Abstract
Background
Gastric acid suppressants frequently are used in cats with acid‐related gastric disorders. However, it is not known if these drugs effectively increase intragastric pH in cats.
Objectives
To examine the effects of PO administered ranitidine and omeprazole on intragastric pH in cats and to compare the efficacy of once‐daily versus twice‐daily dosage regimens for omeprazole.
Animals
Eight domestic shorthair cats.
Methods
Using a randomized 4‐way cross‐over design, cats were given enteric‐coated omeprazole granules (1.1–1.3 mg/kg q24h and q12h), ranitidine (1.5–2.3 mg/kg q12h), and placebo. Intragastric pH was monitored continuously for 96 hours using the Bravo™ system1, starting on day 4 of treatment, followed by a median washout period of 12 days. Mean percentage of time pH was ≥3 and ≥4 was compared among groups using repeated measures ANOVA.
Results
Mean ± SD percentage of time intragastric pH was ≥3 and ≥4 was 67.0 ± 24.0% and 54.6 ± 26.4% for twice‐daily omeprazole, 24.4 ± 22.8% and 16.8 ± 19.3% for once‐daily omeprazole, 16.5 ± 9.0% and 9.6 ± 5.9% for ranitidine, and 9.4 ± 8.0% and 7.0 ± 6.6% for placebo administration. Twice‐daily omeprazole treatment significantly increased intragastric pH, whereas pH after once‐daily omeprazole and ranitidine treatments did not differ from that of placebo‐treated cats.
Conclusion and Clinical Importance
Only twice‐daily PO administered omeprazole significantly suppressed gastric acidity in healthy cats, whereas once‐daily omeprazole and standard dosages of ranitidine were not effective acid suppressants in cats.
Keywords: Acid suppressant, Feline, Omeprazole, Ranitidine
Abbreviations
- PO
per os
- PBS
phosphate‐buffered saline
Acid‐related gastric disorders result from an imbalance between gastric acid secretion and gastric acid mucosal defense mechanisms.1 In humans, gastric acidity plays an important role in gastric ulcer development, and numerous studies have been conducted on the efficacy of PO administration of acid‐suppressing drugs.2 In veterinary medicine, the role of gastric acidity in the pathogenesis of gastric erosive and ulcerative disease has received limited attention, and the most appropriate extent of gastric acid inhibition for acid‐related diseases has yet to be determined in small animals.3 Although gastric erosive disease is commonly suspected in cats, especially in critically ill and stressed cats, it is difficult to confirm because of the inherent invasiveness of gastric mucosal visual inspection. Instead, gastric acid suppressants including histamine‐2 receptor antagonists, such as ranitidine or famotidine, and proton pump inhibitors, such as omeprazole, are widely used empirically in this species.
Although some information on the antisecretory effects of commonly used acid suppressants (e.g. omeprazole, famotidine, ranitidine) is available for dogs,4, 5, 6 the clinical efficacy of antisecretory drugs in cats is largely unknown. The Existing data on feline gastric pH, derived from studies that used the cat as a model for humans, are difficult to interpret because gastric acid secretion was pharmacologically modified or experiments were carried out in anesthetised cats that had been vagotomized, and pH measurements were determined for only a few hours.7, 8, 9, 10 Despite the relative paucity of studies in cats, acid suppressant medications are commonly used in clinical practice, using dosages extrapolated from studies performed in dogs.4, 5
The recent introduction of pH monitoring devices such as the Bravo™ system1 has allowed noninvasive, continuous assessment of intragastric pH over prolonged periods. This technique has been evaluated for extended recordings of intragastric pH in dogs.4, 11 The effects of twice‐daily omeprazole versus standard dosages of famotidine on intragastric pH in cats recently have been reported using this new technique. The Results of this study indicated that omeprazole administration provided superior acid suppression compared with famotidine.12
The goals of the present study were to determine normal gastric acid profiles in healthy cats, to investigate the effect of omeprazole and ranitidine on intragastric pH in a placebo‐controlled study and to compare once‐daily and twice‐daily dosage regimens for omeprazole.
We hypothesized that omeprazole would be superior to ranitidine for achieving a sustained increase in gastric pH and that omeprazole administered twice daily would provide superior gastric acid control compared with a once‐daily dosage regimen.
