Abstract
Background
Up to 60% of foals develop diarrhea within 6 months after birth. Preventive measures are limited but potentially probiotics could be used.
Objective
To evaluate the effect of a newly designed probiotic on the incidence of foal diarrhea in a randomized field trial.
Animals
Seventy‐two healthy neonatal foals.
Methods
Randomized, placebo‐controlled field trial. Foals were administered a placebo or probiotic for 3 weeks and monitored for an additional week. A total of 3 fecal samples were taken from each foal at biweekly intervals. Statistical modeling was applied for comparison of incidence and duration of diarrhea and fecal shedding of Clostridium perfringens and Clostridium difficile between treatment and age groups.
Results
The overall incidence of diarrhea was 41 of 72 (59%) and did not differ (P = 0.37) between treatment groups. Foals treated with probiotics were more likely to develop diarrhea requiring veterinary intervention (P = 0.007). Age had a significant effect on incidence of diarrhea (P < 0.001); foals 8–15 days old having the highest probability of developing diarrhea. Duration of diarrhea and soft feces were not significantly different between groups. The prevalence of C. perfringens shedding was 55% with no difference between treatment groups (P = 0.23). The prevalence of C. difficile shedding was 11%.
Conclusion and Clinical Importance
There was no benefit of administering a 3‐week course of probiotics, but potential adverse effects were noted. Whether the probiotics lacked a clinical effect, or the choice of strains or dose was inadequate, is unknown. Clostridial shedding was not influenced by probiotics despite in vitro activity of probiotics.
Keywords: Bifidobacterium, Lactobacillus
Abbreviations
- cdtA
gene coding for part of the binary toxin of Clostridium difficile
- cfu
colony‐forming units
- cpb2
gene coding for beta2 toxin of C. perfringens
- cpe
gene coding for enterotoxin of C. perfringens
- tcdA
gene coding for toxin A of Clostridium difficile
- tcdB
gene coding for toxin B of Clostridium difficile
Up to 60% of foals develop diarrhea in their first 6 months of life.1 The etiology is often undetermined and could be multifactorial; however, infectious agents, particularly bacteria such as Clostridium difficile and Clostridium perfringens could play important roles.1 Preventive measures are currently limited and novel approaches are needed.2 Probiotics have received increasing interest in veterinary medicine to prevent and treat enteric disease.3, 4, 5 Probiotics exert their beneficial effect through several pathways, including production of antimicrobial compounds targeting intestinal pathogens and their toxins, as well as general immune stimulation and colonization resistance.6 Few studies in horses have been performed to date to evaluate the efficacy of probiotics in prevention or treatment of enteric disease.4, 7, 8 The 2 studies on probiotic use to prevent neonatal foal diarrhea failed to show a lack of significant effect of probiotic treatment.3, 9 Potential reasons for this include the bacterial strain(s) chosen, the dose administered, the duration of administration, and timing of administration. A 1‐week treatment course was chosen in both of the above studies but probiotics are typically considered unable to persist in the gastrointestinal tract after cessation of administration.10 Longer periods of treatment could therefore be needed. Commercial probiotic products often contain unreliable and varying amounts of active ingredients and scientific evidence supporting these formulations is lacking.11 Recently, several commercial bacterial strains (Lactobacillus rhamnosus LHR 19 and SP1, L. plantarum LPAL and BG112 and Bifidobacterium animalis lactis) have been shown to inhibit growth of C. difficile and C. perfringens in vitro and to grow in the presence of acid and bile, making them suitable for further evaluation as animal probiotics.12
The objective of this study was to determine the effect of a probiotic formulation consisting of the 4 above described 4 Lactobacillus species and Bifidobacterium animalis lactis on the incidence of diarrhea and prevalence of fecal pathogen shedding in neonatal foals in a 4‐week randomized, placebo‐controlled clinical field trial.
Materials and Methods
The study was conducted as a randomized, placebo‐controlled blinded field trial on breeding farms in Southern Ontario, Canada. The study was conducted under approval of the Animal Use and Care Committee of the University of Guelph (AUP 1455).
Reporting of this trial follows the CONSORT 2010 guidelines.13
Study Design
A sample size calculation was performed assuming a diarrhea incidence of 50% in neonatal foals. A sample size of 93 animals was calculated to detect a 20% difference and achieve a power of 0.8 with an alpha error of 0.05. Foals from 7 breeding farms were enrolled at the time of birth with an anticipated 10–20 foals per farm. Inclusion criteria for farms included the birth of more than 10 foals per season and willingness to participate in the study.
