Abstract
Background
There is an unmet clinical need for a cat‐specific formulation of amlodipine to treat hypertensive cats.
Objectives
To assess the efficacy of chewable amlodipine tablets in reducing systolic blood pressure (SBP) in cats diagnosed with systemic arterial hypertension.
Animals
Seventy‐seven client‐owned cats with systemic hypertension were included (median age 14 years).
Methods
The study was randomized, double‐blinded, and placebo‐controlled. Forty‐two cats received 0.125–0.50 mg/kg amlodipine once daily for 28 days; 35 cats received placebo. After 28 days all cats continued with amlodipine for 2–3 months in an open‐label phase. Blood pressure was measured using high definition oscillometry. A responder was defined as a cat showing a decrease of SBP to <150 mmHg at 28 days or a decrease from baseline ≥15%.
Results
Sixty‐one cats completed the study. The responder rate was 63% in amlodipine group and 18% in placebo group. Cats receiving amlodipine were 7.9 (95% CI 2.6–24.1) times more likely to be classified as responders when compared to those receiving placebo (P < .001). From a mean (±SD) baseline value of 181 (±12) mmHg, SBP decreased to 154 (±17) mmHg with amlodipine and to 170 (±21) mmHg with placebo (P < .001). The voluntary acceptance rate of amlodipine formulation was 73%.
Conclusions and Clinical Importance
The chewable amlodipine tablet effectively reduced SBP compared with placebo in hypertensive cats, and was well‐tolerated. It can be used concomitantly with angiotensin‐converting enzyme inhibitors and in cats with chronic kidney disease.
Keywords: Calcium channel blocker, Cardiovascular agents, Evidence based medicine, Feline, High definition oscillometry
Abbreviations
- ACE
angiotensin converting enzyme
- ACVIM
American College of Veterinary Internal Medicine
- AE
adverse event
- BP
blood pressure
- CCB
calcium channel blocker
- CKD
chronic kidney disease
- DM
diabetes mellitus
- HDO
high definition oscillometry
- SBP
systolic blood pressure
- TOD
target organ damage
- QoL
quality of life
Cats usually develop secondary hypertension with an underlying disease triggering increased blood pressure (BP), although idiopathic hypertension, where no underlying disease can be recognized, is seen in about 1 in 5 cases. Systemic hypertension in cats is most commonly associated with acute or chronic kidney disease (CKD). Other conditions associated with the development of secondary hypertension in cats include hyperthyroidism, diabetes mellitus (DM), primary hyperaldosteronism, and pheochromocytoma.1 Chronically sustained increases in BP cause injury to various tissues, mainly to kidneys, eyes, brain, and heart. This is commonly referred to as target organ damage (TOD).
According to the guidelines of the American College of Veterinary Internal Medicine (ACVIM) Hypertension Consensus Panel,1 hypertension is categorized according to its risk of TOD: minimal risk (<150/95 mmHg), mild risk (150–159/95–99 mmHg), moderate risk (160–179/100–119 mmHg), and severe risk (>180/120 mmHg).
The goal of antihypertensive treatment is to maximally decrease the risk of TOD, which is achieved with persistent BP reduction to values <150/95 mmHg. Currently, no drugs are approved for treatment of hypertension in cats, but calcium channel blockers (CCB) and angiotensin converting enzyme (ACE) inhibitors are the most widely used antihypertensive agents in practice.1
Amlodipine has been considered the treatment of choice for hypertension in cats for more than a decade. Amlodipine at a dose of 0.125–0.25 mg/kg once daily has been shown to significantly decrease BP in spontaneously hypertensive cats in several clinical trials.2, 3, 4, 5, 6 According to the ACVIM consensus statement on hypertension,1 standard veterinary textbooks, and other publications, CCBs are the first choice for antihypertensive treatment in cats.
There is, however, no amlodipine product approved for veterinary use for treatment of hypertension in cats; therefore amlodipine approved for human use has been used off‐label in veterinary medicine. The product approved for human use has certain disadvantages such as difficult dosing because of high amlodipine concentration and challenging administration because of lack of palatable formulation. Thus, veterinary profession lacks an approved medication effective in majority of cases for feline hypertension.
The present study was undertaken to determine the efficacy of chewable amlodipine tablets in cats diagnosed with systemic arterial hypertension. The formulation used in this study has been shown to have similar in vitro dissolution profile (immediate release tablet) to that of the human formulation.