Materials and Methods
Cats
Eight healthy European shorthair cats (2 intact females, 3 spayed females, 3 intact males), aged 5–6 years (median, 5.7 years) and weighing 4.3–6.8 kg (median, 5.3 kg) with median body condition score of 5/9, were used. All cats were research colony cats from the Institute of Animal Nutrition of our institution. The cats had no clinical signs of gastrointestinal disease for the past 6 months and were deemed healthy based on physical examination findings as well as the results of CBC, serum biochemistry profile and urinalysis. The study was approved by the Cantonal Veterinary Office of Zürich and conducted in accordance with the guidelines established by the Animal Welfare Act of Switzerland (permission no. 527/2013). Permission for the use of animals in our study specified that cats with anorexia, vomiting persisting >24 hours, weight loss exceeding 10% of body weight, or some combination of those would be excluded. During intragastric pH recording periods, pairs of cats were housed in 140 × 105 × 100 cm cages and had daily physical exercise.
Study Design
Using a randomized cross‐over design, cats received 1 of the following treatments PO for 7 consecutive days: placebo (empty gelatin capsule)2 q12h, ranitidine3 (1.5–2.3 mg/kg; median, 1.9 mg/kg) q12 h, omeprazole4 (1.1–1.3 mg/kg; median, 1.2 mg/kg) q24h, or omeprazole4 (1.1–1.3 mg/kg; median, 1.2 mg/kg) q12 h. The goal of treatment was to achieve a dosage of approximately 1 mg/kg for omeprazole, thus, 1 enteric‐coated granule containing 1.1 mg omeprazole was given per kg body weight (e.g. a 10 mg omeprazole capsule4 contained 9 enteric‐coated granules each consisting of 1.1‐mg omeprazole).5 The dosage of each drug was consistent among treatments for each cat. All drugs were administered in hard gelatin capsules.2 To facilitate swallowing, approximately 1 teaspoon of a highly palatable feline food6 was fed immediately after administration of the capsule. Cats were medicated daily at 6:30 am and 6:30 pm, 30 minutes before a standardized morning and evening meal.7 The once‐daily omeprazole treatment was given in the morning. One of the authors (ŠS) stayed with the cats for a minimum of 45 minutes after treatment to ensure that medication was not regurgitated or vomited. Each treatment period was followed by a median washout period of 12 days (range, 7–24 days). Attitude, appetite, body weight, number of defecations, and fecal consistency were recorded daily. Feces were graded from 1 to 7 (1, very hard; 7, watery) according to a standardized fecal scoring system.8
On day 4 of each treatment period, cats were anesthetized after a 12‐hour fast for endoscopy‐assisted placement of a pH capsule.1 Cats were premedicated with butorphanol9 (0.2 mg/kg IM) and medetomidine10 (5 μg/kg IM), an IV catheter was placed, and general anesthesia was induced with propofol and maintained with isoflurane. Before the first pH capsule placement, routine gastric and duodenal endoscopic biopsy was performed. The biopsy samples were fixed in 10% buffered formalin, embedded in paraffin, cut into 2‐μm sections and stained using a routine protocol with hematoxylin and eosin. Gastrointestinal biopsy specimens were assessed by a board‐certified pathologist (MR) according to World Small Animal Veterinary Association guidelines.13 All pH capsules were placed under direct endoscopic guidance by the same investigator (PHK). Immediately before placement, the capsules were calibrated with commercial buffer solutions (pH 1.07 and 7.01) according to the manufacturer's instructions.1 A drift of 0.1 pH units was tolerated. All pH capsules were anchored in the fundic area using the supplied delivery system that combined suction and a lock‐and‐pin mechanism.
The approach for gastric capsule placement was similar to what has been described recently in dogs,4, 11 with the exception that the external vacuum suction (510 mm Hg) applied to the capsule delivery system was decreased from approximately 30 seconds to a median of 20 seconds during the study.11 After capsule placement, gastric pH recordings were obtained telemetrically at 6‐second sampling intervals for 4 days (96 hours). The receiver was kept in close proximity outside of the feline's cage. After acquisition, pH data were uploaded from the receiver to the computer using the manufacturer software.7 Percentage of time intragastric pH was ≥3 and ≥4 and in 1 of each of 8 categories (pH 0–1, pH 1–2, up to pH 7–8) was calculated by the computer software. Throughout the study, all cats were subjected to visual inspection 4 times daily and were allowed to play in a separate enclosure twice daily. A chaperone (ŠS) was present during these times to entertain the cats and to ensure that the distance to the receiver was adequate.
