Abstract
Background
Transsphenoidal hypophysectomy is an effective treatment for dogs with pituitary‐dependent hypercortisolism (PDH). However, long‐term recurrence of hypercortisolism is a well‐recognized problem, indicating the need for reliable prognostic indicators.
Objectives
The aim of this study was to evaluate the prognostic value of perioperative plasma ACTH and cortisol concentrations for identifying recurrence of hypercortisolism after transsphenoidal hypophysectomy.
Animals
A total of 112 dogs with PDH that underwent transsphenoidal hypophysectomy met the inclusion criteria of the study.
Methods
Hormone concentrations were measured preoperatively and 1–5 hours after surgery. Both absolute hormone concentrations and postoperative concentrations normalized to preoperative concentrations were included in analyses. The prognostic value of hormone concentrations was studied with Cox's proportional hazard analysis.
Results
Median follow‐up and disease‐free period were 1096 days and 896 days, respectively. Twenty‐eight percent of patients had recurrence, with a median disease‐free period of 588 days. Both absolute and normalized postoperative cortisol concentrations were significantly higher in dogs with recurrence than in dogs without recurrence. High ACTH 5 hours after surgery, high cortisol 1 and 4 hours after surgery, high normalized ACTH 3 hours after surgery, high normalized cortisol 4 hours after surgery and the random slope of cortisol were associated with a shorter disease‐free period.
Conclusions and clinical importance
Individual perioperative hormone curves provide valuable information about the risk of recurrence after hypophysectomy. However, because no single cutoff point could be identified, combination with other variables, such as the pituitary height/brain area (P/B) ratio, is still needed to obtain a good estimate of the risk for recurrence of hypercortisolism after hypophysectomy.
Keywords: Canine, Cushing's disease, Hypercortisolism, Survival analysis
Abbreviations
- ACTH
adrenocorticotropic hormone
- AUC
area under the curve
- CD
Cushing's disease
- CRH
corticotropin‐releasing hormone
- CT
computed tomography
- MRI
magnetic resonance imaging
- P/B ratio
pituitary height to brain area ratio
- PDH
pituitary‐dependent hypercortisolism
- RI
random intercept
- ROC
receiver operating curve
- RS
random slope
- UCCR
urinary corticoid‐to‐creatinine ratio
Pituitary‐dependent hypercortisolism (PDH) is a common endocrinopathy in dogs, with an estimated prevalence of 1 or 2 in 1000 dogs/y.1 It is caused by an adrenocorticotropic hormone (ACTH)‐secreting pituitary adenoma. Pituitary carcinomas are rare.2 Clinical signs are caused by hypercortisolism and include polyuria, polydipsia, polyphagia, panting, heat intolerance, and enlargement of the abdomen caused by centripetal fat storage, atrophy of the skin, alopecia, muscle weakness, and lethargy.3 Pituitary‐dependent hypercortisolim in dogs has many similarities to Cushing's disease (CD) in humans, which has a much lower incidence, affecting only 1–2 persons per million.4, 5
Pituitary surgery is the treatment of choice for CD in human patients, in whom preferably an adenomectomy is performed.6 In dogs, transsphenoidal hypophysectomy has been shown to be an effective treatment for PDH.7, 8 Despite high initial remission rates, long‐term recurrence is a well‐recognized problem in both humans9, 10, 11, 12, 13 and dogs,7, 14 with reported recurrence rates of 8.5% in humans and 25% in dogs.7, 12 Therefore, much effort has been expended to identify reliable predictors for completeness of tumor removal and recurrence, either preoperatively or postoperatively.
