Abstract
Background
Hypertension is a common problem in older cats, most often associated with chronic kidney disease (CKD). Cross‐sectional studies have suggested that blood pressure in cats increases with age.
Hypothesis/Objectives
To determine whether blood pressure in cats increases with age and whether this occurs independently of the presence of CKD. To investigate risk factors for developing hypertension.
Animals/Subjects
Two hundred and sixty‐five cats with CKD and 133 healthy cats ≥9 years were retrospectively identified.
Methods
Four groups were created according to status at initial evaluation (CKD or healthy) and blood pressure at the last included visit (normotensive [NT] or developed hypertension [DH]): Healthy‐NT, Healthy‐DH, CKD‐NT and CKD‐DH. Systolic blood pressure (SBP) over time slopes were compared with 0 and between groups. Risk factors for the development of hypertension were investigated, and associations of biochemical and clinical variables with SBP were examined.
Results
Cats that were hypertensive at CKD diagnosis (n = 105) were not included in further analyses. Twenty‐seven cats with CKD and 9 healthy cats developed hypertension ≥3 months after diagnosis of CKD or their first visit. Systolic blood pressure significantly increased with age in all cats (P < .001). Healthy cats were at less risk than cats with CKD to become hypertensive (hazard ratio 0.2, P < .001), with creatinine being an independent risk factor for the development of hypertension.
Conclusions and Clinical Importance
The high prevalence of hypertension in azotemic cats in this study shows the importance of monitoring of SBP in elderly cats, and in particular in cats with CKD.
Keywords: Feline, Hypertension, Renal disease, Risk factors
Abbreviations
- BPM
beats per minute
- CKD
chronic kidney disease
- DH
developed hypertension
- IQR
interquartile range
- NT
remained normotensive
- PCV
packed cell volume
- RAAS
renin‐angiotensin‐aldosterone system
- SBP
systolic blood pressure
- USG
urine specific gravity
Hypertension is a common problem in older cats but, in contrast to humans, where 95–99% cases of hypertension are considered to be primary or essential hypertension, hypertension in cats is most often associated with underlying diseases such as chronic kidney disease (CKD).1 Increased serum creatinine concentrations occur in up to 74% of hypertensive cats and 19–65% of cats with CKD are hypertensive.2, 3, 4, 5 In humans, the prevalence of CKD increases with increasing age, and the same is true for cats.6, 7 Mean values of systolic blood pressure (SBP) also increase with age in most human populations, as does the prevalence of clinical hypertension.8, 9 In cats, it has been suggested that age is a predisposing factor for the development of hypertension. A cross‐sectional study grouping healthy cats into 3 age groups (<5, 5–10 and older than 10 years) showed higher SBP in the older age groups, although renal function was not assessed in all cats that were classified as healthy.10 There is a positive correlation between age and mean arterial pressure, but the age distribution of the cats included in this study is not known, which precludes interpretation of the results.11
Chronic kidney disease and hypertension are both common in older cats and current consensus is that hypertension is secondary to CKD, although the possibility still exists that the hypertension occurs independently of the presence of CKD or actually causes the renal injury.12 The question can be raised whether this increase in prevalence of hypertension and CKD in older cats is purely age related, or whether the development of hypertension in most cats is secondary to an inability of the kidney to regulate blood pressure.12 Investigations into the risk factors for blood pressure to increase in both healthy older cats and cats with CKD may aid in answering this question. The hypothesis of this study is that the blood pressure increases with age in senior cats and that the rate of this increase, and therefore the occurrence of clinically significant hypertension, is greater in cats with CKD.
Materials and Methods
Case Selection
Cats that were 9 years of age or older and examined for the first time at 2 first opinion practices in central London (People's Dispensary for Sick Animals in Bow and Beaumont Sainsbury's Animal Hospital in Camden) between August 2000 and August 2012 were retrospectively identified. All cats had a follow‐up period of ≥3 months and had visited 1 of the 2 veterinary practices a minimum of 3 times. On every visit, a full history was obtained and a physical examination was performed. Systolic blood pressure was measured at all visits using a noninvasive Doppler technique1 after a period of acclimatization and the SBP was calculated as the average of 5 consecutive readings. Indirect fundoscopy after applying 1 drop of tropicamide 1% to both eyes was performed at the end of the consultation if average SBP was ≥160 mmHg. At recruitment into the study, the owner consented to collection of blood samples via jugular venipuncture and urine samples by cystocentesis. Blood samples were collected into lithium heparin and held on ice (4°C) for a maximum of 6 hours before centrifugation2 and separation. Plasma biochemistry was performed at an external laboratory3. Total thyroxine concentration was assessed in all cats that showed clinical signs of hyperthyroidism (eg, polyphagia, weight loss, tachycardia, and palpable goiter) or had plasma biochemical findings that raised concern (increased alanine aminotransferase or alkaline phosphatase activity). If the bladder was palpable and a urine sample could be collected, a dipstick evaluation was performed and a urine specific gravity (USG) was measured in all cats. In addition to this, the sediment of samples was evaluated microscopically and, if bacteria or an active sediment was found, urine was sent for bacterial culture and sensitivity testing. All cats diagnosed with hyperthyroidism (total T4 > 55 nmol/L) were excluded from the study, unless a thyroidectomy had been performed ≥90 days before inclusion, to allow stabilization of glomerular filtration rate.13 Visits from cats that were being treated with glucocorticoids, nonsteroidal anti‐inflammatory drugs, antihypertensive medications, erythropoietin, or intravenous fluid therapy were excluded.
