Abstract
Background
Medullary elevation (ie, medullary kinking) at the craniocervical junction (CCJ) is reported in dogs with Chiari‐like malformations (CM), but its diagnostic criteria and clinical relevance are unclear.
Objective
To describe the position of the medulla at the CCJ in mature cavalier King Charles spaniels (CKCS), and evaluate its relationship with clinical status and the presence of syringomyelia.
Animals
Thirty‐six CKCS, 5–12 years of age, including 16 asymptomatic dogs.
Methods
Dogs were assigned a neurologic grade; magnetic resonance imaging (MRI) of the CCJ then was performed. The presence of a CM and syringomyelia was recorded and syringomyelia severity was quantified. Medullary position was quantified using the medullary kinking index, the elevation angle and obex position relative to the foramen magnum. The relationship between medullary position measures and presence and severity of neurologic signs and syringomyelia was investigated.
Results
Chiari‐like malformation was found in 33 dogs; 26 of them had syringomyelia. Mean medullary kinking index was 46.4% (SD, 10.3), elevation angle was 132° (SD, 12) and obex position was 3.5 mm (SD, 0.8). A higher medullary kinking index was associated with the presence of neurologic signs (P = .0368). Obex position was associated with the presence (P = .0018) and severity of syringomyelia (P = .0164).
Conclusions and clinical importance
There is a significant association between medullary elevation and clinical signs, whereas more caudal brainstem positions appear related to the presence of syringomyelia.
Keywords: Cervicomedullary, Chiari‐like, Medullary elevation, Medullary kinking
Abbreviations
- CKCS
cavalier King Charles spaniels
- CM
Chiari‐like malformation
- MRI
magnetic resonance imaging
Chiari‐like malformations (CM) are defined by cerebellar herniation and crowding of the foramen magnum, with the absence of cerebrospinal fluid at the craniocervical junction; these frequently are associated with syringomyelia.1, 2 Medullary elevation3, 4 or “kinking,”5, 6, 7, 8, 9, 10, 11 also is considered a component of canine CM that occurs in conjunction with this disease with a reported prevalence of 40–68% in toy and small breed dogs,7, 12 and of 66–100% in Cavalier King Charles spaniels (CKCS).5, 8, 9 It can be identified on magnetic resonance imaging (MRI) studies as a ventrally concave and elevated appearance of the caudal medulla oblongata at its junction with the cranial cervical spinal cord (ie, the cervicomedullary junction), independent of bony structures (ie, exclusive of direct medullary compression).5, 13, 14 Compression of the subarachnoid space may be seen at this level in up to 70% of cases.9 Medullary “elongation”8 and herniation through the foramen magnum1 also have been proposed as components of CM in dogs.
Medullary kinking and herniation are well recognized in humans with Chiari type II malformations, and indeed, the terminology in dogs appears to have been adopted from the human medical literature. In humans, medullary kinking is described as herniation or distal displacement of the medulla oblongata and fourth ventricle through the foramen magnum and into the spinal canal, resulting in downward “kinking” or “buckling” of the medulla oblongata at the level of the gracile and cuneate nuclei.15, 16 Herniation of the brainstem and medullary kinking can occur concurrently, and are known to impact the clinical presentation of affected patients. In particular, the condition in humans has been linked to both neuropathic pain and cervicomedullary dysfunction, particularly if kinking is located distal to the fourth cervical vertebra.15
The characteristics of the “kink” differ, however, between the conditions in dogs and humans. In the latter, at the level of the s‐shaped “kink,” the medulla oblongata lies caudal to (ie, “behind”) the upper cervical spinal cord.16 This positioning is observed in 70% of human patients with type II Chiari malformation,15, 16 at the caudal‐most extent of the cerebellar herniation, along with other concurrent anomalies, such as spina bifida, arachnoid diverticulae, and diastematomyelia. The distal‐most aspect of the kink occurs most commonly at the level of the second to fourth cervical vertebrae, although it can be located as far distally as the upper thoracic vertebrae.15, 16, 17 Brainstem herniation also may be present along with Chiari 1.5 malformations.17
In contrast, with medullary kinking in dogs, the “kinked” medulla oblongata appears to be elevated as a whole, rather than overlapping with the cervical spinal cord,15, 16 and herniation of the brainstem through the foramen magnum is described only as a subjective finding.16 Neither dorso‐ventral overlapping of the medulla oblongata with the cervical spinal cord, nor positioning of the “kinked” medulla oblongata farther distal than the second cervical vertebrae is described.5, 6, 7, 8, 9, 10, 11, 12, 14 Thus, the term “medullary elevation” more accurately may reflect the morphologic changes than the term “medullary kinking.”