Investigation of Capsule Dissolution
Because all medications were administered in hard gelatin capsules2, an experiment was conducted to examine the drug release time at different pH levels. Dissolution of gelatin capsules containing enteric‐coated omeprazole granules4 and ranitidine3 was examined visually in transparent cups containing 35 ml of phosphate‐buffered saline (PBS) buffer solution that was warmed to body temperature (37.5 °C) and had a pH of 1, 2, 3, 4, 5, 6, or 7.
Statistical Analyses
Commercially available software8 was used for analysis. Repeated measures ANOVA was used to analyze differences among the 4 treatment arms regarding (1) percentage of time intragastric pH was ≥3 and ≥4 during the 96‐hour period after pH capsule placement (days 4–7 of treatment), (2) percentage of time intragastric pH was in 1 of 8 pH categories (0–1, 1–2, 2–3, 3–4, 4–5, 5–6, 6–7, 7–8) for days 4–7 of treatment; and (3) adverse effects of treatments by comparing the 7‐day mean number of defecations with fecal scores ≥3 and ≥4.
In a second analysis, the different treatment arms were assessed separately and the effect of day was evaluated with repeated measures ANOVA. Assumption of sphericity was examined by Mauchly's test of sphericity and a Bonferroni correction was applied to multiple comparisons. Differences were considered significant at P < 0.05.
Results
Cats
All cats were alert and active and had a normal appetite throughout the study. A single episode of vomiting occurred 2 days after Bravo™ capsule placement in 1 of the cats in the twice‐daily omeprazole treatment arm.
Assessment of Fecal Scores
The occurrence of fecal scores >3 and >4 did not differ among treatment arms. The median fecal score was 3 for the placebo, 4 for ranitidine, 4 for once‐daily treatment with omeprazole, and 4 for twice‐daily administration of omeprazole.
Experience with the Bravo™ System1 in Cats
Overall, 32 Bravo™ capsules were successfully attached to the fundic mucosa. Total procedure times for endoscopy‐assisted capsule placement ranged from 5 to 8 minutes, with most procedures taking <5 minutes. In 1 cat, the stomach still contained food, and mucosal capsule attachment was unsuccessful because food remnants obstructed the suction hole. Capsule placement and pH measurement were repeated at a later time point. In 4 cases, the previously attached capsule was still in place at the time of the subsequent pH measurement (Fig. 1). Because endoscopic removal of the capsule with the help of endoscopic foreign body retrieval devices and polypectomy forceps was unsuccessful in the first case, a new capsule was attached next to the other 1 in these 4 cats. No problems were encountered with this approach. Passing of pH capsules through the digestive tract was verified by daily fecal examination in all cats. A total of 2,909 hours and 20 minutes of intragastric pH recording time (equivalent to 1,745,580 pH readings) was obtained. The maximum possible data acquisition was 3,072 hours (96 hours per cat per treatment). Therefore, the overall missing data rate was 5.3%. Missing data were caused by signal interference in the radiotelemetric system, which occurred intermittently in all treatment groups.
Figure 1.
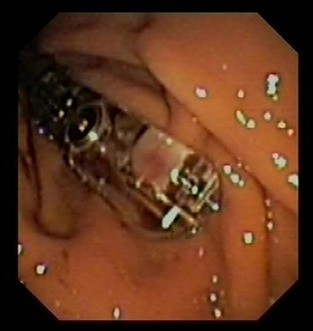
View of the Bravo™ capsule attached to the feline gastric mucosa. Compared to dogs,11 comparatively large parts of the gastric mucosa became lodged in the suction well of the capsule.
Endoscopic and Histologic Assessment of Gastrointestinal Biopsy Samples
Gastroduodenoscopic evaluation was normal in all cats. Histologic examination of endoscopic gastric biopsy samples showed severe colonization of the mucosal surface with spiral‐shaped organisms interpreted as gastric Helicobacter spp. in all of the cats. There were no concurrent lesions in the mucosa or lamina propria. The villi of the intestine were slender and had normal architecture with a crypt‐to‐villi ratio of 1:4. The epithelium was normal but in the lamina propria, small clusters of 9–12 neutrophils per high power field (hpf) were observed in 5 cats. The remaining 3 cats had smaller clusters of 4–5 neutrophils per hpf scattered in the lamina propria. The final histologic diagnosis was gastric colonization by Helicobacter spp. Duodenal tissue was deemed normal.