For the purposes of this study, systemic hypertension was diagnosed when cats had a SBP ≥170 mmHg on 2 consecutive visits (reliably placing them in the category that is at moderate risk to develop target organ damage)12 or SBP ≥160 mmHg with concurrent evidence of hypertensive retinopathy or choroidopathy on indirect fundoscopy. All hypertensive cats were started on amlodipine at a dose of 0.625 mg once daily at the time of diagnosis. CKD was diagnosed in cats with renal azotemia (plasma creatinine concentration ≥2.0 mg/dL either on 2 consecutive visits or in conjunction with USG <1.035). All owners of cats diagnosed with CKD were advised to feed their cat a commercially available renal diet. Cats were considered healthy if no significant clinical signs were reported by the owner, no abnormalities were found on clinical exam, and the blood results showed no evidence of CKD or other pathologies. Cats were classified according to the diagnosis of CKD and their SBP—the CKD‐normotensive group (CKD‐NT‐group) was composed of cats diagnosed with CKD that did not meet the hypertensive criteria during follow‐up, cats in the CKD‐developed‐hypertension group (CKD‐DH‐group) developed hypertension ≥3 months after diagnosis of CKD, cats that met the criteria for being healthy were included in the healthy‐normotensive group (Healthy‐NT‐group), and cats were included in the healthy‐developed‐hypertension group (Healthy‐DH‐group) if the criteria for being healthy were met, but the cat was diagnosed with idiopathic hypertension during follow‐up of ≥3 months.
Re‐examination of cats diagnosed with CKD was offered at a maximum of 8 week intervals and blood‐ and urine samples were obtained at a maximum of 16 week intervals. Re‐examination of healthy cats was offered at a maximum of 6 month intervals, at which point blood‐ and urine samples were obtained. If a healthy cat was diagnosed with CKD, its penultimate visit before developing azotemia was excluded from the analysis as it is likely that nonazotemic CKD was already present at that stage.
Statistical Analyses
All statistical analyses were performed using commercially available software4. P‐values ≤.05 were considered significant. Descriptive statistics are presented to define the population, SBP, biochemical and clinical variables at the first and last visit, and are presented as median and interquartile range (IQR). Comparisons between groups were performed using a nonparametric Kruskall‐Wallis or parametric ANOVA with post‐hoc comparisons where appropriate. Continuous variables were graphically assessed for normality and values were log transformed if normality criteria were not met to allow use in the linear mixed effects model and the time‐dependent Cox proportional hazards model.
Systolic blood pressure at the first visit between all 4 groups was compared using a Kruskal–Wallis test with a Bonferroni adjusted post‐hoc comparison. The rates of change in SBP over time and associations between SBP and biochemical (sodium, chloride, phosphate, total calcium, potassium, creatinine, packed cell volume (PCV), albumin, cholesterol) and clinical (weight, heart rate) variables over time were compared between all groups using a linear mixed effects model with subjects as random effects, and time, group and sex as fixed effects. In the CKD‐DH‐group and Healthy‐DH‐group, the actual SBP measurement that resulted in the diagnosis of hypertension was not included in the analysis. In the CKD‐NT‐group and Healthy‐NT‐group all available visits were included. Not all cats had all information available at all visits as, in general, blood samples were taken every other visit, but having a SBP measurement was a requirement for the visit to be included in the models. Change in SBP over time is expressed as ∆mmHg/100 days. A time‐dependent Cox proportional hazards model and Kaplan–Meier curves were used to assess the association of the biochemical and clinical variables with the risk of becoming hypertensive. The assumption of proportional hazards was checked for all variables included in the model and cases were censored if they died, were lost to follow‐up (defined as not seen for >6 months and not contactable by telephone), or if the study end point was reached. To investigate the independent association of the significant variables with SBP (linear mixed model) and the risk of becoming hypertensive (time dependent Cox proportional hazards model), all significant variables at the P < .10 level were included in a multivariable analysis.