Until recently, “medullary kinking” had been reported as a subjective finding (ie, present or absent), rather than as an objective evaluation of medullary position. Although it was suspected of influencing the clinical status of affected dogs,9 studies evaluating this relationship in young CKCS did not find any associations between its presence and that of either clinical signs or syringomyelia.5 More recently, a medullary kinking index was described7 as an objective measure of medullary elevation at the craniocervical junction. Our study aims to utilize the newly described index to evaluate the relationship of this and other novel measures of medullary position to both clinical signs and syringomyelia in a group of mature CKCS. We also aim to assess the position of the medulla oblongata with respect to the foramen magnum (ie, medullary herniation) and the relationship of this measurement with clinical signs, syringomyelia, or both.
Methods
Inclusion Criteria
Thirty‐six CKCS were prospectively recruited. Dogs enrolled in this study also took part in a separate concurrent study. Full recruitment details and inclusion criteria are described in that study.5
Clinical and MRI Assessment
Each owner was interviewed in person at the time of imaging and completed a questionnaire inquiring about the dog's behavior at home, signs of neuropathic pain or signs of neurologic dysfunction. Specifically, the questionnaire determined whether the owner had noted the following: gait change; scratching or rubbing of the neck, head, or shoulders, decreased interactivity with owners compared to littermates or housemates; episodes of crying out; or, other indicators of apparent pain seen at home. When scratching or rubbing was seen, owners were asked which area was involved, frequency of occurrence, and factors inciting the behavior. Owners also were questioned about past and current medical or surgical management (eg, medications, dosing regimen, and response to treatment). A full neurologic examination then was performed by 1 of the investigators (SCG or NJO). Findings were used to assign each dog a neurologic grade between 0 and 5, according to previously published criteria.5
In preparation for imaging, each dog was anesthetized (see companion study) then positioned for MRI1 in sternal recumbency with the craniocervical junction in an extended position (ie, neck lying flat on the table), in a typical position for imaging of the head and neck. Acquired sequences included: T1‐ and T2‐weighted sagittal images and T2‐weighted transverse images of the craniocervical junction and cervical spine. Images were uploaded into OsiriX Medical Imaging Software (open source software, www.osirix-viewer.com) and assessed for the presence of CM (ie, cerebellar indentation, herniation, or both through the foramen magnum, and loss of cerebrospinal fluid at the craniocervical junction) and syringomyelia. In dogs with syringomyelia, a grade between 0 and 3 was assigned to denote severity (Table 1).
Table 1.
Grading criteria for syringomyelia severity.
| Grade | Severity of SM |
|---|---|
| 0 | None |
| 1 | <33% of spinal cord |
| 2 | 33–60% of spinal cord |
| 3 | >60% of spinal cord |
SM, syringomyelia.
The position of the medulla oblongata was quantified using 2 objective measures, as follows: the recently described medullary kinking index,7 and the angle formed between the ventral and caudal margins of the medulla oblongata (ie, the medullary elevation angle), to quantitate medullary elevation at the cervicomedullary junction (Fig 1). The former was evaluated by measuring the distance between the ventral margin of the ventral subarachnoid space and the ventral margin of the cervico‐medullary junction, at its point of maximal elevation. This distance was divided by the height of the nearest normal spinal cord, to obtain a percentage value. Lastly, to evaluate the position of the brainstem in relation to the foramen magnum, the distance between the obex (ie, caudodorsal‐most border of the fourth ventricle) and a line drawn parallel to the foramen magnum was measured (termed obex position, Fig 2). Identification of the obex as the caudal border of the brainstem has been previously described.6 Assessments were made by a single observer (SCG).
Figure 1.