Materials and Methods
Study Design
The randomized, multicenter, double‐blinded, placebo‐controlled, parallel group study was conducted at 20 private veterinary clinics in 3 European countries (Finland, France, and Germany). This study was conducted in accordance with the principles of Good Clinical Practice.7 Informed consent was obtained from each animal owner prior to enrollment. The welfare, treatment, and care of study animals at study sites were ensured by veterinary supervision. Permission to conduct the study was received from each National Regulatory Agency prior to commencement.
Study Animals
Cats included in the study were identified by the participating veterinarians in the course of their routine clinical practice. Veterinary practices were encouraged to screen cats at risk (old cats, including those with CKD or other diseases related to hypertension). To be eligible for the study, cats had to weigh between 2.5 and 10.0 kg, and have a systolic blood pressure (SBP) ≥165 mmHg on 2 separate visits within 2 weeks, to rule out white coat hypertension. If the cat had a primary disease associated with hypertension (e.g. CKD, hyperthyroidism, primary hyperaldosteronism, DM, or pheochromocytoma), the primary disease had to be stable with no need for immediate initiation of other medication or dose adjustment of current medication.
Cats were excluded from study if they met any of the following criteria: use of systemic treatment with CCBs, vasodilators, alpha‐1 adrenergic antagonists, angiotensin receptor blockers, beta‐blockers, aldosterone antagonists within 30 days of screening; use of long‐acting glucocorticoids or continuous use of short‐acting glucocorticoids within 3 months of screening, initiation or change in dosing of methimazole, carbimazole, phenylpropalamine, nonsteroidal anti‐inflammatory drugs, diuretics, short‐acting systemic glucocorticoids, or any other medication for primary disease within 14 days of screening; starting on ACE inhibitors or insulin treatment for DM or a change in dosing of existing ACE inhibitors or insulin or renal diet within 30 days of screening and; presence of ocular or neurological signs, which were deemed to be caused by hypertension and requiring immediate medical treatment. Cats were also excluded if SBP >200 mmHg was recorded, unless the investigator estimated that the cat could be enrolled in the study. Other reasons for exclusion were presence of clinically relevant liver failure or impaired hepatic function and unstable CKD that was expected to worsen markedly during the study.
The dose of amlodipine was determined from data found in literature. In previous studies, most cats received an initial single dose of 0.625 mg amlodipine daily. This dosage regimen was chosen mainly for practical reasons as this corresponds to 1/8 of the 5 mg tablet registered for human use.
The amlodipine product used in this study was a chewable chicken flavored tablet designed for cats. Placebo tablets were equal in size and shape, contained the same excipients but no active ingredient.
The overall study design and plan is presented in Figure 1. The study consisted of 2 phases with differing designs. Phase 1 (blinded efficacy period, 28 ± 3 days) was double‐blind. Cats were randomized to receive amlodipine 0.125 mg/kg (range 0.125–0.25 mg/kg) or placebo given PO by the owner once daily at home. If after 2 weeks SBP was ≥150 mmHg or had decreased <15% from baseline value, the dose was doubled.
Figure 1.
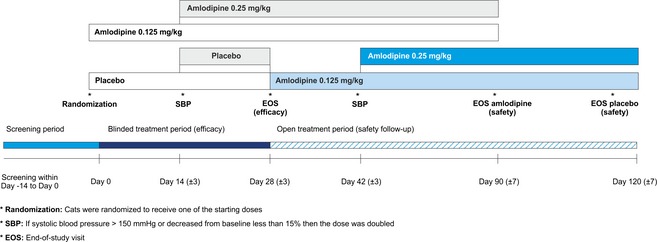
Study design.
Phase 2 (safety period) was an open‐label safety follow‐up with all cats receiving amlodipine. The cats that had received amlodipine during phase 1 continued their medication for 2 months, while all placebo cats started receiving amlodipine 0.125 mg/kg (range 0.125–0.25 mg/kg) for 3 months. The dose was doubled after 2 weeks if SBP did not meet the same criteria as in phase 1.