Assessment of Capsule Dissolution
The capsules started to swell within 2 minutes of being placed in the PBS buffer solution. Complete disintegration of the capsules and release of the contents occurred within 5 minutes at all pH levels.
Intragastric pH Recordings
With respect to percentage of time intragastric pH was ≥3 and ≥4, twice‐daily omeprazole had a significantly greater effect than once‐daily omeprazole, ranitidine, and placebo administration. There were no significant differences among the latter 3 treatment arms.
Mean ± SD percentage of time intragastric pH was ≥3 and ≥4 was 67.0 ± 24.0% and 54.6 ± 26.4% for twice‐daily omeprazole administration, 24.4 ± 22.8% and 16.8 ± 19.3% for once‐daily omeprazole administration, 16.5 ± 9.0% and 9.6 ± 5.9% for ranitidine administration, and 9.4 ± 8.0 and 7.0 ± 6.6% for placebo administration (Fig. 2).
Figure 2.
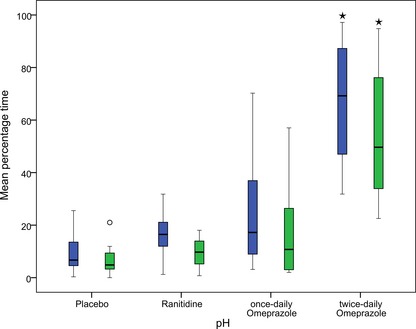
Comparison of efficacy of various treatments on intragastric pH. Box plots show variation in percentage of time intragastric pH was ≥3 (blue) and ≥4 (green) for 8 cats given placebo, ranitidine, once‐daily omeprazole and twice‐daily omeprazole. *Results were significantly increased compared with placebo, ranitidine, and once‐daily omeprazole (P = 0.011 for pH ≥3; P = 0.044 for pH ≥4).
The treatment arms differed with respect to the distribution of intragastric pH over pH categories 1–8 (Fig. 3). For pH category 1–2, twice‐daily omeprazole administration differed significantly from the other treatment arms (placebo, P = 0.02; once‐daily omeprazole, P = 0.024; ranitidine, P = 0.040). For categories 3–4, 4–5, 5–6, and 6–7, twice‐daily omeprazole differed significantly from placebo, and for pH categories 4–5, 5–6, and 6–7, ranitidine differed significantly from placebo. To summarize, intragastric pH ranged widely across all pH categories with all treatments; but twice‐daily omeprazole resulted in the largest amount of time intragastric pH was in categories 3–4 to 6–7.
Figure 3.
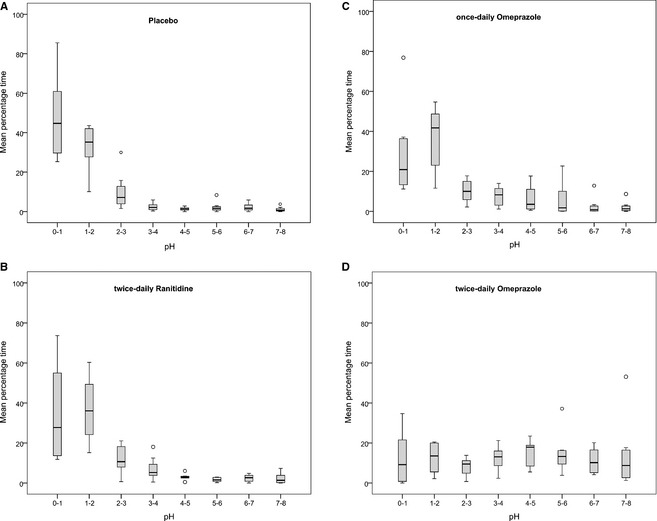
Box plots representing the intragastric pH distribution for 8 cats receiving placebo (A), ranitidine (B), once‐daily omeprazole (C), and twice‐daily omeprazole (D). Circles represent outliers.
Comparison of treatment days within a given treatment identified a significant increase in intragastric pH between days 4 and 7 only for twice‐daily omeprazole (P = 0.011 for pH ≥3; P = 0.044 for pH ≥4).