All foals that were born by natural delivery between March 1st 2013 and July 30th 2014 and were clinically normal at the time of enrollment were eligible for inclusion. Determination of clinical status was made by an experienced farm manager on each farm. Exclusion criteria included presence of gastrointestinal disease or illness that would preclude the administration of probiotics as judged by the farm manager, as well as prior administration of antimicrobials or probiotics. No other probiotics were administered to the foals during the study and routine farm management was maintained.
Treatment and placebo packages were indistinguishable and were prepared with sequentially labeled numbers for each farm by one of the authors (AS). The treatment group of the first package for each farm was determined by coin toss, with subsequent packages assigned in an alternating fashion (consecutive stratified randomization per farm). Farm personnel were provided with the numbered treatment packages and instructed to assign the first foal born on the farm to treatment package 1 and continue in consecutive fashion, and were so blinded to the treatment groups.
The probiotic product contained 1 × 109 colony‐forming units (cfu) of each of L. rhamnosus SP1, L. rhamnosus LRH19, L. plantarum LPAL, L. plantarum BG112, and 1 × 1010 cfu of Bifidobacterium animalis lactis strain with starch added for a total weight of 2 g. The placebo product contained the starch without the bacterial cultures. Both products had indistinguishable visual appearance and odor. The products were distributed in individual 2 g dose sachets for each foal. Farm personnel were instructed to mix the contents with 10 mL of water or syrup and administer the solution via an oral dosing syringe.
The treatments were administered once daily for 21 days starting on day 3 of life. Farm personnel monitored the foals daily for 28 days. Farm personnel were instructed to record abnormal fecal consistency (firm, soft feces, diarrhea). If diarrhea occurred, farm personnel were instructed to record rectal temperature, appetite, and attitude. If the farm personnel considered the clinical signs severe enough for need of veterinary examination, the farm veterinarian was asked to assess the foal and decide on necessary treatments. If treatments were administered, they were recorded. A medical record sheet containing all of the above variables for each foal was supplied.
Fecal samples were collected from foals by rectal swab at 0–2, 2–4, and 4–6 weeks of age. The exact day of sampling was decided by farm personal. Samples were frozen at −80°C until analysis.
Bacterial Culture and Typing
Selective enrichment culture and molecular typing of C. perfringens and C. difficile was performed as previously reported.14 Please refer to the supporting document (Appendix S1) for further information.
Quality Control
Samples of probiotic and placebo sachets were analyzed for their content. Serial dilutions were performed in phosphate‐buffered saline and inoculated onto Man‐Rogosa‐Sharp agar.1 The plates were incubated anaerobically for 24 hours at 37°C and resulting colonies were counted.
Statistical Analysis
Outcomes of interest included the incidence of diarrhea, incidence of soft feces, and the incidence of diarrhea severe enough to require veterinary treatment. A generalized linear mixed model with random effects was used to examine the above outcomes of interest and fecal shedding of Clostridia spp. in relation to the age of the foal. A general linear mixed model was used to predict differences in duration of diarrhea and soft feces between groups. Farm and year were combined into 1 factor and were considered a random effect, whereas sex, treatment, and age group were considered fixed effects. Repeated measures were accounted for. Various error structures were examined (ar1, arh1, toep2‐3, toeph2‐3, un2‐3). The best‐fit error structure was chosen based on the Akaike information criterion or whether the model converged. Random effects, fixed effects, and their interactions with a P < 0.25 were retained in the final model. The correlation between a positive culture results and presence of abnormal feces was tested using the Pearson's correlation coefficient. Analysis was conducted by a commercial statistical software package.2 Significance was set at P < 0.05.
Descriptive statistics were used when data were not present in sufficient numbers for statistical analysis.
Results
A total of 93 foals from 7 farms met the inclusion criteria. One farm lost all medical records and the foals (10 in each treatment group) had to be removed from analysis. One foal (probiotic group) sustained a traumatic stifle injury requiring euthanasia and was removed. This left 72 foals from 6 farms for analysis, 36 foals in each treatment group. Of the resulting 72 foals, 68 (94%) were Thoroughbreds and 4 of 72 (6%) were Standardbreds. Twenty‐six (36%) foals were female and 46 of 72 (64%) were male. The initial plan to enroll more than 10 foals per farm was not accomplished on all farms because of human error. Each farm contributed between 4 and 20 foals. Clinical signs were inconsistently reported and therefore removed from analysis.