Results
A total of 265 cats were diagnosed with azotemic CKD between August 2000 and August 2012 and were seen for 3 or more visits, over the course of more than 3 months. Of these cats, 105 were hypertensive at diagnosis of CKD or were diagnosed with systemic hypertension within 3 months of CKD diagnosis. These cats were excluded from further analyses. Of the remaining 160 cats with CKD, 133 (83%) remained normotensive throughout follow‐up (CKD‐NT group), and 27 cats of the 160 cats that were initially normotensive (17%) developed hypertension ≥3 months after CKD diagnosis (CKD‐DH group). A total of 133 cats were included as healthy cats in this study of which 9 (7%) developed hypertension (Healthy‐DH group), and 124 healthy cats remained normotensive throughout follow‐up (Healthy‐NT group).
Demographic Data
An overview of the clinical and biochemical variables at baseline and at the last visit (NT‐groups: last included visit; DH‐groups: hypertensive visit) is presented in Tables 1 and 2. The majority of cats included in the 4 groups were domestic short hair (n = 229), followed by domestic long hair (n = 34), and 147 cats were female (of which 4 not neutered) and 146 cats male (of which 4 not neutered). Of the 105 cats that were diagnosed as hypertensive at or within 3 months of CKD diagnosis, 73 (70%) had visible retinal lesions, whereas of the CKD‐DH group 52% (14 cats) and of the Healthy‐DH group 3 cats (33%) had evidence of hypertensive retinopathy.
Table 1.
Clinicopathologic variables for all groups at initial visit
| Variable | CKD‐NT (n = 133) | CKD‐DH (n = 27) | Healthy‐NT (n = 124) | Healthy‐DH (n = 9) |
|---|---|---|---|---|
| Age (years) | 14 (12, 16)a | 14 (13, 15)a | 12 (10, 13)b | 11 (10, 14)ab |
| Weight (kg) | 3.9 (3.3, 4.7)a | 3.7 (3.4, 4.7)ab | 4.6 (3.6, 5.3)b | 4.7 (3.9, 5.8)ab |
| SBP (mmHg) | 133.2 (121.2, 144.6)a | 147.2 (140.4, 156.1)b | 131.6 (115.0, 143.7)c | 145.6 (139.5, 154)ab |
| Heart rate (bpm) | 186 (165, 204) | 184 (164, 204) | 180 (168, 197) | 180 (176, 190) |
| PCV (%) | 34 (32, 38)a | 36 (31, 39)a | 39 (36, 41)b | 36 [34, 38)ab |
| Albumin (g/dL) | 3.1 (3.0, 3.3)a | 3.1 (2.9, 3.2)ab | 3.2 (3.1, 3.4)b | 3.3 (2.9, 3.5)ab |
| Creatinine (mg/dL) | 2.4 (2.2, 2.9)a | 2.5 (2.3, 2.8)a | 1.5 (1.4, 1.7)b | 1.5 (1.5, 1.6)b |
| Urea (mmol/L) | 16.9 (14.4, 20.2)a | 17.0 (13.6, 20.0)a | 9.9 (8.9, 11.2)b | 9.5 (8.4, 10.5)b |
| Phosphate (mg/dL) | 4.22 (3.63, 5.12)a | 3.97 (3.43, 4.73)ab | 3.84 (3.3, 4.73)b | 4.12 (3.47, 4.46)ab |
| Total calcium (mg/dL) | 10.2 (9.9, 10.4)a | 9.9 (9.7, 10.6)ab | 9.8 (9.5, 10.3)b | 10.1 (9.8, 10.6)ab |
| Sodium (mEq/L) | 153.0 (151.3, 154.8)a | 151.7 (150.5, 152.5)a | 152.4 (151.3, 153.7)a | 151.0 (150.0, 152.5)b |
| Potassium (mEq/L) | 4.10 (3.80, 4.40) | 4.10 (3.90, 4.38) | 3.90 (3.70, 4.20) | 3.80 (3.60, 4.25) |
| Chloride (mEq/L) | 117.8 (116.3, 120.2)ab | 116.0 (114.8, 118.9)a | 119.3 (117.2, 120.6)b | 120.4 (116.5, 121.2)ab |
| Cholesterol (mg/dL) | 200 (154, 239) | 201 (160, 234) | 178 (151, 212) | 166 (154, 205) |
| USG | 1.020 (1.017, 1.024)a | 1.017 (1.016, 1.022)a | 1.050 (1.038, 1.060)b | 1.042 (1.039, 1.050)b |
CKD‐DH, CKD‐developed‐hypertension; CKD‐NT, CKD‐remained normotensive; SBP, systolic blood pressure; PCV, packed cell volume; USG, urine specific gravity.
Parameters shown are at first visit for all cats. Superscript letters identify groups, which differed significantly.
Table 2.