Discussion
Although more commonly encountered in dogs, gastric erosive and ulcerative disease can exacerbate a range of gastrointestinal, metabolic, or neoplastic conditions14 or be a complication of nonsteroidal anti‐inflammatory drug administration in cats.15 Gastric acid suppressants currently are the treatment of choice for these conditions. Because information on the efficacy of antisecretory drugs is scarce in cats,9 , 2 the goal of the present study was to compare the effects of PO administered gastric acid suppressants commonly used in cats. Our study clearly demonstrated that twice‐daily omeprazole administration provided superior gastric acid suppression compared with a once‐daily dosage regimen and with standard dosages of ranitidine and placebo based on percentage of time intragastric pH was ≥3 and ≥4. The conceptual basis for these cut‐offs has been explored by meta‐analysis of many trials in human medicine, and these cut‐offs are now considered ideal for promoting optimal gastrointestinal ulcer healing.16, 17 Comparison of once‐daily and twice‐daily omeprazole administration was chosen because additional drug administration could pose a substantial problem for sick cats and would likely result in poor owner compliance. A twice‐daily dosage regimen used in small animals has been largely extrapolated from studies of humans.18 A simplified omeprazole suspension (enteric‐coated granules suspended in 8.4% sodium bicarbonate) administered to dogs as a bolus through a stomach tube resulted in superior gastric acid suppression over 24 hours compared with once‐daily omeprazole.5 Another study in dogs found that the effect of once‐daily administration of reformulated omeprazole paste used in horses for gastric acid secretion waned substantially during 24 hours after administration.4 It therefore was postulated that a twice‐daily dosage regimen was more beneficial. It was surprising that the once‐daily dosage of omeprazole in the present study had poor results in cats because comparable omeprazole dosages in dogs yielded a mean percentage of time with intragastric pH ≥3 of 70.2% and ≥4 of 52.3%.5 Species‐specific differences such as increased de novo biosynthesis of proton pumps may account for this difference because restoration of acid secretion is dependent on pump biosynthesis.18 Delayed gastric release of omeprazole from the gelatin capsule with inadequate intestinal absorption caused by variability in the hardness of the capsule wall9 was ruled out as a cause of the unexpected low intragastric pH because the capsules were shown to dissolve quickly at all pH levels.
The effects of ranitidine and placebo on intragastric pH did not differ in the present study, which is in contrast to a recent report that PO administered famotidine in cats had better antisecretory efficacy than placebo.2 Ranitidine and famotidine were shown to have poor antisecretory properties in dogs.4, 5 We anticipated similar results, based on observations that plasma gastrin concentrations did not increase in cats undergoing long‐term ranitidine treatment.9 This indicates that ranitidine has weak antisecretory properties because gastrin release is inhibited by the presence of acid in the stomach by a negative feedback mechanism. Nevertheless, we felt it was important to provide substantial evidence that ranitidine also is a weak acid suppressant in cats and therefore should not be used for treatment of acid‐related gastric disorders in cats.
We used enteric‐coated omeprazole granules rather than splitting tablets because a dosage of 1 mg/kg can be more easily approximated by administering 1 granule per kg body weight (1 granule contains 1.1 mg omeprazole),4 which is convenient in small patients. Omeprazole granules are also used in human pediatric patients and when medication must be given through a feeding tube.19, 20, 21 Moreover, it was felt that splitting enteric‐coated omeprazole tablets may adversely affect drug efficacy. However, results published during the course of the present investigation showed that fractionated enteric‐coated omeprazole tablets remained effective acid suppressants in cats despite disruption of the enteric coating.12
To facilitate swallowing of the capsule, a teaspoon of highly palatable canned feline food6 was fed immediately after pill administration. Administration of water by syringe usually is recommended after oral drug administration in cats.22 However, the cats in our study did not tolerate syringe feeding of water, but readily ate the small amount of food6. This procedure is likely more practical for owners who administer drugs to their cats at home, and we do not believe that the small amount of food compromised the efficacy of the medication. The administration of omeprazole granules to children in an acidic or alkaline solution or mixed with apple sauce resulted in acid suppression comparable with that of intact capsules in humans.19, 23 Similarly, a study of omeprazole absorption in humans indicated that the area under the curve of omeprazole was similar, and that the total amount of drug absorbed was not affected when the granules were given immediately before or after breakfast.24
We chose the Bravo™ pH monitoring system1 because we found it reliable and minimally invasive for extended continuous gastric pH monitoring in dogs.11 This system allows longer measurement periods than catheter‐based pH probes.5 Compared with dogs, a shorter vacuum application time (approximately 20 seconds) worked better for pH capsule placement in cats. In the 4 cats with pH capsules still in place at the time of second capsule application, the vacuum was applied for ≥25 seconds, and we believe a larger part of the gastric mucosa became lodged in the suction well of the capsule (Fig. 1) compared with what we observed in dogs.11 Inclusion of the lamina muscularis may have added to the rigidity of the attachment. Attempts to remove the first capsule failed, and we left them in place. We were aware of a case of gastric perforation related to endoscopic removal of a Bravo™ capsule,25 , 1 and it is possible that our attempts at removal were too conservative.