In both humans and dogs, recurrence is more common in patients with an enlarged pituitary gland.10, 15 For postoperative evaluation of pituitary surgery in humans, different protocols have been used including intraoperative and postoperative measurement of plasma cortisol9, 16, 17, 18, 19, 20 and ACTH21, 22, 23 concentrations, as well as stimulation tests with corticotropin‐releasing hormone (CRH)6, 24, 25 and desmopressin.25, 26, 27 Overall, postoperative basal hormone concentrations in humans are the most important predictor for recurrence,12 but a test that is predictive for the individual patient remains to be developed.28
For postoperative evaluation of transsphenoidal hypophysectomy in dogs, the urinary corticoid‐to‐creatinine ratio (UCCR) has been used to define residual disease, remission, and recurrence. Urinary corticoid‐to‐creatinine ratio values in the upper half of the reference range 6–8 weeks after surgery are associated with a higher frequency of recurrence at long‐term follow‐up, whereas low UCCR values predict long‐term remission.15 The UCCR is an indirect reflection of ACTH production by the pituitary gland, and therefore the plasma ACTH concentration may give more direct information about the completeness of hypophysectomy and risk for recurrence. Because of the short plasma half‐life of ACTH (approximately 20 minutes in both humans and dogs), the plasma ACTH concentration should decrease within a few hours after hypophysectomy.29, 30 This makes direct postoperative evaluation of ACTH concentrations potentially interesting and it is hypothesized that very low ACTH concentrations within a few hours after hypophysectomy will be associated with long‐term remission. Indeed, in humans, it was shown that patients with increased plasma ACTH concentrations developed recurrence, although their postoperative serum cortisol concentrations were low.22 In dogs with PDH, a combined stimulation test, including CRH and 3 other hypophysiotropic hormones, performed at 8 weeks after hypophysectomy, failed to identify dogs in which the disease would recur,31 but plasma ACTH and cortisol concentrations collected immediately after hypophysectomy have not been evaluated yet.
The aim of this study was to evaluate the prognostic value of perioperative plasma ACTH and cortisol concentrations for recurrence of hypercortisolism after transsphenoidal hypophysectomy in dogs with PDH.
Materials and Methods
Animals
Between 2001–2013, 204 dogs with PDH, referred to the Department of Clinical Sciences of Companion Animals, Utrecht University, the Netherlands, underwent transsphenoidal hypophysectomy as primary treatment for PDH. All dogs were operated by the same veterinary neurosurgeon (BM). Medical records of these dogs were evaluated retrospectively, and dogs were included in this study when the postoperative follow‐up period was ≥1 year and postoperative remission of PDH was confirmed within 8 weeks after surgery. Thirty‐eight dogs were excluded because follow‐up was <1 year, 25 dogs were excluded because remission was not confirmed within 8 weeks after surgery and 29 dogs were excluded because the immediate postoperative hormone measurements were not performed or were not available. In total, 112 dogs met the inclusion criteria, consisting of 10 Labrador Retrievers, 9 Maltese dogs, 8 Beagles, 8 Dachshunds, 5 Boxers, 4 Jack Russell Terriers, 3 Yorkshire Terriers, 3 Stabyhouns, 3 German Pointers, 2 Bearded Collies, 2 Cavalier King Charles Spaniels, 2 Hovawarts, 2 Scottish Terriers, 1 dog each of 33 other breeds, and 18 crossbred dogs. There were 53 male dogs (21 castrated) and 59 female dogs (45 spayed). Age at time of surgery ranged from 3.7 to 14 years (median, 8.5 years). Body weight ranged from 3.8 to 61 kg (median, 19.5 kg).
Diagnosis
The diagnosis of hypercortisolism was based on clinical signs and increased UCCRs (reference range, 0.3–8.3 × 10−6 32) in 2 consecutive morning urine samples collected at home.33 After collection of the second urine sample, 3 doses of 0.1 mg dexamethasone per kg body weight were administered PO at 6–8 hours intervals, and the next morning a third urine sample was collected. When the UCCR in the third sample was <50% of the mean of that of the first 2 samples, the dog was categorized as being responsive to dexamethasone suppression, and PDH was diagnosed.33 The median UCCR of the 112 dogs included in this study was 55 × 10−6 (range, 6–652 × 10−6), and the median suppression after dexamethasone was 80% (range, −162 to 99%). In 36 cases, there was <50% suppression of the UCCR in the third sample, and in these dogs dexamethasone‐resistant PDH was demonstrated by measurement of plasma ACTH concentration. The diagnosis of PDH was further supported by visualization of the adrenal glands by ultrasonography and pituitary gland imaging using computed tomography (CT) or magnetic resonance imaging (MRI).34, 35 In dogs with PDH, enlarged pituitary glands were distinguished from nonenlarged pituitary glands by their pituitary height/brain area (P/B) ratio.36 A microadenoma in a nonenlarged pituitary gland can be visualized indirectly by the displacement of the ‘pituitary flush’ on dynamic CT imaging.35 For the dogs included in this study, the median P/B ratio was 0.43 (range, 0.13–1.1). There were 24 dogs with nonenlarged pituitary glands (P/B ≤0.31) and 88 dogs with enlarged pituitary glands (P/B >0.31).