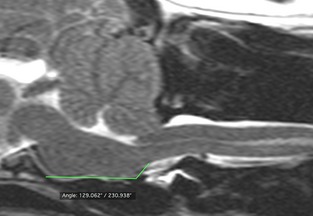
The angle formed between the ventral and caudal margins of the medulla oblongata was evaluated (medullary elevation angle) and used to quantitate medullary elevation at the cervicomedullary junction.
Figure 2.
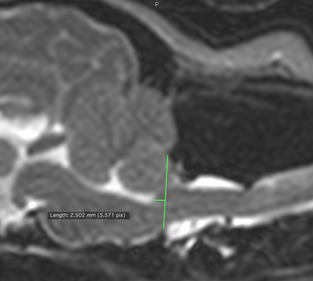
The position of the brainstem was evaluated by measuring the distance between the obex (ie, caudodorsal‐most border of the fourth ventricle) and a line drawn parallel to the foramen magnum. This was termed the obex measurement.
Statistical Analysis
Data were evaluated using SAS software2 and included the following parameters: the presence of a CM (Y or N), the variables (Y or N) and severity (grade 0–5) of clinical signs, syringomyelia presence (Y or N) and severity (grade 0–3), medullary kinking index, medullary elevation angle, and obex position. Ordinal factors, such as neurologic and syringomyelia grades were examined using contingency tables. The significance of relationships between ordinal variable pairs was established using Chi Square tests. Associations between disease severity and continuous measurements (eg, medullary kinking index, medullary elevation angle, obex position) were investigated using Spearman correlation coefficients. Continuous measurements were compared across 2 groups (ie, presence of syringomyelia) using Wilcoxon nonparametric tests, whereas Kruskal–Wallace tests were used to compare across 2 groups (ie, neurologic sign severity). The alpha level was decreased from 0.10 to 0.035 to control for the increased chance of false positives resulting from multiple comparisons. Receiver Operator Characteristic (ROC) curves were created to determine whether the medullary kinking index and obex position measurements could be used to predict the presence of neurologic signs or syringomyelia, respectively.
Results
Patient Characteristics
Thirty‐six CKCS were screened for craniocervical junction anomalies, including 15/36 male dogs (41.7%) ranging in age from 5 to 12 years (mean, 8.8 years; median, 9 years). Sixteen of these (44%) were asymptomatic; the remaining 20 (56%) had varying degrees of neck pain and dysesthesia. In 6 dogs, neck pain alone was found, without other sensory or neurologic signs. A single dog was assigned a neurologic grade of 5 (ie, ataxia and tetraparesis), but was excluded from statistical analysis because of the high likelihood of postoperative sequelae impacting the dog's clinical signs.18 Foramen magnum decompression had been performed in two other dogs without complications.
Imaging Findings
Chiari‐like malformations were found in 32 dogs (91.4%). Measurements could be performed in all dogs, including medullary index, angle, and obex position (Tables 2 and 3). Medullary kinking index measurements ranged from 31.1 to 92% (mean, 46.4; median, 46.4; SD, 10.3). Medullary elevation angles of 107–158 degrees were found (mean, 132; median, 130; SD, 12). Obex measurements ranged between 1.7 and 5.3 mm (mean, 3.5 mm; median, 3.4 mm; SD, 0.8) rostral to the foramen magnum.
Table 2.
Medullary kinking index, medullary angle, and obex position values per neurologic grade. Values are listed as mean/median.
| Neurologic Grade | 0 | 1 | 2 | 3 | 4 | 5 |
|---|---|---|---|---|---|---|
| Number of dogs per neurologic grade | 14 | 4 | 3 | 13 | 0 | 1 |
| Medullary kinking index | 41.8/39.5 | 47/46.6 | 59.5/50.9 | 47.9/48.4 | – | 51.4 |
| Medullary angle | 131/131 | 135/136 | 128/130 | 134/133 | – | 134 |
| Obex position | 3.9/3.6 | 3.1/3.1 | 3.3/3.3 | 3.4/3.3 | – | 3.5 |
Table 3.