Soft feces has been associated with PO administered omeprazole treatment in humans and dogs,4, 5, 26 but how suppression of gastric acid secretion predisposes patients to this adverse effect is not well understood. We used fecal score cut‐offs of 3 and 4 and found that the treatments did not differ with respect to feces consistency.
Histologic examination of gastric and duodenal biopsy samples was done to rule out occult gastrointestinal disease that could have interfered with parietal cell function or duodenal drug absorption. In all cats, the gastric mucosa was heavily colonized with Helicobacter spp., but no evidence of gastritis was observed. In humans, the relationship between gastritis caused by H. pylori and gastric acid secretion is controversial, and normal acid secretion as well as hyper‐ and hypo‐chlorhydria have been reported.1 Although the severity of gastritis did not seem to be correlated with gastric acid secretion, omeprazole treatment resulted in higher gastric pH in human patients infected with H. pylori than in patients free of H. pylori.27 This effect of omeprazole is believed to be present because of the neutralization of substances produced by H. pylori.27 We believe this scenario to be unlikely in the cats of our study because H. pylori infection is extremely rare in cats28 and evidence of gastritis was not detected in the present study. Given the overall high prevalence of gastric mucosal colonization with Helicobacter spp. without concurrent gastritis in cats,28 we concluded that gastric histology results were typical of what is seen in cats. The principal goal of histologic examination was to rule out occult gastric disease, which could have affected gastric acid secretion, and small bowel disease,29 which could have affected drug absorption. The relationship between gastric acid secretion and Helicobacter spp. colonization in cats requires further study.
A significant increase in intragastric pH between days 4 and 7 was only recorded after twice‐daily omeprazole administration, which was similar to reports in humans in which multiple omeprazole doses were needed for optimal effect.23, 30 True drug efficacy might be greater after multiple doses than what we were able to document during the 96‐hour study period. Considering the strong and long‐lasting attachment of the capsule to the gastric mucosa in cats when using a vacuum time >25 seconds, it should be possible to study the efficacy of gastric acid suppressants for longer than 4 days.
We saw considerable individual variation in response to treatment with omeprazole and much less so with ranitidine. For example, 1 cat consistently had close to 100% acid suppression (e.g. percentage of time pH was ≥4 was 95% when treated with twice‐daily omeprazole), whereas another had extremely poor acid suppression (percentage of time pH was ≥4 was 3% when treated with twice‐daily omeprazole). These findings are similar to those observed in humans23, 31 and may be a consequence of genetic polymorphism in the hepatic cytochrome P‐450 system involved in the metabolism of omeprazole, which is a well‐established explanation for people who do not respond to gastric acid suppressants.32 In fact, recent studies on the acid suppressant effects of proton pump inhibitors in humans include analysis of cytochrome 450 genes and report pH measurements based on results of genotyping (i.e. extensive, intermediate, and poor metabolizers).33
The washout periods used in the present study varied slightly among treatments subject to staff availability. However, we feel that the protocols used in our experiments precluded drug carryover effects. The minimum washout period of 7 days (n = 5) pertained to 3 examinations preceded by placebo and 2 preceded by ranitidine. The next shortest washout period was 9 days (n = 2), pertaining to 2 examinations preceded by once‐daily omeprazole. A minimum of 1 week washout period was chosen because full restoration of gastric acid secretion was shown 7 days after long‐term administration of ranitidine in cats9 and 5 days after long‐term administration of omeprazole in people.34
In conclusion, twice‐daily administration of omeprazole granules appears to be the treatment of choice for cats with acid‐related gastrointestinal disease. Ranitidine and once‐daily omeprazole cannot be recommended as acid suppressants in cats.
Acknowledgments
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The study was conducted at the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty, University of Zurich, and was supported by a grant from the Stiftung für Kleintiere, Vetsuisse Faculty, University of Zurich, Zurich, Switzerland.
Footnotes
Bravo™ pH monitoring system, Given Imaging, Yoqneam, Israel.