Treatment and Follow‐up
Transsphenoidal hypophysectomy was performed according to a microsurgical technique described previously.37 After removal of the pituitary gland, treatment was started with 1 drop of 0.01% desmopressin1 and continued every 8 hours in the conjunctival sac. Intravenous administration of 1 mg hydrocortisone2 per kg body weight was started 5 hours after removal of the pituitary gland and continued every 6 hours until the dog resumed eating and drinking. Postoperative treatment followed the same protocol as described previously.38 The dogs were kept on lifelong substitution therapy with cortisone acetate3 at a dosage of 0.25 mg/kg PO q12h, and thyroxine4 at a dosage of 15 μg/kg PO q12h. Desmopressin was administered routinely for 2 weeks and continued if polyuria because of central diabetes insipidus persisted.38 The dogs were re‐examined after 8 weeks. After surgery, UCCR was measured (in duplicate) at 2 weeks, 8 weeks, 6 months and thereafter once a year, or more frequently in dogs with suspected recurrence. All morning urine samples for UCCR measurements were collected at home by the owner 24 hours after cortisone acetate treatment. Follow‐up reports were obtained from the routine follow‐up examinations in the clinic, or during telephone conversations with the owner, referring veterinarian, or both. Remission was defined as UCCR <10 × 10−6 and resolution of clinical signs of hypercortisolism. Recurrence was defined as UCCR ≥10 × 10−6 and reappearance of clinical signs of hypercortisolism after initial remission.
Blood Sampling and Analysis
The protocol was approved by the Ethical Committee of the Faculty of Veterinary Medicine (Utrecht University, Utrecht, The Netherlands).
Two basal blood samples were collected on the day before surgery before 2008 and within 2 hours before the surgery after 2008. The average of the 2 values was included in this study as the preoperative value. After hypophysectomy, the first blood sample was collected when the dog was still on the operating table, approximately 1 hour after extraction of the pituitary gland. The remaining samples were collected at 2, 3, 4, and 5 hours after hypophysectomy when the dog was recovering in the intensive care unit. Desmopressin was started when the dog left the operating table. Hydrocortisone medication was started after the 5‐hour sample was collected. Blood samples were collected in prechilled EDTA tubes and kept on ice, and centrifuged for 10–12 minutes at 4°C. Plasma was stored at −20°C until analyzed.
Plasma ACTH concentration was measured by a commercially available 2‐site immunoradiometric assay.,5,6 Plasma cortisol concentration was measured by a solid phase 125I radioimmunoassay.7 39
Data Analysis
Calculations were performed with SPSS8 and R Studio.9 For all postoperative time points, normalized plasma ACTH and cortisol concentrations were calculated by dividing the absolute plasma concentration by the preoperative concentration. These normalized plasma ACTH and cortisol concentrations also were used for analysis. Also, for each dog the area under the curve (AUC), random slope (RS), and random intercept (RI) of the ACTH and cortisol curves were calculated. Normality was assessed with the Kolmogorov–Smirnov Test, and nonparametric tests were used accordingly. Correlations were calculated using the Spearman's rho test. Differences between dogs with and without recurrence were evaluated with Mann–Whitney U‐tests. Longitudinal comparisons were made using the Wilcoxon rank‐sum test. Bonferoni corrections for multiple comparisons were applied.
The disease‐free period was calculated for all dogs and was defined as the interval between the date of surgery and the date on which, the dog was last known to be remission, or the date of recurrence. Dogs that had died from nonrelated causes and dogs that were still alive and in remission at the time of follow‐up were counted as censored cases. To assess the prognostic value of the different factors, variables were first analyzed with univariate Cox's proportional hazard analysis. Variables with a P‐value <.05 were then entered in a multivariate Cox's proportional hazard analysis (Reverse Stepwise [Conditional LR]). For variables significant in the multivariate analysis, cutoff values to create the binary variables ‘low’ versus ‘high’ were calculated based on a receiver operating curve (ROC).40 Disease‐free analysis was performed using Kaplan–Meier curves and the Log‐rank test was used to assess significance between ‘low’ and ‘high’ values. Significance was set at P < .05.
Results
Follow‐up Data
Median follow‐up for the 112 patients included in this study was 1096 days (range, 373–3799 days). At last follow‐up, 35 dogs were alive. Median disease‐free period was 896 days (range, 80–3340 days). Of the 112 patients, 31 (28%) had a recurrence of hypercortisolism, whereas 81 dogs were still in remission at last follow‐up (alive or dead because of nonrelated cause). Median disease‐free period for the dogs with recurrence was 588 days (range, 80–1688 days).