Blood Pressure Measurements
Blood pressure was measured in accordance with the ACVIM guidelines1 using a high definition oscillometry (HDO) device.1 Measurements were made at each visit before performing any other examination or manipulation. Cats were allowed to acclimatize in a quiet room 5–10 minutes before measurement, if needed. All measurements were obtained using the same cuff size, place (tail), and body position (standing or lying on the owner's lap). The cuff was placed at the level of heart, regardless of the position of cat. Measurement quality was visually verified from computer trace and recorded. The average of 5 consistent measurements, within 15 mmHg of each other, was used. All measurements were verified by the same person.
All investigators were trained in the measurement technique and use of the HDO device.
Other Variables
Quality of Life (QoL) was evaluated on a 4‐point scale (Table 1). The questionnaire consisted of 4 items: appetite; drinking and urinating; mobility and owner interactions; and self‐grooming habits. Additionally, owner evaluated overall improvement in the cat's condition.
Table 1.
Numerical rating scale for the assessment of quality of life
| Score | Description |
|---|---|
| Appetite | |
| 0 | Excellent, always consumes whole meal |
| 1 | Good, consumes most of the meal |
| 2 | Moderate, consumes some but usually leaves some |
| 3 | Poor, consumes little and leaves most of the meal |
| Drinking and urinating | |
| 0 | Normal |
| 1 | Occasionally and/or mildly increased drinking/urinating |
| 2 | Moderate, consumes more water and urinates more |
| 3 | Consumes water and urinates in excess |
| Mobility and owner interactions | |
| 0 | Excellent; moves around, plays and interacts with joy/ as a healthy cat of the breed in question |
| 1 | Good; moves around, plays and interacts with joy but may occasionally seem tired |
| 2 | Moderate; reluctant to move around, play or interact |
| 3 | Poor; refuses to move around, play or interact |
| Self‐grooming habits | |
| 0 | Grooms itself normally |
| 1 | Grooms somewhat less often |
| 2 | Grooms markedly less often |
| 3 | Has stopped grooming |
Palatability was scored on a 3‐point scale: tablet taken spontaneously from hand or from empty bowl (1); tablet taken with food from bowl or administered within palatable food (2); tablet administered directly into mouth (3). Scores 1 and 2 were considered as palatable, while score 3 was considered not palatable. The investigator evaluated palatability and possible changes based on owner's interview and diary data.
Safety was assessed by recording adverse events (AE), physical examination findings, cardiorespiratory status, and laboratory variables. An AE was any observation in animals that was unfavorable and unintended and occurred after the use of the investigational veterinary product, whether considered to be product related.
Statistical Methods
The target number of cats in the study was 72 with group sizes of 36 giving 90% power to detect superiority, assuming 70% and 30% response rates in amlodipine and placebo groups respectively. Block randomization was used with different block size for cats with CKD than for cats without CKD.
Comparison between groups was made using Wilcoxon rank sum test for numeric data and Fisher's exact test for categorical data. The change from baseline SBP at the end of blinded efficacy period (Day 28) was evaluated as a dichotomous variable, where a responder was defined as decrease of SBP to <150 mmHg or decrease from baseline of at least 15%. This was the primary efficacy variable and factors influencing its attainment were analyzed with a logistic regression model, where baseline SBP, CKD (present or absent), and concomitant ACE inhibitor use (yes or no) were used as covariates.
A repeated measures analysis of covariance model was used to analyze absolute changes in SBP. The model included the same fixed effects as logistic regression model together with visit as a repeated factor and the treatment‐by‐visit interaction. All other variables were tabulated with descriptive statistics. A P‐value of <.05 was taken to indicate statistical significance.
Results
A total of 128 cats were assessed for eligibility for the study; 77 cats were enrolled. Forty‐two cats were randomized to receive amlodipine and 35 cats received placebo. Sixteen cats discontinued the study, 3 of which discontinued during the blinded efficacy period. The most common reason for discontinuation was an AE. Thus, 61 cats completed the study.
No clinically or statistically significant differences in demographic and baseline characteristics were found between the 2 groups at entry to the study (Table 2).
Table 2.