Clinicopathologic variables for all groups at last visit
| Variable | CKD‐NT (n = 133) | CKD‐DH (n = 27) | Healthy‐NT (n = 124) | Healthy‐DH (n = 9) |
|---|---|---|---|---|
| Follow‐up (days) | 316 (225, 512) | 379 (281, 771) | 666 (495, 1065) | 939 (685, 1217) |
| Age (years) | 15 (13, 17)a | 15 (14, 18)a | 14 (12, 16)b | 15 (14, 15)ab |
| Weight (kg) | 3.6 (3.0, 4.5)a | 3.7 (3.2, 4.3)ab | 4.3 (3.4, 5.1)b | 4.7 (3.4, 5.7)b |
| SBP (mmHg) | 138.4 (120.5, 148)a | 181.6 (175.0, 188.8)b | 133.4 (121.1, 146.1)a | 181.2 (174.8, 190)b |
| Heart rate (bpm) | 186 (168, 210) | 180 (160, 204) | 184 (176, 204) | 192 (186, 208) |
| PCV (%) | 34 (30, 38)a | 36 (29, 39)ab | 37 (34, 40)b | 37 (36, 40)ab |
| Albumin (g/dL) | 3.1 (2.9, 3.3) | 3.1 (2.9, 3.3) | 3.1 (3.0, 3.4) | 3.2 (3.1, 3.4) |
| Creatinine (mg/dL) | 2.5 (2.1, 3.3)a | 2.7 (2.4, 3.0)a | 1.4 (1.3, 1.7)b | 1.6 (1.4, 1.9)b |
| Urea (mmol/L) | 16.9 [13.6, 22.0)a | 17.1 (15.2, 21.1)a | 10.5 (8.8, 12)b | 10.9 (9, 11.8)b |
| Phosphate (mg/dL) | 4.15 (3.55, 5.04)a | 4.28 (3.72, 5.16)a | 3.81 (3.50, 4.31)b | 4.06 (3.53, 4.56)ab |
| Total calcium (mg/dL) | 10.4 (10.0, 10.8)a | 10.6 (10.1, 11.0)a | 9.9 (9.5, 10.2)b | 9.9 (9.8, 10.2)ab |
| Sodium (mEq/L) | 154.3 (152.2, 156.4)a | 153.8 (151.7, 154.9)ab | 153.6 (152.0, 154.9)b | 152.7 (151.0, 153.6)ab |
| Potassium (mEq/L) | 4.20 (3.95, 4.50)a | 4.10 (3.95, 4.55)ab | 4.05 (3.80, 4.30)b | 4.20 (3.79, 4.40)ab |
| Chloride (mEq/L) | 119.1 (117.4, 120.8) | 117.7 (115.8, 119.6) | 118.9 (117.2, 120.8) | 118.7 (117.3, 119.4) |
| Cholesterol (mg/dL) | 200 (157, 247)a | 212 (177, 256)ab | 177 (147, 219)b | 201 (151, 236)ab |
| USG | 1.018 (1.016, 1.022)a | 1.018 (1.015, 1.019)a | 1.047 (1.032, 1.054)b | 1.034 (1.034, 1.038)b |
CKD‐DH, CKD‐developed‐hypertension; CKD‐NT, CKD‐remained normotensive; SBP, systolic blood pressure; PCV, packed cell volume; USG, urine specific gravity.
Parameters shown are at last included visit for the‐NT groups and first hypertensive visit for the‐DH groups. Superscript letters identify groups, which differed significantly.
Initial Visit
Chronic kidney disease‐cats that remained normotensive during follow‐up had significantly lower mean SBP at their first visit than CKD‐cats that developed hypertension, and Healthy‐NT cats had a significantly lower SBP than Healthy‐DH cats (P < .05) (Table 1). The SBP at baseline between the NT‐groups did not significantly differ, and neither did the DH‐groups (Table 1). Cats included in the Healthy‐NT group were significantly younger than both groups of cats with CKD (P < .001) but the other groups did not significantly differ in age (Table 1).
Rate of Change in Systolic Blood Pressure over Time
The CKD‐DH, CKD‐NT and Healthy‐NT cats all had a significant increase in SBP over time, although the rates of change were not significantly different from each other (P = .09; CKD‐NT: 0.5 ± 0.1, Healthy‐NT: 0.4 ± 0.1 mmHg, CKD‐DH: 1.1 ± 0.3, Healthy‐DH: 0.3 ± 0.5 mmHg/100 days). All rates of change, aside from the Healthy‐DH group, were significantly different from 0 (P < .001).