Incidence of Diarrhea and Soft Feces
The incidence of diarrhea was 41 of 72 (59%) and soft feces were identified in 47 of 72 (65%). The distribution for each treatment group is shown in Figure 1.
Figure 1.
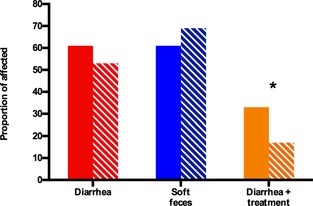
Proportional incidence of diarrhea, soft feces, and diarrhea in need of veterinary intervention, compared between 36 probiotic‐ and 36 placebo‐treated foals in a 4‐week randomized, blinded field trial. The solid bars represent the percentage of probiotic‐treated foals, the striped bars represent the percentage of placebo‐treated foals that developed the specified outcome. *Designates a significant difference between placebo‐ and probiotic‐treated foals (P = 0.007)
Age group had a significant effect on occurrence of diarrhea (P < 0.001), with a significantly higher incidence in foals between 8 and 15 days of age (Fig. 2). All pairwise comparisons are shown in Table 1. The factor farm/year was retained in the final model (P = 0.1). Sex (P = 0.06) and treatment (P = 0.37) did not have a significant effect on incidence of diarrhea.
Figure 2.
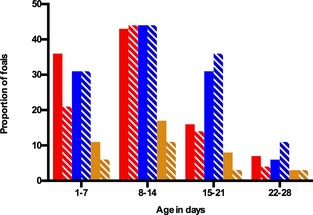
Incidence of diarrhea, soft feces, and diarrhea in need for veterinary intervention compared between 36 placebo‐ and 36 probiotic‐treated foals based on age group in a randomized, blinded field trial. The solid bars represent the percentage of probiotic‐treated foals, the striped bars represent the percentage of placebo‐treated foals that developed the specified outcome. Red bars represent foals that had diarrhea, blue bars represent foals with soft feces and orange bars represent foals with diarrhea requiring treatment. Age group had a significant effect on diarrhea (P < 0.001)
Table 1.
Odds ratios, confidence intervals, and P values from selected outcomes of a randomized, placebo‐controlled field trial in neonatal foals as determined by generalized linear mixed model analysis
| Outcome | Variable | Relationship Between Variables | Odds Ratio | Confidence Interval | P‐Value |
|---|---|---|---|---|---|
| Diarrhea | Sex | Female/Male | 2.12 | 0.04–1.54 | 0.06 |
| Treatment | Control/Probiotic | 0.71 | 0.35–1.48 | 0.37 | |
| Age group | 1/2 | 2.06 | 1.24–3.44 | 0.005 | |
| 1/3 | 7.81 | 4.01–15.20 | <0.001 | ||
| 1/4 | 43.34 | 13.90–135.04 | <0.001 | ||
| 2/3 | 3.78 | 2.11–7.76 | <0.001 | ||
| 2/4 | 20.99 | 7.25–60.75 | <0.001 | ||
| 3/4 | 5.56 | 2.08–14.80 | <0.001 | ||
| Soft feces | Sex | Not included in final model | |||
| Treatment | Control/Probiotic | 1.19 | 0.56–2.37 | 0.62 | |
| Age group | 1/2 | 2.49 | 1.40–4.43 | 0.002 | |
| 1/3 | 4.25 | 2.26–8.02 | <0.001 | ||
| 1/4 | 34.12 | 12.21–95.31 | <0.001 | ||
| 2/3 | 1.70 | 0.95–3.03 | 0.007 | ||
| 2/4 | 13.66 | 5.43–34.36 | 0.07 | ||
| 3/4 | 8.01 | 3.33–19.24 | <0.001 | ||
| Diarrhea and treatment | Sex | Not included in final model | |||
| Treatment | Control/Probiotic | 0.35 | 0.16–0.75 | 0.007 | |
| Age group | 1/2 | 3.46 | 1.85–6.46 | <0.001 | |
| 1/3 | 10.76 | 4.26–27.20 | <0.001 | ||
| 1/4 | 25.75 | 7.63–86.86 | <0.001 | ||
| 2/3 | 3.10 | 1.33–7.22 | 0.008 | ||
| 2/4 | 7.43 | 2.25–24.48 | 0.001 | ||
| 3/4 | 2.39 | 2.39–0.79 | 0.12 | ||
| Clostridium perfringens shedding | Sex | Not included in final model | |||
| Treatment | Control/Probiotic | 0.65 | 0.32–1.31 | 0.23 | |
| Age at fecal sampling (weeks) | 0–2/2–4 | 1.15 | 0.58–2.26 | 0.68 | |
| 0–2/4–6 | 1.75 | 0.80–3.80 | 0.15 | ||
| 2–4/4–6 | 1.52 | 0.72–3.20 | 0.27 | ||
1, age group 0–7 days; 2, age group 8–14 days; 3, age group 15–21 days; 4, age group 22–28 days.