Demographic and baseline characteristics of cats enrolled in the study
| Variables | Amlodipine N = 42 | Placebo N = 35 | Total N = 77 | P‐valuea |
|---|---|---|---|---|
| Systolic blood pressure | 177 (165–220) | 177 (166–204) | 177 (165–220) | .45 |
| Age (years) | 14.0 (7–20) | 14.0 (9–18) | 14.0 (7–20) | .31 |
| Weight (kg) | 3.7 (2.5–7.5) | 4.2 (2.5–6.8) | 4.0 (2.5–7.5) | .17 |
| Breed | ||||
| Domestic | 16 (38.1) | 14 (40.0) | 30 (39.0) | .13 |
| European | 17 (40.5) | 14 (40.0) | 31 (40.3) | |
| Persian | 5 (11.9) | – | 5 (6.5) | |
| Other | 4 (9.5) | 7 (20.0) | 11 (14.2) | |
| Sex (all neutered) | ||||
| Female | 19 (45.2) | 17 (48.6) | 36 (46.8) | .82 |
| Male | 23 (54.8) | 18 (51.4) | 41 (53.2) | |
| S‐creatinine (mg/dL) | 1.6 (0.6–4.7) | 1.8 (0.6–4.4) | 1.7 (0.6–4.7) | .74 |
| U‐specific gravity | 1.02 (1.01–1.07) | 1.03 (1.01–1.06) | 1.03 (1.01–1.07) | .81 |
| U‐protein/creatinine ratio | 0.2 (0.1–1.9) | 0.2 (0.1–0.8) | 0.2 (0.1–1.9) | .15 |
| Primary disease | ||||
| Renal disease | 14 (33.3) | 12 (34.3) | 26 (33.8) | .63 |
| Hyperthyroidism | 9 (21.4) | 10 (28.6) | 19 (24.7) | |
| Idiopathic hypertension | 11 (26.2) | 10 (28.6) | 21 (27.3) | |
| Other | 8 (19.0) | 3 (8.6) | 11 (14.3) | |
| Angiotensin converting enzyme inhibitors | ||||
| Yes | 7 (16.7) | 5 (14.3) | 12 (15.6) | >.99 |
| No | 35 (83.3) | 30 (85.7) | 65 (84.4) | |
Data are median (range) or number (%).
P‐value for comparison of groups using Wilcoxon rank sum test for numeric data and Fisher's exact test for categorical data.
The protocol allowed the use of ACE inhibitors; 12 cats continued to use an ACE inhibitor (9 benazepril, 2 imidapril, and 1 ramipril) during the study.
Systolic Blood Pressure
Responder rate at the end of the blinded efficacy period (Day 28) was significantly higher (adjusted odds ratio 7.9; 95% CI 2.6–24.1) in the amlodipine group (63%) than in the placebo group (18%). Individual responses for each cat are shown in Figure 2.
Figure 2.
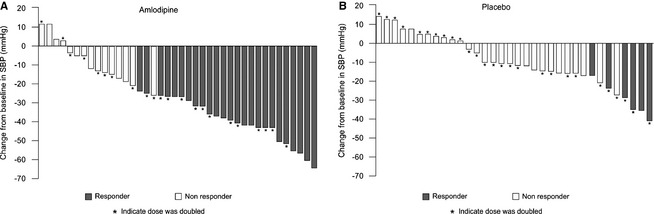
Individual responses (responder or nonresponder) for each cat after blinded efficacy period (Day 28).
In the first 14 days of treatment, the median dose of amlodipine was 0.179 (range 0.125–0.25) mg/kg. On Day 14, 19 of 41 (46%) cats allocated to receive amlodipine met criteria of responding to treatment (SBP <150 mmHg or a reduction in SBP of ≥15%) and 22 (54%) did not. This contrasted with 7 of 35 (20%) cats from placebo group that responded and 28 (80%) that did not. For the nonresponding cats, median dose of amlodipine was increased to 0.379 (range 0.25–0.50) mg/kg and by Day 28, 25 of 40 cats (63%) remaining in study were responders. This contrasted with 6 of 34 cats receiving placebo (18%) that were responders. Cats receiving amlodipine were 7.9 (95% CI 2.6–24.1) times more likely to be responders than cats receiving placebo, which was statistically significant (P < .001). Logistic regression analysis showed that treatment (amlodipine versus placebo) was the only significant factor. An unadjusted logistic regression analysis confirmed results of the adjusted model.
Decrease in mean SBP was significantly (P < .001) greater in the amlodipine group than in the placebo group (Fig 3). A 10 mmHg reduction in SBP was seen in the placebo group which stabilized within 14 days, whereas amlodipine treatment led to a 28 mmHg reduction in SBP after 28 days.
Figure 3.