Basal Hormone Concentrations and Clinical Variables
Correlation was calculated between preoperative plasma ACTH and cortisol concentrations and clinical variables (P/B ratio, preoperative UCCR, survival time and disease‐free period). A significant correlation was only found between preoperative plasma ACTH concentration and P/B ratio (ρ = 0.28, P = .003) and between preoperative plasma cortisol concentration and preoperative UCCR (ρ = 0.31, P = .003).
Hormone Profiles after Hypophysectomy
For each dog, plasma ACTH and cortisol concentrations were plotted as an individual curve (Fig 1) and compared with follow‐up information. A significant correlation was found between absolute plasma ACTH and cortisol concentrations at 1–5 hours after surgery, normalized plasma ACTH and cortisol concentrations at 1–5 hours after surgery, AUC of ACTH and cortisol, and RI of ACTH and cortisol. For each time point of ACTH and cortisol measurement, plasma concentrations, normalized plasma concentrations, and curve characteristics were compared between the group of dogs with recurrence and the group of dogs without recurrence. Plasma cortisol concentrations at 1–5 hours after surgery were significantly higher in dogs with recurrence than those in dogs without recurrence (Fig 2). Also, significant differences were found in normalized plasma cortisol concentrations at 1–5 hours after surgery and for RS and RI of cortisol. When comparing the lowest postoperative result, dogs with recurrence had a significantly higher plasma cortisol concentration (P = .008), and plasma ACTH concentration tended to be higher in these dogs (P = .07). Data also were analyzed longitudinally, both for dogs with recurrence and dogs without recurrence. Plasma ACTH and cortisol concentrations decreased significantly over time after surgery in both groups (Fig 2).
Figure 1.
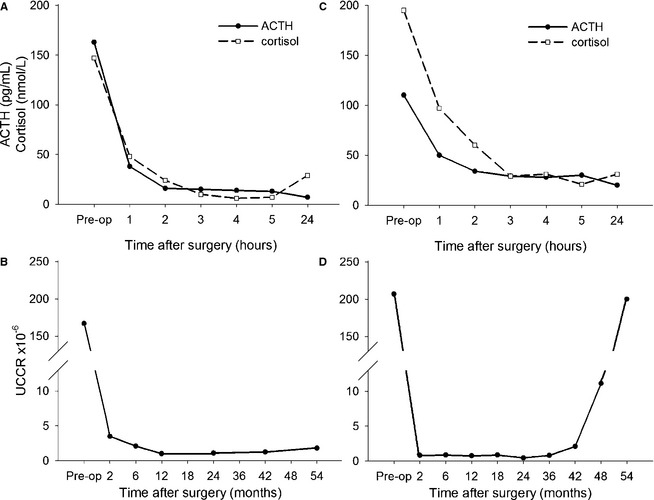
Typical example of perioperative individual profiles of plasma ACTH and cortisol concentrations in dogs that underwent hypophysectomy for treatment of PDH . Panels A and C show the plasma concentrations of ACTH and cortisol from 24 hours (Preoperative) before to 5 hours after surgery. Panels B and D show the UCCRs of the same dogs over time. The dog in panel A and B remained in remission for 54 months, and the plasma ACTH and cortisol concentrations decreased within 5 hours after hypophysectomy to values close to zero. The dog in panel C and D had a recurrence of hypercortisolism at 48 months after surgery. In this dog plasma, ACTH and cortisol concentrations did not decrease to values close to zero within 5 hours after hypophysectomy.
Figure 2.
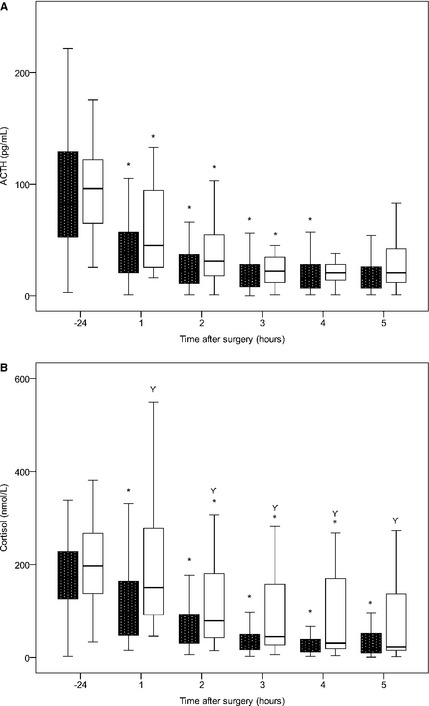
Boxplots demonstrating the change in plasma ACTH (A) and cortisol (B) concentrations over time in dogs with recurrence (open boxes, n = 31) and dogs without recurrence (black boxes, n = 81) of hypercortisolism after hypophysectomy. * indicates a significant difference to the previous time point.  indicates a significant difference between plasma cortisol concentrations in dogs with and without recurrence.