There was a significant association between age and the occurrence of soft feces (P < 0.001). Pairwise comparisons are shown in Table 1. The factor farm/year was retained in the final model (P = 0.08). Sex (P = 0.88) and treatment (P = 0.62) did not have a significant effect.
Eighteen (25%) foals had diarrhea severe enough to require veterinary intervention of which 12 of 18 (66%) foals were in the probiotic and 6 of 18 (33%) were in the placebo group (Fig. 1), respectively. Placebo‐treated foals were less likely to develop diarrhea in need of veterinary treatment than probiotic‐treated foals (OR: 0.35, CI: 0.16–0.75, P = 0.007). There was a significant effect of age (P < 0.001). Pairwise comparisons are shown in Table 2. The factor farm/year was retained in the final model (P = 0.06). Sex (P = 0.88) did not have a significant effect. Medical treatments of foals included antimicrobial treatment (metronidazole, trimethoprim sulfonamide, ceftiofur, oxytetracycline, penicillin, and chloramphenicol) and analgesic treatment (flunixin meglumine). Additional treatments recorded were di‐tri‐octahedral smectite, butylscopolamine, ranitidine, omeprazole, and sucralfate.
Table 2.
Longitudinal fecal shedding of Clostridium difficile and Clostridium perfringens of foals treated with placebo or a probiotic formulation for 3 weeks
| Age at Fecal Sampling (weeks) | Overall | Probiotic | Placebo | |
|---|---|---|---|---|
| C. difficile | 0–2 | 8/71 (11%) | 5/35 (14%) | 3/36 (8%) |
| 2–4 | 10/60 (17%) | 5/30 (17%) | 5/30 (17)% | |
| 4–6 | 2/47 (4%) | 1/22 (5%) | 1/25 (4%) | |
| Overall | 20/178 (11%) | 11/87 (13%) | 9/91 (10%) | |
| C. perfringens | 0–2 | 43/71 (61%) | 24/35 (69%) | 19/36 (53%) |
| 2–4 | 34/60 (57%) | 17/30 (57%) | 17/30 (57)% | |
| 4–6 | 21/47 (45%) | 10/22 (46%) | 11/25 (44%) | |
| Overall | 98/178 (55%) | 51/87 (59%) | 47/91 (52%) |
Odds ratios and their confidence intervals are reported in Table 2.
Duration of Diarrhea
Duration of diarrhea ranged from 0 to 11 days in both groups. The distribution per group is shown in Figure 3. The factor farm/year (P = 0.19) and the interaction of the factor farm/year and treatment (P = 0.17) were retained in the final model. There was no impact of treatment (P = 0.31) or sex (P = 0.06) on duration of diarrhea. There was no apparent impact of treatment (P = 0.8) or age (P = 0.2) on the occurrence of soft feces.
Figure 3.
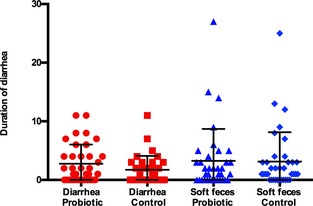
Duration of diarrhea and soft feces in 36 placebo‐ and 36 probiotic‐treated foals are shown. Mean and standard deviation are shown. There was no significant effect of treatment on duration of either soft feces or diarrhea.
Bacteriologic Cultures
A total of 178 fecal samples were collected, each foal had at least 2 samples collected. The time prevalence rate was 20 of 178 (11%) and 98 of 178 (55%) for C. difficile and C. perfringens, respectively. This corresponded to a foal‐level time prevalence rate of 20 of 72 (28%) for C. difficile and 45 of 72 (63%) for C. perfringens. Prevalence data for Clostridia spp. based on age are presented in Table 3.