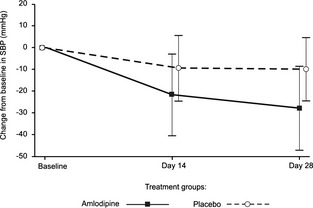
Mean (SD) changes from baseline in systolic blood pressure during the blinded efficacy period.
In subgroup analysis by disease etiology, a similar proportion of cats with CKD (71.4%), hyperthyroidism (75%), and other diseases (75%) responded to amlodipine treatment by Day 28, whereas only 30% of cats with idiopathic hypertension were responders.
Other Efficacy Variables
Palatability (i.e. voluntary acceptance of the tablet with or without food) during the first 4 weeks was 80% with amlodipine and 59% with placebo. Palatability was stable throughout the study in cats that started with amlodipine but increased somewhat in placebo cats when they started amlodipine treatment. Overall palatability with amlodipine during the 3‐month treatment period was 73%. There were no statistical differences between the groups.
The QoL score improved somewhat during the study (from 2.8 to 2.4 with amlodipine, and from 3.1 to 2.4 with placebo), but there were no statistical differences in QoL between the groups.
According to owner's evaluation, a higher proportion of cats seemed to feel better in the amlodipine group than in the placebo group (35% versus 15%, respectively, P = .066).
Adverse Events
There were no differences in frequency of AEs between the amlodipine and placebo groups during 28‐day blinded efficacy period (Table 3). AEs were followed for the whole 3–4 month study period during which the most common AEs were emesis (13%), anorexia/appetite disorder (8%), hyperthyroidism (7%), dehydration and lethargy (5%).
Table 3.
Most common (n > 1) AEs during blinded efficacy period (up to 28 days)
| System Organ Class | Amlodipine | Placebo | P‐valuea | ||||
|---|---|---|---|---|---|---|---|
| N = 42 | N = 35 | ||||||
| f | n | % | f | n | % | ||
| Any event | 23 | 12 | 28.6 | 17 | 10 | 28.6 | >.99 |
| Digestive tract disorders (ascites, diarrhea, emesis, gingival disorder, tooth disorder) | 6 | 5 | 11.9 | 5 | 4 | 11.4 | >.99 |
| Renal and urinary disorders (cystitis, nephritis, renal insufficiency, urine abnormalities) | 4 | 4 | 9.5 | 4 | 4 | 11.4 | >.99 |
| Systemic disorders (abscess, anorexia, death, lethargy, trauma, weight loss) | 2 | 2 | 4.8 | 4 | 2 | 5.7 | >.99 |
| Endocrine system disorders (hyperthyroidism, unspecified thyroid gland disorder) | 4 | 3 | 7.1 | 1 | 1 | 2.9 | .62 |
f, number of events; n, number of subjects; %, % of subjects.
P‐value from comparison of subject counts using Fisher's exact test.
Laboratory Variables
There were generally few appreciable changes in laboratory values. Creatinine remained essentially unchanged during the study in the amlodipine group (decreased by 0.02 mg/dL [±0.26], P = .77), while it increased somewhat in the placebo group (by 0.18 mg/dL [±0.54], P = .012). The median baseline values were fairly high (about 1.7 mg/dL in both groups) and an increase >25% from baseline to Day 28 was observed in 10% of 40 amlodipine cats and in 15% of 34 placebo cats. At the end of study, 9% of 67 amlodipine treated cats had creatinine increases >25%. There were no appreciable changes in urea in either group. Potassium decreased somewhat in the amlodipine group, from 4.3 (±0.50) to 4.2 (±0.44) mmol/L (P = .082), and increased in the placebo group, from 4.4 (±0.59) to 4.6 (±0.68) mmol/L (P = .020), but both of these changes were not deemed clinically relevant. No notable change was seen in urine protein to creatinine ratio.
Discussion
The present study describes the effects of amlodipine in hypertensive client‐owned cats compared to placebo. It confirms findings of the first placebo‐controlled study which involved just 9 cats3 and other uncontrolled studies.2, 5 It increases our confidence because of the fact that changes induced by amlodipine were compared to placebo in a large cohort of cats seen in primary care practices and improves understanding of the dose required to produce a clinically relevant reduction in arterial BP. Precision in dosing amlodipine in the present study was facilitated by a cat‐specific formulation of amlodipine and a tablet size that facilitated dosing between 0.125 and 0.5 mg/kg body weight.
