill DG, Elliott J, Church DB, et al. Chronic kidney disease in dogs in UK veterinary practices: Prevalence, risk factors, and survival. J Vet Intern Med 2013;27:814–821. [DOI] [PubMed] [Google Scholar]
- 21. Mattin M, O'Neill D, Church D, et al. An epidemiological study of diabetes mellitus in dogs attending first opinion practice in the UK. Vet Rec 2014;174:349. [DOI] [PubMed] [Google Scholar]
- 22. Egenvall A, Bonnett BN, Haggstrom J. Heart disease as a cause of death in insured Swedish dogs younger than 10 years of age. J Vet Intern Med 2006;20:894–903. [DOI] [PubMed] [Google Scholar]
- 23. Olsen LH, Fredholm M, Pedersen HD. Epidemiology and inheritance of mitral valve prolapse in Dachshunds. J Vet Intern Med 1999;13:448–456. [DOI] [PubMed] [Google Scholar]
- 24. Olsen LH, Martinussen T, Pedersen HD. Early echocardiographic predictors of myxomatous mitral valve disease in dachshunds. Vet Rec 2003;152:293–297. [DOI] [PubMed] [Google Scholar]
- 25. VeNom coding group . VeNom Veterinary Nomenclature. Available at http://venomcoding.org 2013. Accessed August 20, 2013.
- 26. StataCorp . Stata: Release 13. Statistical Software. College Station, TX: StataCorp LP; 2013. Available at: http://www.stata.com/manuals13/u.pdf. Accessed August 6, 2014. [Google Scholar]
- 27. Heinze G, Schemper M. A solution to the problem of separation in logistic regression. Stat Med 2002;21:2409–2419. [DOI] [PubMed] [Google Scholar]
- 28. Bussadori C, Pradelli D, Borgarelli M, et al. Congenital heart disease in boxer dogs: Results of 6 years of breed screening. Vet J 2009;181:187–192. [DOI] [PubMed] [Google Scholar]
- 29. Fabrizio F, Baumwart R, Iazbik MC, et al. Left basilar systolic murmur in retired racing greyhounds. J Vet Intern Med 2006;20:78–82. [DOI] [PubMed] [Google Scholar]
- 30. Bavegems VC, Duchateau L, Polis IE, et al. Detection of innocent systolic murmurs by auscultation and their relation to hematologic and echocardiographic findings in clinically normal Whippets. J Am Vet Med Assoc 2011;238:468–471. [DOI] [PubMed] [Google Scholar]
- 31. The Kennel Club . Kennel Club's Breed Information Centre. Available at: http://www.thekennelclub.org.uk/services/public/breed/Default.aspx. Accessed December 5, 2014.
- 32. Borgarelli M, Buchanan JW. Historical review, epidemiology and natural history of degenerative mitral valve disease. J Vet Cardiol 2012;14:93–101. [DOI] [PubMed] [Google Scholar]
- 33. Borgarelli M, Haggstrom J. Canine degenerative myxomatous mitral valve disease: Natural history, clinical presentation and therapy. Vet Clin North Am Small Anim Pract 2010;40:651–663. [DOI] [PubMed] [Google Scholar]