indicates a significant difference between plasma cortisol concentrations in dogs with and without recurrence.
Disease‐free Period Analysis
Clinical variables (P/B ratio, age, body weight, sex), absolute plasma ACTH and cortisol concentrations, normalized plasma ACTH and cortisol concentrations and curve characteristics (AUC, RI and RS) were entered in the univariate Cox's proportional hazard analysis for disease‐free period of the 112 dogs. The variables that were significant (P < .05, Table 1) were entered in a multivariate Cox's proportional hazard model, grouped in absolute plasma hormone concentrations, normalized plasma hormone concentrations, and curve characteristics. The P/B ratio was excluded from further analysis, for a better appreciation of the influence of the hormone concentrations in the different models. High plasma ACTH concentration 5 hours after surgery, high plasma cortisol concentrations 1 and 4 hours after surgery, high normalized plasma ACTH concentration 3 hours after surgery, high normalized plasma cortisol concentration 4 hours after surgery and the RS of cortisol were associated with shorter disease‐free period (Hazard Ratio >1.0, P < .05); (Table 2). No significant differences were found between Kaplan–Meier survival curves for disease‐free periods of these variables (ACTH 5 hours after surgery [P = .36], cortisol 1 hours after surgery [P = .12], cortisol 4 hours after surgery [P = .07, Fig 3], normalized cortisol 4 hours after surgery [P = .15], and RS of cortisol [P = .13]). The ROC curve of normalized ACTH 3 hours after surgery was not of sufficient quality to calculate a cutoff value.
Table 1.
Significant variables (P < .05) in the univariate Cox's proportional hazard analysis for disease‐free period after transsphenoidal hypophysectomy. Variables with P > .05 are not included in this table
| Variable | HR | 95% CI | P‐Value | |
|---|---|---|---|---|
| P/B value | 22.664 | 4.478 | 114.712 | <.001 |
| ACTH2 | 1.008 | 1.002 | 1.014 | .007 |
| ACTH3 | 1.007 | 1.003 | 1.010 | <.001 |
| ACTH4 | 1.016 | 1.008 | 1.025 | <.001 |
| ACTH5 | 1.019 | 1.004 | 1.035 | .014 |
| Cort1 | 1.004 | 1.002 | 1.005 | <.001 |
| Cort2 | 1.004 | 1.002 | 1.005 | <.001 |
| Cort3 | 1.002 | 1.001 | 1.004 | <.001 |
| Cort4 | 1.004 | 1.003 | 1.006 | <.001 |
| normACTH2 | 4.477 | 1.416 | 14.152 | .011 |
| normACTH3 | 2.241 | 1.469 | 3.420 | <.001 |
| normACTH4 | 4.788 | 1.770 | 12.954 | .002 |
| normCort1 | 1.826 | 1.318 | 2.530 | <.001 |
| normCort2 | 1.888 | 1.286 | 2.772 | .001 |
| normCort3 | 1.498 | 1.176 | 1.908 | .001 |
| normCort4 | 2.067 | 1.407 | 3.035 | <.001 |
| RI ACTH | 1.011 | 1.003 | 1.018 | .005 |
| AUC Cort | 1.000 | 1.000 | 1.000 | <.001 |
| RI Cort | 1.005 | 1.003 | 1.008 | <.001 |
| RS Cort | 1.171 | 1.093 | 1.256 | <.001 |
AUC Cort, area under the curve for cortisol; CI, confidence interval; HR, hazard ratio; normACTH, normalized plasma ACTH concentration to preoperative value; normCort, normalized plasma cortisol concentration to preoperative value; P/B value, pituitary height/brain area value; RI, random intercept; RS, random slope.
Table 2.