The design of the present study demonstrates that when BP of 34 cats is measured repeatedly, the administration of a placebo tablet led to an apparent reduction of about 10 mmHg (5% reduction from baseline) after 14 days. No further reduction was seen after further 14 days in the placebo group suggesting this decrease in BP might be accounted by a training effect although other explanations are possible. This contrasted with change in SBP seen in group of 40 cats receiving amlodipine which decreased by more than twice the reduction seen in the placebo group. Doubling the amlodipine dose resulted in further significant reduction in SBP over next 14 days increasing the difference between the 2 groups after 28 days of treatment. The effect of amlodipine was further demonstrated in placebo group after they were switched to receive amlodipine. Their SBP reduced to very similar levels to those seen in amlodipine treated cats by Day 42 of the study.
In addition to having the gold standard design of a randomized, controlled, double‐blinded clinical trial, the present study had a number of other strengths which set it apart from previous published studies. Firstly, primary efficacy measure was defined a priori as a reduction in SBP to below 150 mmHg or a decrease from baseline pressure of more than 15%. Furthermore, number of cats recruited was determined by a power calculation which assumed that 70% of amlodipine treated and 30% of placebo treated cats would attain this primary efficacy endpoint. The power calculation showed that 72 cats would need to be recruited if these assumptions were correct, in order to determine whether amlodipine was superior to placebo in achieving this endpoint. In the 2 placebo‐controlled studies in the published literature,3, 8 one was very much a pilot study involving just 9 client‐owned cats3 and other was an experimental model study where hypertension was induced by subtotal nephrectomy.8 Neither of these studies had determined a target BP reduction a priori and no power calculations are mentioned in these papers.
Another factor that distinguishes the present study is its multicentric design. It shows that the results are applicable across a range of different clinics in 3 European countries. The fact that the study was well‐designed with clear inclusion/exclusion criteria was essential for it to work as a multicenter study. Furthermore, it was essential to standardize the method of BP measurement and ensure all personnel measuring BP were well‐trained in the selected method. None of the commonly used indirect BP measurement techniques used in clinical practice performs sufficiently well that they would be approved by the Association of Medical Instruments as being valid. High definition oscillometry was selected for the present study and recent data9 suggests that this method compares favorably with direct telemetry measurements made in young healthy cats. It has the advantage of providing a digital output that can be stored and examined after the measurement session by an expert to quality control the values taken in clinic. Thus, the method of BP measurement selected for this trial minimized intraoperator variability by standardization through training and additional quality control measures.
The primary efficacy endpoint used in the present study was based on expert opinion of the risk of TOD resulting from persistently elevated BP. A reduction of SBP below 150 mmHg would decrease the risk of TOD from moderate to negligible according to the ACVIM consensus statement on hypertension.1 Cats entering the study with a baseline SBP between 165 and 176 mmHg, would respond if their SBP was reduced below 150 mmHg (15% of the baseline SBP). Responders with starting SBP between 177 and 187 mmHg would notice a reduction in SBP below 160 mmHg (going from moderate or severe risk to mild risk) and those between 188 and 211 mmHg baseline SBP, would be taken from severe risk to moderate risk (160–180 mmHg) of TOD. Thus, achievement of primary efficacy endpoint, by consensus, should lead to protection of hypertensive cats against TOD.
Two‐thirds of the recruited cats (63%) randomized to receive amlodipine were responders whereas just 1 in 6 cats (18%) apparently responded to placebo. The reason why administration of placebo led to a reduction in BP relative to baseline is unclear. One possible explanation is that this represented a training effect in response to repeated BP measurement sessions over a period of 3–4 months. Classification of a cat as a responder was not influenced by factors other than amlodipine treatment (e.g. baseline SBP, concomitant ACE inhibitor treatment, underlying disease). In previously published studies, administration of amlodipine has been associated with reductions in SBP of over 40 mmHg.2, 3, 6 The most likely reasons for larger reduction in BP achieved in previous studies, is that a higher proportion of cats enrolled in these studies had baseline SBP values above 200 mmHg. Although practitioners could use their discretion in enrolling cats with such high SBP in the present study, it seems likely that as this was a randomized placebo‐controlled trial, the veterinarians and owners were reluctant to enroll cats with such high SBP particularly where there was evidence of TOD (usually hypertensive retinopathy). Indeed, in the present study, secondary analysis showed that the absolute magnitude of reduction in SBP was influenced by baseline SBP value as well as whether the cat received amlodipine, supporting the conclusion that smaller reduction in SBP seen in the present study is explained by the relatively lower baseline SBP of the cats enrolled.