Neither treatment (P = 0.23) nor age (P = 0.35) had a significant effect on prevalence of C. perfringens shedding. The factor farm/year (P = 0.08) was retained in the model. Odds ratios and confidence intervals are presented in Table 1. Clostridium difficile shedding decreased over time (Fig. 4). The prevalence was too low for statistical comparisons. Please see the supporting document for results of the clostridial typing (Appendix S1).
Figure 4.
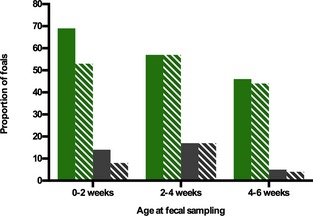
Longitudinal prevalence of fecal shedding of Clostridium perfringens and C. difficile in a randomized, placebo‐controlled field trial in neonatal foals. The solid bars represent the percentage of probiotic‐treated foals, the striped bars represent the percentage of placebo‐treated foals that were shedding either C. difficile or C. perfringens. The green bars represent data for C. perfringens; the gray bars represent data for C. difficile.
Association of Culture Status and Presence of Diarrhea
Fecal consistency could only be determined for the 131 of 178 (74%) fecal samples taken during the 4‐week monitoring period. Thirty‐nine of 131 (30%) times the foals had diarrhea when sampling occurred, of which 22 (56%) were C. perfringens positive. There was no statistically significant correlation of having diarrhea and a C. perfringens positive fecal culture (r 2 = 0.007, P = 0.13). Clostridium perfringens Type C was isolated from 4 samples, 2 of 4 (50%) were taken when the foals had clinical signs of soft feces, 1 (25%) was taken from a foal without clinical signs. The remaining isolate (25%) was taken after the monitoring period had ended; therefore, the fecal consistency was unknown. The 3 C. perfringens strains containing the gene coding for beta2 toxin (cpb2) were isolated from foals with normal feces. Five of 39 (13%) diarrheic samples were C. difficile positive. There was no statistically significant correlation between the presence of clinical signs and C. difficile‐positive fecal culture result (r 2 = 0.0012, P = 0.13).
Quality Control of Probiotics
The placebo sachets yielded no bacterial growth. The probiotic sachets yielded between 3–4 × 103 colony‐forming units Lactobacillus spp. colonies and 1.3–4.2 × 103 − 104 Bifidobacterium spp. colonies.
Discussion
This study showed that a 3‐week course of prophylactic probiotic treatment did not reduce the incidence or duration of diarrhea in neonatal foals and could have even contributed to foals developing more severe disease. The probiotic strains were selected for their in vitro ability to inhibit pathogens; however, there was no effect on fecal shedding of C. perfringens in vivo. The cumulative prevalence of C. difficile and C. perfringens fecal shedding in healthy foals was similar to previous reports.
Probiotics have been shown to be unable to colonize the gastrointestinal tract of horses and it is therefore unlikely that they can act beyond their period of administration.7, 10 The 1 week of treatment course chosen in previous studies therefore might not be sufficient to see an effect.3, 5, 9 A treatment period of 3 weeks was chosen for this study. The lack of a beneficial effect of probiotic treatment, despite the longer administration period, during the time of highest diarrhea incidence between 8 and 15 days, is disappointing and raises further concern about their potential efficacy. In 1 previous study, a multistrain probiotic product derived from equine gastrointestinal contents was studied in a 1‐week randomized, placebo‐controlled, double‐blinded clinical trial. Foals in the probiotic group showed statistically significant larger weight gain after treatment and a significantly lower incidence of diarrhea.9 These effects, however, were only significant at one time point (2–3 weeks of age), which was after probiotic administration had ceased and the relevance of a single period of increased weight gain, with no discernable clinical impact, is debatable. In another randomized, placebo‐controlled clinical trial, assessing an equine‐derived strain of Lactobacillus pentosus, probiotic administration was associated with a significantly higher incidence of diarrhea, presence of clinical and the need for veterinary examination and treatment.3 In that study, an equine‐derived strain was chosen as probiotic and the authors postulated that the increased incidence of severe diarrhea seen could have been caused by excessive organic acid (eg, lactic acid) production by overgrowing lactobacilli in the relatively poorly developed neonatal intestinal microbiota.3 As this probiotic led to an increased incidence of diarrhea, we chose to evaluate probiotic strains used for human probiotic formulations and with in vitro evidence for inhibitory activity against C. difficile and C. perfringens.12 The reasons for the increased incidence of diarrhea requiring treatment in our study are unclear, but could also relate to an increase in lactic acid‐producing bacteria. C. perfringens did not appear to be involved in pathogenesis of the diarrhea as shedding rates of C. perfringens were not significantly different between the treatment and placebo groups.