Grouped variables entered in multivariate Cox's proportional hazard analysis (Reverse LR Conditional) for disease‐free period after transsphenoidal hypophysectomy resulted in a final model
| Grouped Variables Entered | Variables in Final Model | HR | 95% CI | P‐Value | |
|---|---|---|---|---|---|
| ACTH2, ACTH3, ACTH4, ACTH5, Cort1, Cort2, Cort3, Cort4 | ACTH3 | 1.032 | 0.999 | 1.066 | .060 |
| ACTH4 | 0.921 | 0.845 | 1.005 | .064 | |
| ACTH5 | 1.055 | 1.006 | 1.106 | .026* | |
| Cort1 | 1.005 | 1.000 | 1.010 | .041* | |
| Cort2 | 0.993 | 0.986 | 1.001 | .072 | |
| Cort4 | 1.007 | 1.002 | 1.013 | .008* | |
| normACTH2, normACTH3, normACTH4, normCort1, normCort2, normCort3, normCort4 | normACTH3 | 2.082 | 1.237 | 3.504 | .006* |
| normCort4 | 1.805 | 1.182 | 2.756 | .006* | |
| RI ACTH, RI Cort, RS Cort, AUC Cort | RI ACTH | 1.011 | 0.999 | 1.022 | .067 |
| RS Cort | 1.150 | 1.064 | 1.244 | <.001* | |
AUC Cort, area under the curve for cortisol; CI, confidence interval; HR, hazard ratio; normACTH, normalized plasma ACTH concentration to preoperative value; normCort, normalized plasma cortisol concentration to preoperative value; RI, random intercept; RS, random slope.
Significant variables (P < .05) are marked with *.
Figure 3.
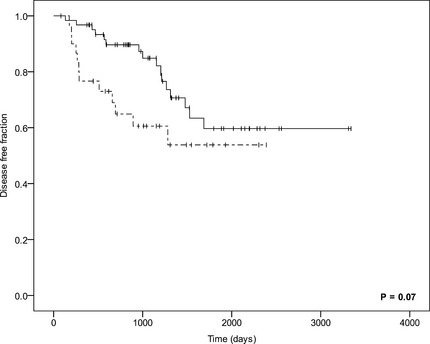
Kaplan–Meier curves comparing the disease‐free period in dogs with high (>33 nmol/L), dotted line, n = 31) and low (<33 nmol/L), continuous line, n = 62) plasma cortisol concentrations at 4 hours after hypophysectomy (P = .07). Censored cases are represented by vertical bars.
Discussion
We studied the prognostic value of perioperative ACTH and cortisol concentrations in predicting recurrence of hypercortisolism in dogs after hypophysectomy. With multivariate analysis, we identified several variables with significant prognostic value (ACTH 5 hours after surgery, cortisol 1 and 4 hours after surgery, normalized ACTH 3 hours after surgery, normalized cortisol 4 hours after surgery, and the RS of cortisol). However, Kaplan–Meier survival analysis showed that single time point measurements were not sufficient to predict a significant difference in disease‐free period, as was also shown in a meta‐analysis of studies of humans.12 Therefore, perioperative hormone concentrations must be combined with other clinical information, such as pituitary size (P/B ratio), to provide reliable information about the prognosis of dogs with PDH.
The long‐term recurrence rate in this study was 28% (31 in 112 dogs). The dogs included in this study had a median P/B ratio of 0.43, indicating a high proportion of dogs with enlarged pituitary glands. We previously showed that high P/B ratio is predictive of recurrence,15 and also in this study, the P/B ratio had a high hazard ratio in the Cox's proportional hazard's analysis.
We showed a significant decrease in the plasma ACTH concentrations after surgery in both dogs with and without recurrence. The short half‐life of ACTH explains this rapid decrease. Plasma ACTH concentrations have been measured intra‐operatively in humans,21, 22, 23 but are not commonly used as prognostic indicators. A recent study of humans did however show the usefulness of the combination of ACTH and cortisol measurements.22 Plasma cortisol has a longer half‐life (in humans approximately 50 minutes41), and the decrease in plasma cortisol concentration after hypophysectomy is expected to be less steep than that of ACTH. In humans, plasma and urinary cortisol concentrations most commonly are used as prognostic variables.9, 12, 16, 17, 18, 19, 20 We showed also that cortisol concentrations decreased significantly in the postoperative period, with the median plasma cortisol concentration being significantly higher in dogs that developed recurrence than in dogs without recurrence at all postoperative time points.