Gelatin capsules size 5, Interdelta SA, 1762 Givisiez, Switzerland.
Ranitidin 20 mg, Christoffel‐Apotheke, Christoffelgasse 3, 3001 Bern, Switzerland.
Omezol‐Mepha MT 10, Mepha Pharma AG, 4010 Basel, Switzerland.
email communication with Ms. Linda Kötter‐Spirgi (07.24.2014), Mepha Pharma AG, 4010 Basel, Switzerland.
Hill's Prescription Diet Canine/Feline a/d.
Hill's Science Diet Optimal Care Original Adult Cat Food.
Faecal Scoring System, Nestle Purina PetCare Company, St Louis, MO, USA.
Morphasol, Graeub AG, Rehhagstrasse 83, 3018 Bern, Switzerland
Dorbene, Graeub AG, Rehhagstrasse 83, 3018 Bern, Switzerland.
email communication with Dr. Katie Tolbert (21.10.2013)
Polygram Net Software, Given Imaging, Yoqneam, Israel.
SPSS, version 11, SPSS Inc, Chicago, IL, USA.
References
- 1. Schubert ML, Kaunitz JD. Gastric Secretion In: Feldman M, Friedman LS, Brandt LJ, eds. Sleisenger and Fordtran's Gastrointestinal and liver disease, 9th ed Philadelphia, PA: WB Saunders; 2010;817–832. [Google Scholar]
- 2. Sachs G, Shin JM, Howden CW. Review article: the clinical pharmacology of proton pump inhibitors. Aliment Pharmacol Ther 2006;23(Suppl 2):2–8. [DOI] [PubMed] [Google Scholar]
- 3. Bersenas AME. Antacid Therapy In: ed. Kirk's Current Veterinary Therapy, 15th ed Philadelphia, PA: WB Saunders; 2014; 505–508. [Google Scholar]
- 4. Tolbert K, Bissett S, King A, et al. Efficacy of oral famotidine and 2 omeprazole formulations for the control of intragastric pH in dogs. J Vet Intern Med 2011;25:47–54. [DOI] [PubMed] [Google Scholar]
- 5. Bersenas A, Mathews K, Allen D, et al. Effects of ranitidine, famotidine, pantoprazole, and omeprazole on intragastric pH in dogs. Am J Vet Res 2005;66:425–431. [DOI] [PubMed] [Google Scholar]
- 6. Williamson KK, Willard MD, Payton ME, et al. Efficacy of omeprazole versus high‐dose famotidine for prevention of exercise‐induced gastritis in racing Alaskan sled dogs. J Vet Intern Med 2010;24:285–288. [DOI] [PubMed] [Google Scholar]
- 7. Chiavarini M, Barocelli E, Ballabeni V, et al. Omeprazole‐like compounds on histamine‐stimulated acid and peptic secretions in conscious dog and cat. Boll Soc Ital Biol Sper 1992;68:429–436. [PubMed] [Google Scholar]
- 8. Scarpignato C, Tramacere R, Tangwa M, et al. Effect of the new H2‐receptor antagonist mifentidine on gastric acid secretion in the cat: Comparison with cimetidine and ranitidine. Arch Int Pharmacodyn Ther 1985;276:142–151. [PubMed] [Google Scholar]
- 9. Coruzzi G, Bertaccini G. Increased parietal cell sensitivity after chronic treatment with ranitidine in the conscious cat. Agents Actions 1989;28:215–217. [DOI] [PubMed] [Google Scholar]
- 10. Fändriks L, Jönson C. Effects of acute administration of omeprazole or ranitidine on basal and vagally stimulated gastric acid secretion and alkalinization of the duodenum in anaesthetized cats. Acta Physiol Scand 1990;138:181–186. [DOI] [PubMed] [Google Scholar]
- 11. Kook PH, Kempf J, Ruetten M, et al. Wireless ambulatory esophageal pH monitoring in dogs with clinical signs interpreted as gastroesophageal reflux. J Vet Intern Med 2014;28:1716–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Parkinson S, Tolbert K, Messenger K, et al. Evaluation of the effect of orally administered acid suppressants on intragastric pH in cats. J Vet Intern Med 2015;29:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008;138(Suppl 1):S1–43. [DOI] [PubMed] [Google Scholar]
- 14. Liptak JM, Hunt GB, Barrs VR, et al. Gastroduodenal ulceration in cats: Eight cases and a review of the literature. J Feline Med Surg 2002;4:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Cariou MP, Halfacree ZJ, Lee KC, et al. Successful surgical management of spontaneous gastric perforations in three cats. J Feline Med Surg 2010;12:36–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Howden CW. Appropriate acid suppression in the treatment of acid‐related conditions. Pharmacol Ther 1994;63:123–34. [DOI] [PubMed] [Google Scholar]