Probiotic treatment decreased incidence of diarrhea by 45% in a study where 130 healthy neonatal foals were administered probiotics once a week for up to 20 weeks.5 The evidence for a beneficial effect should however be considered weak, as the study had several limitations, including lack of blinding. It is further unclear how randomization and monitoring of the foals was performed and why an unequally sized control group was used. Quality control of the administered probiotic was also not performed.
The probiotic was designed to contain 1 × 107–9 bacterial colonies per 2 g. Only 3–4 × 103–4 of bacteria per 2 g were grown when the probiotics were assessed for content. The testing was performed 18 months after the manufacturing date but within the expiry date. While this is disappointing, it has been shown before that many veterinary and human probiotic preparations are not being accurately represented by label claims.15 It is arguable that the lack of a positive effect in this study could be because of an inadequate dose; however, presence of a significantly higher incidence of diarrhea requiring veterinary treatment in the probiotic group suggests that there was an effect of the probiotic, albeit an undesirable one.
There was no impact of treatment on shedding of C. difficile and C. perfringens. However, data obtained here provide more insight into the epidemiology of these potentially important pathogens in foals. A previous study of C. difficile reported isolation of the bacterium from 30% of foals shortly after birth, decreasing to 3% by 4 weeks of age and 0% at 6 weeks.16 Similarly in this study, initial prevalence in the first 2 weeks after birth was 17% and decreased to 13% at 4 weeks and was 0% at 6 weeks of age. A high prevalence of C. difficile colonization in healthy neonates followed by a rapid decline as the animal (and its microbiota) matures has also been reported in other species.17 As the prevalence dropped to <1% at 4–6 weeks of age, this approaches prevalence of 0–8% in most studies of healthy adult horses.14, 16, 18, 19, 20
The prevalence of 57% for fecal shedding of C. perfringens is similar to a previous study where a prevalence of 62% in 0‐ to 2‐month‐old foals was reported.21 Unlike with C. difficile, the prevalence did not decrease substantially at 6 weeks of age, which is consistent with a previous study where prevalence rates remained at 30% at 1–2 months of age.21 Clostridium perfringens shedding in healthy adults other than broodmares is uncommon, with most studies reporting rates <1%,14, 22, 23, 24 so further studies are needed to determine when prevalence of C. perfringens shedding decreases.
The lack of effect of the probiotic formulation on C. difficile and C. perfringens shedding in the current study is disappointing given the in vitro inhibitory activity against these pathogens.12 In vitro results cannot necessarily be extrapolated to in vivo conditions, as was evident here. In vitro testing should be performed to detect potential beneficial and harmful effects, but effects need to be confirmed in randomized, placebo‐controlled clinical trials. The effect of probiotics to decrease shedding of C. difficile and C. perfringens has not been studied in animals previously, but 2 studies have been performed to evaluate the effects of probiotics on Salmonella shedding. In 1 study assessing probiotic administration in the postoperative period after colic surgery, no difference in Salmonella shedding rates was seen between groups.25 In a second study, 5‐day administration of probiotics to horses with colic did not result in significantly reduced shedding rates of Salmonella.26 Please see the supporting document for discussion of the clostridial types.
There were several limitations to this study. Because of the low number of foals born in that remaining foaling season, the study period was extended to the following breeding season. The factor farm/year had to be retained in most statistical models, indicating that a higher number of farms should have been included to decrease the influence of this factor. The selection of farms was made based on location for the convenience of sampling. We could not determine whether foals had other clinical signs at the time of diarrhea as these were recorded inconsistently and were removed from analysis. The farm veterinarians made the decision to treat a foal and reasons for this decision were not recorded. The sample size at the end of the study was smaller than initially calculated because of unforeseen circumstances. It was elected not to extend the study period further as preliminary statistical analysis showed no evidence of a trend toward efficacy, and a negative impact on what is perhaps the most important clinical outcome, requirement of veterinary treatment.