Evaluating individual hormone curves for patients with and without recurrence, we observed a faster decrease in plasma ACTH concentrations in dogs without recurrence, but mean plasma ACTH concentrations were not different between groups, probably because of large variation, especially in the recurrence group. Very low plasma ACTH concentrations suggest complete removal of the corticotroph adenoma, but do not exclude presence of remaining normal pituitary cells, because these cells have been suppressed by the negative feedback of high cortisol concentrations for a long period. Very low plasma ACTH concentrations within 5 hours as an indication of complete hypophysectomy is even more noteworthy because at 1 hour after hypophysectomy desmopressin was administered which, besides replacing the vasopressin deficiency, is a well‐known stimulant of ACTH secretion.25, 26, 27 Because of this, measurement of high postoperative plasma ACTH concentrations indicates remnant functional corticotroph adenoma tissue, increasing the risk for recurrence of hypercortisolism over time. Indeed, evaluating the lowest postoperative plasma concentrations of ACTH and cortisol for each patient, we showed significantly higher plasma cortisol concentrations in dogs with recurrence.
With multivariate analysis, not only absolute hormone concentrations were included but also concentrations normalized to the preoperative result and characteristics of the complete hormone curve. The definitive models included several variables with significant prognostic value. The advantage of hourly repeated measurements is that curve characteristics could be included in the analysis. Kaplan–Meier survival analysis showed that single time point measurements were not sufficient to predict recurrences, as was also shown in a meta‐analysis of studies of humans,12 making a combination of different data essential.
Based on a cohort of 112 patients with a follow‐up of >1 year, a combination of absolute hormone concentrations, normalized hormone concentrations and curve characteristics has predictive value. Therefore, use of the hormone curve of a patient, in combination with other clinical variables (such as P/B ratio) is needed in the postoperative evaluation of dogs with PDH to provide a reliable estimation of the prognosis. Close follow‐up of the patients, over the long‐term, also remains crucial.
Acknowledgments
The authors thank Jeannette Wolfswinkel for assistance with retrieval of hormone data. The authors also thank Hans Vernooij, Theoretical Epidemiology, Department of Farm Animal Health, Faculty of Veterinary Medicine, Utrecht University, the Netherlands, for assistance with statistical analysis.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at the Department of Clinical Sciences of Companion Animals, Faculty of Veterinary Medicine, Utrecht University, Utrecht, the Netherlands.
Footnotes
Minrin, Ferring, Hoofddorp, the Netherlands
Solu‐cortef, Upjohn, Ede, the Netherlands
Cortisoni acetas; Genfarma, Maarssen, the Netherlands
L‐thyroxine; Aesculaap, Boxtel, the Netherlands
Nichols Institute, Wijchen, the Netherlands (until 2007)
Diasorin S.A./N.V., Brussels, Belgium (from 2007)
Coat‐a‐Count® Cortisol, DPC (Siemens healthcare diagnostics), the Hague, the Netherlands
IBM SPSS®Statistics for Windows, Version 20.0, Armonk, NY, USA
R Studio, version 0.98, http://www.rstudio.com
References
- 1. Willeberg P, Priester W. Epidemiological aspects of clinical hyperadrenocorticism in dogs (canine Cushing's syndrome). J Am Anim Hosp Assoc 1982;18:717–724. [Google Scholar]
- 2. Gestier S, Cook R, Agnew W, Kiupel M. Silent pituitary corticotroph carcinoma in a young dog. J Comp Pathol 2012;146:327–331. [DOI] [PubMed] [Google Scholar]
- 3. Galac S, Reusch CE, Kooistra HS, Rijnberk A. Adrenals In: Rijnberk A, Kooistra HS, eds. Clinical Endocrinology of Dogs and Cats, 2nd ed Hannover: Schlütersche; 2010:93–154. [Google Scholar]
- 4. Beckers A. Higher prevalence of clinically relevant pituitary adenomas confirmed. Clin Endocrinol (Oxf) 2010;72:290–291. [DOI] [PubMed] [Google Scholar]