Nevertheless, the population of cats enrolled in the present study appears to be typical of those seen in clinical practice that are diagnosed with systemic arterial hypertension.2, 5, 10, 11, 12, 13 They were typical in terms of age (median age 14 years), sex (equal numbers of neutered males and females), breed (majority of cats being nonpedigree) and underlying disease (CKD International Renal Interest Society (IRIS) stage 2 and 3, hyperthyroidism and idiopathic making up the majority of the diagnoses). The major difference from previous studies was the relatively low prevalence of hypertensive retinopathy seen, again possibly explained by the reluctance of veterinarians and owners to enroll cats into a placebo‐controlled study when there is clear evidence of TOD. The block randomization procedure produced 2 groups well‐matched in terms of all of these factors as well as the baseline SBP. The post‐treatment SBP achieved with amlodipine treatment in the present study was similar to that seen in other studies (median value of 150 mmHg). As discussed above, reduction in SBP achieved relative to that seen in the placebo was clinically significant and highly likely to be protective in the cats responding. Only a small minority of cats (16%) were taking ACE inhibitors and this concomitant treatment did not influence whether a cat was classified as a responder or not.
Looking at SBP and responder rates by disease etiology, cats with idiopathic hypertension showed higher proportion of nonresponders compared to cats with other underlying diseases like CKD or hyperthyroidism. Present medical history, AEs during the study or age of cats gave no obvious explanation for these cats not responding to treatment. However, the conclusion that cats with idiopathic hypertension react less to amlodipine than cats with other diseases cannot be made because of small number of cases.
The factors that meant the minority (just over a third) of cats being nonresponders to amlodipine remain to be determined; possible factors including poor compliance, individual (including genetic) variation in pharmacodynamics or pharmacokinetics of amlodipine or resistance of the underlying pathophysiology of the hypertension to arterial vasodilators. In human medicine, large studies of factors influencing response of hypertensive patients to amlodipine have not identified genetic factors, either through a candidate gene approach14 or genome wide association studies.14, 15 In a human clinical trial,15 low calcium, low cholesterol, and low urinary sodium excretion were all associated with more effective response of patients to the BP lowering effect of amlodipine. In humans, amlodipine is a CYP3A enzyme substrate and some studies have identified certain genotypes (e.g. CYP3A5*3/*3) to be associated with greater response to amlodipine but this is highly dependent on the population studied and complicated by the role of the CYP3A enzyme involved in cortisol and corticosterone metabolism; and the explanation of differences seen between different genotypes of CYP3A may not necessarily relate to pharmacokinetics of amlodipine.14 It is clear that future research should explore factors explaining the variation in response of client‐owned hypertensive cats to amlodipine.
Nevertheless, the present study confirms amlodipine as a highly efficacious antihypertensive agent. The AEs seen in the present study were what might be expected in elderly cats with multiple medical problems. They were very similar in frequency and nature between amlodipine and placebo groups over the first 28 days of study indicating that they were unlikely to be directly related to amlodipine treatment itself.
The present study has confirmed the efficacy and safety of amlodipine as a monotherapy for majority of cats with hypertension. Cats given a placebo showed a small reduction in BP with a small minority being classified as responders demonstrating the importance of including a placebo when assessing antihypertensive drug treatments in hypertensive cats.
Conclusion
The present study shows that amlodipine is superior to placebo in treatment of client‐owned cats with hypertension. The chewable amlodipine formulation effectively reduced SBP, had good palatability and was well‐tolerated. It can be used concomitantly with ACE inhibitors and in cats with CKD.
Acknowledgments
The authors thank all the veterinarians, owners of cats, and study team members who participated in the study.