Probiotics are often approached by owners and veterinarians with the thought that they will at worst be ineffective, something that might not be true. Results of this study do not indicate that the concept of probiotic treatment is futile in prevention of neonatal diarrhea. Yet, these results, combined with those of another study3 that identified a negative impact of probiotics in neonatal foals raise concerns. Selected probiotics have been shown to be effective for some gastrointestinal disorders in humans and domestic animals yet prevention of neonatal diarrhea has not been reported.27, 28 Further, successful probiotic studies have often involved inflammatory, not infectious, disorders,29, 30 raising questions of the potential efficacy of probiotics in foals given the pathophysiology of foal diarrhea. Recent advances in the knowledge of the equine intestinal microbiota could be used to design equine‐specific probiotics and further investigation of this area is required. This study, though, suggests that commercial products that lack published safety and efficacy data should be approached with caution.
Supporting information
Appendix S1. C difficile and C. perfringens typing methods, results, and discussion.
Acknowledgments
The authors thank William Sears for kindly providing help with the statistical analysis and Joyce Rousseau for laboratory assistance. The authors also thank Sacco S.R.L for providing the probiotic and placebo formulations.
Funding: This study was supported by a fellowship of the Danish Centre of Antimicrobial Research and Development (DanCARD) funded by the Danish Council for Strategic Research and an operating grant by Equine Guelph. Neither the funding agencies nor the company providing the treatments had any involvement in study design, collection, analysis, and interpretation of data; in the writing of the manuscript; and in the decision to submit the manuscript for publication.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was performed at the University of Guelph.
Parts of this manuscript have been presented as a research abstract at the Annual Conference of the European College for Equine Internal Medicine.
Footnotes
Insta Gene Matrix, Bio‐Rad, Mississauga, ON, Canada
Proc GLIMMIX, SAS 9.2
References
- 1. Frederick J, Giguere S, Sanchez LC. Infectious agents detected in the feces of diarrheic foals: A retrospective study of 233 cases (2003‐2008). J Vet Intern Med 2009;23:1254–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feary DJ, Hassel DM. Enteritis and colitis in horses. Vet Clin North Am Equine Pract 2006;22:437–479. [DOI] [PubMed] [Google Scholar]
- 3. Weese JS, Rousseau J. Evaluation of Lactobacillus pentosus WE7 for prevention of diarrhea in neonatal foals. J Am Vet Med Assoc 2005;226:2031–2034. [DOI] [PubMed] [Google Scholar]
- 4. Boyle AG, Magdesian KG, Durando MM, et al. Saccharomyces boulardii viability and efficacy in horses with antimicrobial‐induced diarrhoea. Vet Rec 2013;172:128. [DOI] [PubMed] [Google Scholar]
- 5. Tanabe S, Suzuki T, Wasano Y, et al. Anti‐inflammatory and intestinal barrier‐protective activities of commensal lactobacilli and bifidobacteria in thoroughbreds: Role of probiotics in diarrhea prevention in neonatal thoroughbreds. J Equine Sci 2014;25:37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Oelschlaeger TA. Mechanisms of probiotic actions—A review. Int J Med Microbiol 2010;300:57–62. [DOI] [PubMed] [Google Scholar]
- 7. Desrochers AM, Dolente BA, Roy MF, et al. Efficacy of Saccharomyces boulardii for treatment of horses with acute enterocolitis. J Am Vet Med Assoc 2005;227:954–959. [DOI] [PubMed] [Google Scholar]
- 8. Schoster A, Weese JS, Guardabassi L. Probiotic use in horses—What is the evidence for their clinical efficacy? J Vet Intern Med 2014;28:1640–1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yuyama T. Evaluation of a host‐specific Lactobacillus probiotic in neonatal foals. J Appl Res Vet Med 2004;18:26–32. [Google Scholar]
- 10. Weese JS, Anderson ME, Lowe A, Monteith GJ. Preliminary investigation of the probiotic potential of Lactobacillus rhamnosus strain GG in horses: Fecal recovery following oral administration and safety. Can Vet J 2003;44:299–302. [PMC free article] [PubMed] [Google Scholar]
- 11. Weese JS, Martin H. Assessment of commercial probiotic bacterial contents and label accuracy. Can Vet J 2011;52:43–46. [PMC free article] [PubMed] [Google Scholar]