Association Between Systolic Blood Pressure and Biochemical Variables over Time
Using measurements from all time points available for each cat, no significant association between creatinine, sodium, phosphate, total calcium, potassium, and weight and SBP over time could be found. Cholesterol, PCV, albumin and heart rate were significantly and positively associated with SBP and chloride was negatively associated with SBP in the univariable analysis (Table 3). In the multivariable model heart rate (0.07 ± 0.02 mmHg/bpm, P < .001) and PCV (0.2 ± 0.1 mmHg/L/L, P < .05) remained significantly and positively associated with SBP.
Table 3.
Linear mixed model investigating the association between biochemical and clinical variables and systolic blood pressure over time
| Estimate ± SE | P‐value | |
|---|---|---|
| Creatinine (mg/dL) | −3.0 ± 2.2 | .17 |
| Sodium (mEq/L) | −0.03 ± 0.1 | .75 |
| Chloride (mEq/L) | −0.2 ± 0.08 | .04 |
| Phosphate (mg/dL) | 0.4 ± 0.4 | .34 |
| Total calcium (mg/dL) | 0.6 ± 0.6 | .30 |
| Potassium (mEq/L) | 0.6 ± 1.1 | .56 |
| Cholesterol (mg/dL) | 0.02 ± 0.009 | .04 |
| PCV (%) | 0.30 ± 0.10 | .001 |
| Albumin (g/dL) | 4.5 ± 1.8 | .013 |
| Weight (kg) | 0.08 ± 0.07 | .27 |
| Heart rate (bpm) | 0.07 ± 0.01 | <.001 |
PCV, packed cell volume; SE, standard error.
Values depicted in bold were significantly associated with SBP in the univariable analysis and were included in the multivariable linear mixed model. PCV and heart rate remained significantly associated with SBP.
Risk of Becoming Hypertensive
The Cox proportional hazards model showed that at all time points, cats that were healthy were at less risk than cats with CKD of becoming hypertensive (Hazard ratio = 0.2 [95% confidence interval 0.08–0.36], P < .001) (Fig 1). Sodium, chloride, total calcium, potassium, cholesterol, PCV, albumin, weight and heart rate were not significant risk factors for the development of hypertension (Table 4). Increased phosphate and creatinine concentrations were found to be significant risk factors for the development of hypertension in the univariable analysis (P < .05). Creatinine concentration was the only independent risk factor for a cat becoming hypertensive (Hazard ratio = 7.3 [95% confidence interval 1.2–45.4], P = .03).
Figure 1.
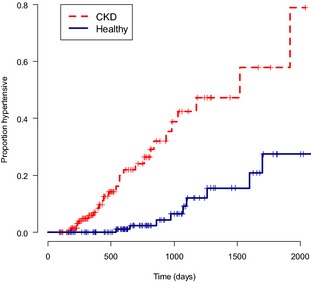
Kaplan–Meier curve of probability to become hypertensive. CKD; chronic kidney disease, time is in days from first visit. Cats with CKD have a greater probability to be hypertensive at each time point than healthy cats (P < .001). Censored cases are represented by ticks.
Table 4.
Cox proportional hazards model investigating the risk factors for the development of hypertension
| HR | 95% Confidence interval | P‐value | |
|---|---|---|---|
| Creatinine (mg/dL) | 9.39 | 2.06–42.82 | .004 |
| Sodium (mEq/L) | 1.07 | 0.90–1.27 | .47 |
| Chloride (mEq/L) | 1.00 | 0.92–1.08 | .93 |
| Phosphate (mg/dL) | 1.34 | 1.03–1.74 | .03 |
| Total calcium (mg/dL) | 0.88 | 0.51–1.53 | .65 |
| Potassium (mEq/L) | 0.91 | 0.28–2.98 | .87 |
| Cholesterol (mg/dL) | 1.00 | 1.00–1.01 | .38 |
| PCV (%) | 0.95 | 0.86–1.05 | .3 |
| Albumin (g/dL) | 0.72 | 0.17–3.11 | .66 |
| Weight (kg) | 0.81 | 0.58–1.14 | .22 |
| Heart rate (bpm) | 1.00 | 0.98–1.00 | .16 |
PCV, packed cell volume.
Values depicted in bold were a significant risk factor for the development of hypertension in the univariable analysis and included in the multivariable Cox proportional hazards model. Creatinine remained the only independent risk factor for the development of hypertension.