Medullary kinking index and obex position measurements by clinical status and syringomyelia. Values are listed as (mean/median, SD).
| Medullary Kinking Index (%) | Obex Position (mm) | |
|---|---|---|
| Symptomatic | 49.6/47.8 (12) | 3.4/3.4 (0.7) |
| Asymptomatic | 43.3/42/2 (7.7) | 3.5/3.4 (0.8) |
| SM present | 47/46.9 (11.5) | 4.1/4.2 (0.8) |
| SM absent | 46.2/46.4 (7.3) | 3.2/3.3 (0.6) |
Relationship between Medullary Position and Clinical Signs
The medullary kinking index was found to be significantly associated with the presence of neurologic signs (P = .0368), but measurements of elevation angle (P = 0.5444) and obex measurements (P = .0827) were not. When considering the severity of signs, neurologic grade was not found to be significantly associated with medullary elevation index measurements (P = .2826), elevation angle (P = .9672), or obex measurements (P = .4789). Receiver Operated Characteristic analysis evaluating the medullary kinking index as a predictor of neurologic signs yielded an area under the curve of 0.713. When used as a diagnostic test, a cut‐off value of 44.8% had a sensitivity of 86% and a specificity of 64% for predicting the presence of neurologic signs.
Relationship between Medullary Position and Syringomyelia
Syringomyelia was present in 26 dogs (74.3%), and varied in severity (grade 1, 23%; grade 2, 38.5%; grade 3, 38.5%). Medullary kinking index and medullary angle measurements were not associated with the presence of syringomyelia (P = .9709, P = .9273, respectively) or severity (P = .0847, P = .5993, respectively). In contrast, lower obex position measurements (ie, obex closer to the foramen magnum) were significantly associated with both the presence (P = .0018) and severity (ie, grade; P = .0164) of syringomyelia. Receiver Operated Characteristic analysis evaluating the obex position measurement as a predictor of syringomyelia yielded an area under the curve of 0.846. When used as a diagnostic test, a cut‐off value of 3.5 mm had a sensitivity of 79% and a specificity of 90% for predicting the presence of syringomyelia. When considering the relationship between this measurement and syringomyelia grade, a 1 mm increase in the foramen magnum to obex measurement was associated with an almost 5‐fold increase in the odds of a higher syringomyelia grade (odds ratio, 4.86; confidence limits, 1.7–13.7; P = .0028).
Discussion
Medullary kinking has been reported to occur in the CKCS breed, with up to 100% of dogs subjectively demonstrating medullary elevation in 1 report.8 In addition, it has been suggested that medullary kinking plays a role in determining the presence of clinical signs6 and syringomyelia9 in affected dogs of this breed, although this relationship could not be confirmed using subjective evaluations of medullary position.5 In contrast, use of the medullary kinking index in this study identified a significant association between medullary position and clinical status (ie, the presence of neurologic signs). Specifically, more pronounced elevation of the medulla oblongata was present in dogs with clinical signs in comparison to asymptomatic dogs. Although further research is needed to determine whether this relationship is causal in nature, use of the medullary index may aid in evaluating potential causes of neuropathic pain in CKCS. This is particularly pertinent in light of the frequent diagnosis of >1 concurrent CCJ abnormality in affected dogs, making it difficult to determining the clinical relevance of each finding.5, 13, 19, 20, 21
Similarly, in humans, evaluating the severity of medullary kinking rather than its presence or absence is considered critical for determining whether it is likely to be associated with clinical signs. Specifically, patients with descent of the medulla oblongata (ie, level of “kinking”) below the level of the C3‐C4 intervertebral disk space are likely to be symptomatic, whereas clinical manifestations are not as consistently seen in patients in whom the kink does not extend beyond this disk space.15 However, in contrast to the human condition, the cause of pain associated with differences in medullary position in dogs has yet to be determined. Additional anatomic studies are needed to determine whether compression of C1 nerve roots could be implicated in addition to syringomyelia, as has been suggested in dogs with CM.22
Brainstem herniation also has been proposed to occur with CM, although its clinical relevance has not been fully elucidated.1 Movement of the medulla oblongata has been demonstrated using phase‐contrast MRI, whereby caudal movement of the medulla oblongata through the foramen magnum is seen with the arrival of a systolic pulse.23, 24 In addition, cerebellar pulsation (ie, degree of movement of the cerebellum during the cardiac cycle) was evaluated in a group of CKCS with CM, and was shown to differ significantly between dogs with or without syringomyelia.24 Similarly, although our study did not evaluate medullary movement, we found that static brainstem position measurements (ie, obex position) were associated with both the presence and severity of syringomyelia. In addition, an obex position measurement of ≤3.5 was sensitive (79%) and highly specific (90%) for the presence of syringomyelia. This measurement therefore may be useful as part of a comprehensive clinical evaluation of the craniocervical junction. Additional research is needed to determine the role of both brainstem herniation and pulsation in the development of syringomyelia.