Conflict of Interest Declaration: Authors declare potential conflicts of interest. M. Huhtinen and J. Aspegrén are employees of the sponsor Orion Corp. C. Zemirline is employee of the sponsor Ceva‐Sogeval; Prof. Elliott has the following interest disclosures: Consultancy: Pfizer Animal Health/Zoetis, Ceva Animal Health, Boehringer Ingelheim UK Ltd, Vétoquinol Magny‐Vernois, Orion Corp., Elanco Animal Health, Idexx Laboratories, Inc, Niche Generics Ltd., Triveritas Ltd., Virbac Ltd. Advisory board membership: International Renal Interest Society (supported by Novartis) European Emesis Council (sponsored by Pfizer Animal Health – now Zoetis) Cardiorenal Board—Vétoquinol Magny‐Vernois, Idexx Renal Advisory Board, Research Grants or contracts: Vétoquinol Magny‐Vernois, Novartis Pharmaceuticals UK Ltd, Pfizer Animal Health Ltd (now Zoetis), Royal Canin SAS, Boehringer Ingelheim UK Ltd, Waltham Centre for Pet Nutrition, Ceva Animal Health, Orion Corp.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Funding: This study was sponsored by Orion Corporation Orion Pharma and Ceva‐Sogeval.
The work was done at 20 veterinary practices in Finland, Germany and France.
Key results of this study were presented as an abstract and oral presentation at ECVIM‐CA meeting in Mainz, Germany, September 2014.
Footnote
S+BMedVET GmbH, Babenhausen, Germany.
References
- 1. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation, and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 2. Henik RA, Snyder PS, Volk LM. Treatment of systemic hypertension in cats with amlodipine besylate. J Am Anim Hosp Assoc 1997;33:226–234. [DOI] [PubMed] [Google Scholar]
- 3. Snyder PS. Amlodipine: a randomized, blinded clinical trial in 9 cats with systemic hypertension. J Vet Intern Med 1998;12:157–162. [DOI] [PubMed] [Google Scholar]
- 4. Snyder PS, Sadek D, Jones GL. Effect of amlodipine on echocardiographic variables in cats with systemic hypertension. J Vet Intern Med 2001;15:52–56. [DOI] [PubMed] [Google Scholar]
- 5. Elliott J, Barber PJ, Syme HM, et al. Feline hypertension: clinical findings and response to antihypertensive treatment in 30 cases. J Small Anim Pract 2001;42:122–129. [DOI] [PubMed] [Google Scholar]
- 6. Jepson RE, Elliott J, Brodbelt D, Syme HM. Effect of control of systolic blood pressure on survival in cats with systemic hypertension. J Vet Intern Med 2007;21:402–409. [DOI] [PubMed] [Google Scholar]
- 7. FDA‐CVM . Guidance for Industry #85; Good Clinical Practice – VICH GL9. Rockville, MD: U.S. Department of Health and Human Services; 2001. [Google Scholar]
- 8. Mathur S, Syme H, Brown CA, et al. Effects of the calcium channel antagonist amlodipine in cats with surgically induced hypertensive renal insufficiency. Am J Vet Res 2002;63:833–839. [DOI] [PubMed] [Google Scholar]
- 9. Martel E, Egner B, Brown SA, et al. Comparison of high‐definition oscillometry – a non‐invasive technology for arterial blood pressure measurement – with a direct invasive method using radio‐telemetry in awake healthy cats. J Feline Med Surg 2013;15:1104–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Littman MP. Spontaneous systemic hypertension in 24 cats. J Vet Intern Med 1994;8:79–86. [DOI] [PubMed] [Google Scholar]
- 11. Syme HM, Barber PJ, Markwell PJ, Elliott J. Prevalence of systolic hypertension in cats with chronic renal failure at initial evaluation. J Am Vet Med Assoc 2002;220:1799–1804. [DOI] [PubMed] [Google Scholar]
- 12. Maggio F, DeFrancesco TC, Atkins CE, et al. Ocular lesions associated with systemic hypertension in cats: 69 cases (1985–1998). J Am Vet Med Assoc 2000;217:695–702. [DOI] [PubMed] [Google Scholar]
- 13. Chetboul V, Lefebvre HP, Pinhas C, et al. Spontaneous feline hypertension: clinical and echocardiographic abnormalities, and survival rate. J Vet Intern Med 2003;17:89–95. [DOI] [PubMed] [Google Scholar]
- 14. Zhang YP, Zuo XC, Huang ZJ, et al. CYP3A5 polymorphism, amlodipine and hypertension. J Hum Hypertens 2014;28:145–149. [DOI] [PubMed] [Google Scholar]
- 15. Hiltunen TP, Kontula K. Clinical and molecular approaches to individualize antihypertensive drug therapy. Ann Med 2012;44(Suppl 1):S23–S29. [DOI] [PubMed] [Google Scholar]