Discussion
Of the initially normotensive cats with CKD, 17% developed hypertension, whereas of the initially normotensive healthy cats included in this study, 7% did. In addition to this, 105/264 azotemic cats were hypertensive at or within 3 months of CKD diagnosis. In cross‐sectional studies, 23% of hypertensive cats are nonazotemic and do not have an identifiable cause for their hypertension,14 and 12% of nonazotemic, nonhyperthyroid cats are hypertensive at baseline.7 This study only included healthy cats that developed hypertension at a later time‐point and excluded cats that were presented as an idiopathic hypertensive cat at their first visit to the clinic, which probably explains the lower prevalence. The criteria for defining hypertension and the composition of the study population greatly influence reported prevalence, and the prevalence of hypertension in cats with CKD in the current study is lower than the 65% reported in a study performed in a referral setting,4 and greater than the 19.4% previously reported in a cross‐sectional study using a higher SBP cut‐off.3 In addition to this, all cats included in this study had a minimum follow‐up of 3 visits over the course of 3 months. This could have artificially increased the size of the group of cats that were hypertensive at diagnosis of CKD, as these cats came back for regular blood pressure checks on antihypertensive medication.
Both healthy cats and cats with CKD included in this study showed a significant increase in SBP with increasing age. No significant increase in SBP over time could be found for the healthy‐DH group, although this was most likely because of the small group size. The significant increase in blood pressure with age is comparable to the human situation.15 Studies investigating the age‐effect on blood pressure in cats are conflicting, but all studies published to date commenting on this phenomenon were cross‐sectional in design.5, 10, 16, 17, 18 A moderate correlation between all blood pressure variables and age has been reported in cats,18 although a study including only healthy cats did not find a significant correlation between SBP and age.17 Sansom and co‐workers reported a higher blood pressure in older cats if the cats were grouped according to age (younger than 5, 5–10 years and older than 10). The cats that were included in this study did, however, not all have serum creatinine concentration measured, and the possibility exists that cats with subclinical CKD have been included, especially in the oldest age group.10 Another study performed in a population of healthy cats, however, could not demonstrate a significant difference between the SBP of cats <10 years of age and cats that were 10 years or older.5 A further study including healthy and ill cats did report an age cut‐off, with cats over the age of 11 years having significantly higher blood pressure than cats younger than 11, although renal function was not assessed in the apparently healthy population of cats.18 Hypertensive cats have been reported to be significantly older than their normotensive counterparts, although the study population consisted of a mixture of cats including cats with renal disease and hyperthyroidism.16 Both diseases are associated with hypertension,12 and the prevalence of CKD and hyperthyroidism also increases with age,6, 7, 19 which means that the age effect found could be due to the inclusion criteria of this study. To our knowledge, this study is the first investigating changes in blood pressures in individual healthy cats and cats with CKD over time and is therefore the first to definitely show by longitudinal analysis that pressure rises with age in cats, as it does in humans.
The positive association between heart rate and SBP has commonly been reported in human literature. Mean arterial pressure is determined by cardiac output and peripheral resistance, and the association between heart rate and SBP and can be attributed to the fact that cardiac output is increased with an increased heart rate.20 A previous cross‐sectional study found no significant correlation between heart rate and SBP in healthy cats.21 Heart rate has been reported to not be significantly different in cats with higher blood pressures and CKD when compared to healthy cats with lower blood pressures,11 although a previous study reported 17% of cats with hypertension to be tachycardic.14 To our knowledge, this study is the first to investigate the association between heart rate and SBP over time in individual cats. A positive independent association between PCV and SBP was found in this study. Hematocrit is the most important determinant of blood viscosity, which in turn determines peripheral resistance together with vascular diameter. Human studies have also described this independent positive association between PCV and SBP, and these studies also report an increased PCV in hypertensive subjects compared to normotensive subjects.22
Cats that develop hypertension have a higher baseline blood pressure than their normotensive counterparts. In the human literature the term ‘prehypertension’ is often used to describe a nonoptimal baseline blood pressure. Blood pressure and the incidence of hypertension increases with age, and human subjects with higher baseline blood pressure are at higher risk of becoming hypertensive.23, 24 The CKD‐DH group had a significantly higher blood pressure than both the CKD‐NT group and the healthy‐NT group. The small size of the healthy‐DH group makes interpretation of the result more challenging, although the SBP at time of inclusion in the study seems comparable to the SBP of the CKD‐DH group. The World Health Organisation‐International Society of Hypertension has guidelines to classify nonhypertensive individuals into blood pressure categories (optimum, normal or high‐normal). Based on these categories, screening intervals are recommended for human subjects, although these intervals differ between organizations.24 No optimum recommendations exist yet for screening intervals for individual elderly normotensive cats, but based on the results obtained in this study it could be argued that more frequent blood pressure measurements need to be encouraged if an owner presents a cat with a mean SBP ≥140 mmHg. Cats with CKD have a significantly greater probability of becoming hypertensive, and creatinine is the only independent predictor for becoming hypertensive, which could call for an even shorter interval between screening visits for cats with CKD. Of the cats that were diagnosed as hypertensive, a greater percentage showed signs of target organ damage if they were diagnosed with CKD and hypertension around the same time point (70%), when compared with the cats that already came to the clinic for regular blood pressure checks after their CKD diagnosis (52%). The overall relatively high incidence of target organ damage provides clinical validity to the diagnosis of hypertension in most cats included in this study. A shorter screening interval can aid in early diagnosis and treatment of hypertension and therefore decrease the development of target organ damage.