Finally, the age of dogs in our study (median, 9 years) differed from that of previous reports of medullary kinking (median, 2.5 years).5 The older age of our study population could have influenced our results if the relationship between these measurements and clinical signs or syringomyelia changes as affected dogs age. Changes in craniocervical junction morphology with increasing age are reported in the CKCS breed, including increases in the severity of syringomyelia, the height of the foramen magnum, the length of cerebellar herniation, and the volume of the caudal cranial fossa.25 In addition, clinical signs reportedly worsen over time in 75% of CKCS with a CM and associated syringomyelia.26 Determining whether such a change occurs in medullary position necessitates performing a longitudinal study, however.
Conclusions
Our study provides 3 different objective measurements of medullary position in a cohort of mature CKCS. Using these measurements, we identified a relationship between higher medullary kinking indices and clinical status, but this relationship does not extend to syringomyelia. Lower obex measurements instead were associated with the presence of syringomyelia. Additional investigation is needed to determine whether the relationship between medullary position and clinical signs oe syringomyelia is causal in nature.
Acknowledgments
This work was funded by a grant from the American Cavalier King Charles Spaniel Club Charitable Trust.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The study was a collaborative effort between Cornell University and North Carolina State University's Colleges of Veterinary Medicine. Imaging was performed at both institutions.
Footnotes
MRI was performed on the following 1.5T scanners: 1, North Carolina State University (AnimalScan), MAGNETOM Symphony, Siemens Medical Solutions USA, Inc, Malvern, PA; 2, Cornell University: Vantage Atlas, Toshiba America Medical Systems, Tustin, CA.
Version 9.3, Cary, NC, USA.
References
- 1. Cappello R, Rusbridge C. Report from the chiari‐like malformation and syringomyelia working group round table. Vet Surg 2007;36:509–512. [DOI] [PubMed] [Google Scholar]
- 2. Harcourt‐Brown TR, Campbell J, Warren‐Smith C, et al. Prevalence of chiari‐like malformations in clinically unaffected dogs. J Vet Intern Med 2015;29(1):231–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Gibson KL, Ihle SL, Hogan PM. Severe spinal cord compression caused by a dorsally angulated dens. Prog Vet Neurol 1995;6:55–57. [Google Scholar]
- 4. Churcher RK, Child G. Chiari 1/syringomyelia complex in a king charles spaniel. Aust Vet J 2000;78:92–95. [DOI] [PubMed] [Google Scholar]
- 5. Cerda‐Gonzalez S, Olby NJ, McCullough S, et al. Morphology of the caudal fossa in cavalier king charles spaniels. Vet Radiol Ultrasound 2009;50:37–45. [DOI] [PubMed] [Google Scholar]
- 6. Schmidt MJ, Wigger A, Jawinski S, et al. Ultrasonographic appearance of the craniocervical junction in normal brachycephalic dogs and dogs with caudal occipital (chiari‐like) malformation. Vet Radiol Ultrasound 2008;49:472–476. [DOI] [PubMed] [Google Scholar]
- 7. Marino DJ, Loughin CA, Dewey CW, et al. Morphometric features of the craniocervical junction region in dogs with suspected chiari‐like malformation determined by combined use of magnetic resonance imaging and computed tomography. Am J Vet Res 2012;73:105–111. [DOI] [PubMed] [Google Scholar]
- 8. Rusbridge C, ‘MacSweeny JE, Davies JV, et al. Syringohydromyelia in cavalier king charles spaniels. J Am Anim Hosp Assoc 2000;36:34–41. [DOI] [PubMed] [Google Scholar]