- 17. Sachs G. Proton pump inhibitors and acid‐related diseases. Pharmacotherapy 1997;17:22–37. [PubMed] [Google Scholar]
- 18. Shin JM, Sachs G. Restoration of acid secretion following treatment with proton pump inhibitors. Gastroenterology 2002;123:1588–1597. [DOI] [PubMed] [Google Scholar]
- 19. Zimmermann AE, Walters JK, Katona BG, et al. A review of omeprazole use in the treatment of acid‐related disorders in children. Clin Ther 2001;23:660–679. [DOI] [PubMed] [Google Scholar]
- 20. Israel DM, Hassall E. Omeprazole and other proton pump inhibitors: Pharmacology, efficacy, and safety, with special reference to use in children. J Pediatr Gastroenterol Nutr 1998;27:568–579. [DOI] [PubMed] [Google Scholar]
- 21. Marchetti F, Gerarduzzi T, Ventura A. Proton pump inhibitors in children: A review. Dig Liver Dis 2003;35:738–746. [DOI] [PubMed] [Google Scholar]
- 22. Westfall DS, Twedt DC, Steyn PF, et al. Evaluation of esophageal transit of tablets and capsules in 30 cats. J Vet Intern Med 2001;15:467–470. [DOI] [PubMed] [Google Scholar]
- 23. Mohiuddin MA, Pursnani KG, Katzka DA, et al. Effective gastric acid suppression after oral administration of enteric‐coated omeprazole granules. Dig Dis Sci 1997;42:715–719. [DOI] [PubMed] [Google Scholar]
- 24. Andersson T, Bergstrand R, Cederberg C. Influence of acid secretory status on absorption of omeprazole from enteric coated granules. Br J Clin Pharmacol 1991;31:275–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Tolbert MK, Odunayol A, Craig LE. Gastric perforation following endoscopic removal of a BRAVO pH capsule in a cat. J Feline Med Surg, 2015 Mar 13. pii: 1098612X15576588. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Davis MS, Willard MD, Nelson SL, et al. Efficacy of omeprazole for the prevention of exercise‐induced gastritis in racing Alaskan sled dogs. J Vet Intern Med 2003;17:163–166. [DOI] [PubMed] [Google Scholar]
- 27. Verdú EF, Armstrong D, Idström JP, et al. Effect of curing Helicobacter pylori infection on intragastric pH during treatment with omeprazole. Gut 1995;37:743–748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Lecoindre P, Chevallier M, Peyrol S, et al. Gastric helicobacters in cats. J Feline Med Surg 2000;2:19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Norsworthy GD, Scot Estep J, Kiupel M, et al. Diagnosis of chronic small bowel disease in cats: 100 cases (2008‐2012). J Am Vet Med Assoc 2013;243:1455–1461. [DOI] [PubMed] [Google Scholar]
- 30. Hartmann M, Theiss U, Huber R, et al. Twenty‐four‐hour intragastric pH profiles and pharmacokinetics following single and repeated oral administration of the proton pump inhibitor pantoprazole in comparison to omeprazole. Aliment Pharmacol Ther 1996;10:359–366. [DOI] [PubMed] [Google Scholar]
- 31. Kuo B, Castell DO. Optimal dosing of omeprazole 40 mg daily. Effects on gastric and esophageal pH and serum gastrin in healthy controls. Am J Gastroenterol 1996;91:1532–1538. [PubMed] [Google Scholar]
- 32. Klotz U. Clinical impact of CYP2C19 polymorphism on the action of proton pump inhibitors: A review of a special problem. Int J Clin Pharmacol Ther 2006;44:297–302. [DOI] [PubMed] [Google Scholar]
- 33. Shin JM, Kim N. Pharmacokinetics and pharmacodynamics of the proton pump inhibitors. J Neurogastroenterol Motil 2013;19:25–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Müller P, Dammann HG, Seitz H, et al. Effect of repeated, once daily, oral omeprazole on gastric secretion. Lancet 1983;1:66. [DOI] [PubMed] [Google Scholar]