Having CKD increases the probability a cat develops systemic hypertension, but their blood pressure does not increase at a significantly greater rate than cats that are healthy and become hypertensive, although this result may have been affected by group size. Cats with CKD included in this study were significantly older than healthy cats that remained normotensive, and even though the difference was not significant for the healthy‐DH group, the age of these cats seems comparable to the healthy‐NT group. This is a confounding factor of this study, and difficult to control for as CKD prevalence increases with age.6 There was no correlation between creatinine concentration and SBP when the data from all time points for each cat was used, similar to the result of a cross‐sectional study in cats with CKD.5 A study investigating differences between hypertensive and normotensive cats also reported no significant difference in creatinine concentration between groups.3
Human patients with end stage renal disease almost invariably suffer from hypertension, but in human CKD patients there is no correlation between serum creatinine concentration and blood pressure,25, 26 although a decline in creatinine clearance is mildly correlated with mean blood pressure in healthy subjects.15 It has been demonstrated that baseline creatinine is an independent risk factor for the development of azotemic CKD in cats.7 This study shows that creatinine is also an independent risk factor for the development of hypertension and that CKD cats are more likely to be diagnosed with clinically significant hypertension. Historically it has been suggested that hypertension in most cats is secondary to CKD, and several mechanisms, such as activation of the renin‐angiotensin‐aldosterone system (RAAS) and fluid retention have been described.11, 27, 28 A recent study showed that plasma renin activity is suppressed in hypertensive cats, whereas plasma aldosterone concentration is increased, although there was a substantial overlap among groups.28 However, it could be hypothesized that CKD and hypertension share a pathophysiological basis that is not yet understood. The possibility exists that feline hypertension has a genetic component, like essential hypertension in humans,29 but to date no studies into the genetics of feline hypertension have been published. This is an area that warrants further investigation.
Conclusion
In conclusion, blood pressure increases with age in all cats. Cats that develop clinically significant hypertension have a higher blood pressure at initial evaluation than their normotensive counterparts and cats with CKD are more likely to develop hypertension. The high prevalence of hypertension in azotemic cats in this study shows the importance of monitoring of SBP in elderly cats and, in particular, in cats with CKD. It could, therefore, be suggested that cats with a higher baseline blood pressure at diagnosis of CKD should be more closely monitored than cats with a lower baseline blood pressure. Creatinine concentration is an independent risk factor for the development of hypertension, and early diagnosis of CKD is essential so that appropriate management can be offered.
Acknowledgments
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at the Royal Veterinary College, London, UK.
Results of this study were presented at the ACVIM Forum 2013, Seattle, Washington.
This study was supported by Zoetis.
Footnotes
Parks Electronic Doppler Model 811B, Perimed UK, Bury St Edmunds, UK
Mistral 3000, Sanyo‐Gallenkamp, Leicestershire, UK
IDEXX Laboratories, Wetherby, Yorkshire, UK
R i386 3.0.1; R Foundation for Statistical Computing, Vienna, Austria and GraphPad Prism 6; GraphPad Software, La Jolla, CA, USA.
References
- 1. Elliott J, Fletcher M, Syme H. Idiopatic feline hypertension: Epidemiological study. J Vet Intern Med 2003;17:754. [Google Scholar]
- 2. Littman M. Spontaneous systemic hypertension in 24 cats. J Vet Intern Med 1994;8:79–86. [DOI] [PubMed] [Google Scholar]
- 3. Syme HM, Barber PJ, Markwell PJ, et al. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc 2002;220:1799–1804. [DOI] [PubMed] [Google Scholar]
- 4. Stiles J, Polzin D, Bistner S. The prevalence of retinopathy in cats with systemic hypertension and chronic renal failure or hyperthyroidism. J Am Anim Hosp Assoc 1994;30:564–572. [Google Scholar]
- 5. Kobayashi DL, Peterson ME, Graves TK, et al. Hypertension in cats with chronic renal failure or hyperthyroidism. J Vet Intern Med 1990;4:58–62. [DOI] [PubMed] [Google Scholar]
- 6. Lulich JP, Osborne CA, O'Brien TD, et al. Feline renal failure: Questions, answers, questions. Compend Contin Educ Pract Vet 1992;14:127–153. [Google Scholar]