- 9. Carrera I, Dennis R, Mellor DJ, et al. Use of magnetic resonance imaging for morphometric analysis of the caudal cranial fossa in cavalier king charles spaniels. Am J Vet Res 2009;70:340–345. [DOI] [PubMed] [Google Scholar]
- 10. Cerda‐Gonzalez S, Dewey CW, Scrivani PV, Kline KL. Imaging features of atlanto‐occipital overlapping in dogs. Vet Radiol Ultrasound 2009;50:264–268. [DOI] [PubMed] [Google Scholar]
- 11. Wolfe KC, Poma R. Syringomyelia in the cavalier king charles spaniel (CKCS) dog. Can Vet J 2010;51:95–102. [PMC free article] [PubMed] [Google Scholar]
- 12. Dewey CW, Berg JM, Stefanacci JD, et al. Caudal occipital malformation syndrome in dogs. Compend Contin Edu Pract Vet 2004;26:886–895. [Google Scholar]
- 13. Driver CJ, Volk HA, Rusbridge C, Van Ham LM. An update on the pathogenesis of syringomyelia secondary to chiari‐like malformations in dogs. Vet J 2013;198:551–559. [DOI] [PubMed] [Google Scholar]
- 14. Dewey CW, Marino DJ, Loughin CA. Craniocervical junction abnormalities in dogs. N Z Vet J 2013;61:202–211. [DOI] [PubMed] [Google Scholar]
- 15. Curnes JT, Oakes WJ, Boyko OB. MR imaging of hindbrain deformity in chiari II patients with and without symptoms of brainstem compression. AJNR Am J Neuroradiol 1989;10:293–302. [PMC free article] [PubMed] [Google Scholar]
- 16. Naidich TP, McLone DG, Fulling KH. The chiari II malformation: Part IV. The hindbrain deformity. Neuroradiology 1983;25:179–197. [DOI] [PubMed] [Google Scholar]
- 17. Bollo RJ, Riva‐Cambrin J, Brockmeyer MM, Brockmeyer DL. Complex chiari malformations in children: An analysis of preoperative risk factors for occipitocervical fusion. J Neurosurg Pediatr 2012;10:134–141. [DOI] [PubMed] [Google Scholar]
- 18. Cerda‐Gonzalez S, Olby NJ, Griffith EH. Dorsal compressive atlantoaxial bands and the craniocervical junction syndrome: association with clinical signs and syringomyelia in mature cavalier King Charles spaniels. J Vet Intern Med 2015; doi:10.1111/jvim.12604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Cerda‐Gonzalez S, Dewey CW. Congenital diseases of the craniocervical junction in the dog. Vet Clin North Am Small Anim Pract 2010;40:121–141. [DOI] [PubMed] [Google Scholar]
- 20. Couturier J, Rault D, Cauzinille L. Chiari‐like malformation and syringomyelia in normal cavalier king charles spaniels: A multiple diagnostic imaging approach. J Small Anim Pract 2008;49:438–443. [DOI] [PubMed] [Google Scholar]
- 21. Fernandez AA, Guerrero AI, Martinez MI, et al. Malformations of the craniocervical junction (chiari type I and syringomyelia: Classification, diagnosis and treatment). BMC Musculoskelet Disord 2009;10(Suppl 1):S1‐2474‐10‐S1‐S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Rusbridge C, Jeffery ND. Pathophysiology and treatment of neuropathic pain associated with syringomyelia. Vet J 2008;175:164–172. [DOI] [PubMed] [Google Scholar]
- 23. March PA, Abramson CJ, Smith M, Murakami J. CSF flow abnormalities in caudal occipital malformation syndrome. J Vet Intern Med 2005;19:418–419. [Google Scholar]
- 24. Driver CJ, Watts V, Bunck AC, et al. Assessment of cerebellar pulsation in dogs with and without chiari‐like malformation and syringomyelia using cardiac‐gated cine magnetic resonance imaging. Vet J 2013;198:88–91. [DOI] [PubMed] [Google Scholar]
- 25. Driver CJ, De Risio L, Hamilton S, et al. Changes over time in craniocerebral morphology and syringomyelia in cavalier king charles spaniels with chiari‐like malformation. BMC Vet Res 2012;8:215‐6148‐8‐215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Plessas IN, Rusbridge C, Driver CJ, et al. Long‐term outcome of cavalier king charles spaniel dogs with clinical signs associated with chiari‐like malformation and syringomyelia. Vet Rec 2012;171:501–505. [DOI] [PubMed] [Google Scholar]