- 12. Schoster A, Kokotovic B, Permin A, et al. In vitro inhibition of Clostridium difficile and Clostridium perfringens by commercial probiotic strains. Anaerobe 2013;20:36–41. [DOI] [PubMed] [Google Scholar]
- 13. Schulz KF, Altman DG, Moher D, Group C . CONSORT 2010 statement: Updated guidelines for reporting parallel group randomized trials. Obstet Gynecol 2010;115:1063–1070. [DOI] [PubMed] [Google Scholar]
- 14. Schoster A, Staempfli HR, Arroyo LG, et al. Longitudinal study of Clostridium difficile and antimicrobial susceptibility of Escherichia coli in healthy horses in a community setting. Vet Microbiol 2012;159:364–370. [DOI] [PubMed] [Google Scholar]
- 15. Weese JS. Microbiologic evaluation of commercial probiotics. J Am Vet Med Assoc 2002;220:794–797. [DOI] [PubMed] [Google Scholar]
- 16. Baverud V, Franklin A, Gunnarsson A, et al. Clostridium difficile associated with acute colitis in mares when their foals are treated with erythromycin and rifampicin for Rhodococcus equi pneumonia. Equine Vet J 1998;30:482–488. [DOI] [PubMed] [Google Scholar]
- 17. Costa MC, Stampfli HR, Arroyo LG, et al. Epidemiology of Clostridium difficile on a veal farm: Prevalence, molecular characterization and tetracycline resistance. Vet Microbiol 2011;152:379–384. [DOI] [PubMed] [Google Scholar]
- 18. Weese JS, Staempfli HR, Prescott JF. A prospective study of the roles of Clostridium difficile and enterotoxigenic Clostridium perfringens in equine diarrhoea. Equine Vet J 2001;33:403–409. [DOI] [PubMed] [Google Scholar]
- 19. Medina‐Torres CE, Weese JS, Staempfli HR. Prevalence of Clostridium difficile in horses. Vet Microbiol 2011;152:212–215. [DOI] [PubMed] [Google Scholar]
- 20. Baverud V, Gustafsson A, Franklin A, et al. Clostridium difficile: Prevalence in horses and environment, and antimicrobial susceptibility. Equine Vet J 2003;35:465–471. [DOI] [PubMed] [Google Scholar]
- 21. Tillotson K, Traub‐Dargatz JL, Dickinson CE, et al. Population‐based study of fecal shedding of Clostridium perfringens in broodmares and foals. J Am Vet Med Assoc 2002;220:342–348. [DOI] [PubMed] [Google Scholar]
- 22. Schoster A, Arroyo LG, Staempfli HR, et al. Presence and molecular characterization of Clostridium difficile and Clostridium perfringens in intestinal compartments of healthy horses. BMC Vet Res 2012;8:94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Herholz C, Miserez R, Nicolet J, et al. Prevalence of beta2‐toxigenic Clostridium perfringens in horses with intestinal disorders. J Clin Microbiol 1999;37:358–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Waggett BE, McGorum BC, Wernery U, et al. Prevalence of Clostridium perfringens in faeces and ileal contents from grass sickness affected horses: Comparisons with 3 control populations. Equine Vet J 2010;42:494–499. [DOI] [PubMed] [Google Scholar]
- 25. Parraga ME, Spier SJ, Thurmond M, Hirsh D. A clinical trial of probiotic administration for prevention of Salmonella shedding in the postoperative period in horses with colic. J Vet Intern Med 1997;11:36–41. [DOI] [PubMed] [Google Scholar]
- 26. Kim LM, Morley PS, Traub‐Dargatz JL, et al. Factors associated with Salmonella shedding among equine colic patients at a veterinary teaching hospital. J Am Vet Med Assoc 2001;218:740–748. [DOI] [PubMed] [Google Scholar]
- 27. Bybee SN, Scorza AV, Lappin MR. Effect of the probiotic Enterococcus faecium SF68 on presence of diarrhea in cats and dogs housed in an animal shelter. J Vet Intern Med 2011;25:856–860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Allen SJ, Wareham K, Wang D, et al. Lactobacilli and bifidobacteria in the prevention of antibiotic‐associated diarrhoea and Clostridium difficile diarrhoea in older inpatients (PLACIDE): A randomised, double‐blind, placebo‐controlled, multicentre trial. Lancet 2013;382:1249–1257. [DOI] [PubMed] [Google Scholar]
- 29. Rossi G, Pengo G, Caldin M, et al. Comparison of microbiological, histological, and immunomodulatory parameters in response to treatment with either combination therapy with prednisone and metronidazole or probiotic VSL#3 strains in dogs with idiopathic inflammatory bowel disease. PLoS One 2014;9:e94699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bibiloni R, Fedorak RN, Tannock GW, et al. VSL#3 probiotic‐mixture induces remission in patients with active ulcerative colitis. Am J Gastroenterol 2005;100:1539–1546. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. C difficile and C. perfringens typing methods, results, and discussion.