- 7. Jepson RE, Brodbelt D, Vallance C, et al. Evaluation of predictors of the development of azotemia in cats. J Vet Intern Med 2009;23:806–813. [DOI] [PubMed] [Google Scholar]
- 8. Miall WE, Lovell HG. Relation between change of blood pressure and age. Br Med J 1967;2:660–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Rodriguez BL, Labarthe DR, Huang B, et al. Rise of blood pressure with age. New evidence of population differences. Hypertension 1994;24:779–785. [DOI] [PubMed] [Google Scholar]
- 10. Sansom J, Rogers K, Wood JL. Blood pressure assessment in healthy cats and cats with hypertensive retinopathy. Am J Vet Res 2004;65:245–252. [DOI] [PubMed] [Google Scholar]
- 11. Mishina M, Watanabe T, Fujii K, et al. Non‐invasive blood pressure measurement in cats: Clinical significance of hypertension associated with chronic renal failure. J Vet Med Sci 1998;60:805–808. [DOI] [PubMed] [Google Scholar]
- 12. Brown S, Atkins C, Bagley R, et al. Guidelines for the indentification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 13. Boag AK, Neiger R, Slater L, et al. Changes in the glomerular filtration rate of 27 cats with hyperthyroidism after treatment with radioactive iodine. Vet Rec 2007;161:711–715. [DOI] [PubMed] [Google Scholar]
- 14. Elliott J, Barber PJ, Syme HM, et al. Feline hypertension: Clinical findings and response to antihypertensive treatment in 30 cases. J Small Anim Pract 2001;42:122–129. [DOI] [PubMed] [Google Scholar]
- 15. Lindeman RD, Tobin JD, Shock NW. Association between blood pressure and the rate of decline in renal function with age. Kidney Int 1984;26:861–868. [DOI] [PubMed] [Google Scholar]
- 16. Chetboul V, Lefebvre HP, Pinhas C, et al. Spontaneous feline hypertension: Clinical and echocardiographic abnormalities, and survival rate. J Vet Intern Med 2003;17:89–95. [DOI] [PubMed] [Google Scholar]
- 17. Sparkes AH, Caney SMA, King MCA, et al. Inter‐ and intraindividual variation in doppler ultrasonic indirect blood pressure measurements in healthy cats. J Vet Intern Med 1999;13:314–318. [DOI] [PubMed] [Google Scholar]
- 18. Bodey AR, Sansom J. Epidemiological study of blood pressure in domestic cats. J Small Anim Pract 1998;39:567–573. [DOI] [PubMed] [Google Scholar]
- 19. Stephens MJ, O'Neill DG, Church DB, et al. Feline hyperthyroidism reported in primary‐care veterinary practices in England: Prevalence, associated factors and spatial distribution. Vet Rec 2014;175:458. [DOI] [PubMed] [Google Scholar]
- 20. Kim JR, Kiefe CI, Liu K, et al. Heart rate and subsequent blood pressure in young adults: The CARDIA study. Hypertension 1999;33:640–646. [DOI] [PubMed] [Google Scholar]
- 21. Paepe D, Verjans G, Duchateau L, et al. Routine health screening: Findings in apparently healthy middle‐aged and old cats. J Feline Med Surg 2013;15:8–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Cirillo M, Laurenzi M, Trevisan M, et al. Hematocrit, blood pressure, and hypertension. The Gubbio population study. Hypertension 1992;20:319–326. [DOI] [PubMed] [Google Scholar]
- 23. de Simone G, Devereux RB, Chinali M, et al. Risk factors for arterial hypertension in adults with initial optimal blood pressure: The Strong Heart Study. Hypertension 2006;47:162–167. [DOI] [PubMed] [Google Scholar]
- 24. Vasan RS, Larson MG, Leip EP, et al. Assessment of frequency of progression to hypertension in non‐hypertensive participants in the Framingham Heart Study: A cohort study. Lancet 2001;358:1682–1686. [DOI] [PubMed] [Google Scholar]
- 25. Bulpitt CJ, Hodes C, Everitt MG. The relationship between blood pressure and biochemical risk factors in a general population. Br J Prev Soc Med 1976;30:158–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Perneger TV, Nieto FJ, Whelton PK, et al. A prospective study of blood pressure and serum creatinine. JAMA 1993;269:488–493. [PubMed] [Google Scholar]
- 27. Jensen J, Henik RA, Brownfield M, et al. Plasma renin activity and angiotensin I and aldosterone concentrations in cats with hypertension associated with chronic renal disease. Am J Vet Res 1997;58:535–540. [PubMed] [Google Scholar]
- 28. Jepson RE, Syme HM, Elliott J. Plasma renin activity and aldosterone concentrations in hypertensive cats with and without azotemia and in response to treatment with amlodipine besylate. J Vet Intern Med 2014;28:144–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Levy D, Ehret GB, Rice K, et al. Genome‐wide association study of blood pressure and hypertension. Nat Genet 2009;41:677–687. [DOI] [PMC free article] [PubMed] [Google Scholar]