- 5. Tjörnstrand A, Gunnarsson K, Evert M, et al. The incidence rate of pituitary adenomas in western Sweden for the period 2001–2011. Eur J Endocrinol 2014;171:519–526. [DOI] [PubMed] [Google Scholar]
- 6. Ciric I. Transsphenoidal surgery for Cushing disease: Experience with 136 patients. Neurosurgery 2012;70:70–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Hanson JM, Hoofd MM, Voorhout G, et al. Efficacy of transsphenoidal hypophysectomy in treatment of dogs with pituitary‐dependent hyperadrenocorticism. J Vet Intern Med 2005;19:687–694. [DOI] [PubMed] [Google Scholar]
- 8. Meij BP. Hypophysectomy as a treatment for canine and feline Cushing's disease. Vet Clin North Am Small Anim Pract 2001;31:1015–1041. [DOI] [PubMed] [Google Scholar]
- 9. Atkinson AB, Kennedy A, Wiggam MI, et al. Long‐term remission rates after pituitary surgery for Cushing's disease: The need for long‐term surveillance. Clin Endocrinol (Oxf) 2005;63:549–559. [DOI] [PubMed] [Google Scholar]
- 10. De Tommasi C, Vance ML, Okonkwo DO, et al. Surgical management of adrenocorticotropic hormone‐secreting macroadenomas: Outcome and challenges in patients with Cushing's disease or Nelson's syndrome. J Neurosurg 2005;103:825–830. [DOI] [PubMed] [Google Scholar]
- 11. Mortini P, Losa M, Barzaghi R, et al. Results of transsphenoidal surgery in a large series of patients with pituitary adenoma. Neurosurgery 2005;56:1222–1233. [DOI] [PubMed] [Google Scholar]
- 12. Roelfsema F, Biermasz NR, Pereira AM. Clinical factors involved in the recurrence of pituitary adenomas after surgical remission: A structured review and meta‐analysis. Pituitary 2012;15:71–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Bochicchio D, Losa M, Buchfelder M, et al. Factors influencing the immediate and late outcome of Cushing‐disease treated by transsphenoidal surgery: A retrospective study by the European Cushings‐disease survey group. J Clin Endocrinol Metab 1995;80:3114–3120. [DOI] [PubMed] [Google Scholar]
- 14. Meij B, Voorhout G, Rijnberk A. Progress in transsphenoidal hypophysectomy for treatment of pituitary‐dependent hyperadrenocorticism in dogs and cats. Mol Cell Endocrinol 2002;197:89–96. [DOI] [PubMed] [Google Scholar]
- 15. Hanson JM, Teske E, Voorhout G, et al. Prognostic factors for outcome after transsphenoidal hypophysectomy in dogs with pituitary‐dependent hyperadrenocorticism. J Neurosurg 2007;107:830–840. [DOI] [PubMed] [Google Scholar]
- 16. Chen JC, Amar AP, Choi S, et al. Transsphenoidal microsurgical treatment of Cushing disease: Postoperative assessment of surgical efficacy by application of an overnight low‐dose dexamethasone suppression test. J Neurosurg 2003;98:967–973. [DOI] [PubMed] [Google Scholar]
- 17. Rollin GAFS, Ferreira NP, Junges M, et al. Dynamics of serum cortisol levels after transsphenoidal surgery in a cohort of patients with Cushing's disease. J Clin Endocrinol Metab 2004;89:1131–1139. [DOI] [PubMed] [Google Scholar]
- 18. Simmons NE, Alden TD, Thorner MO, Laws ER Jr. Serum cortisol response to transsphenoidal surgery for Cushing disease. J Neurosurg 2001;95:1–8. [DOI] [PubMed] [Google Scholar]
- 19. Trainer P, Lawrie H, Verhelst J, et al. Transsphenoidal resection in Cushing's disease: Undetectable serum cortisol as the definition of successfuI treatment. Clin Endocrinol (Oxf) 1993;38:73–78. [DOI] [PubMed] [Google Scholar]
- 20. Yap L, Turner H, Adams C, Wass J. Undetectable postoperative cortisol does not always predict long‐term remission in Cushing's disease: A single centre audit. Clin Endocrinol (Oxf) 2002;56:25–31. [DOI] [PubMed] [Google Scholar]
- 21. Czirjak S, Bezzegh A, Gal A, Racz K. Intra‐and postoperative plasma ACTH concentrations in patients with Cushing's disease cured by transsphenoidal pituitary surgery. Acta Neurochir 2002;144:971–977. [DOI] [PubMed] [Google Scholar]
- 22. Abdelmannan D, Chaiban J, Selman WR, Arafah BM. Recurrences of ACTH‐secreting adenomas after pituitary adenomectomy can be accurately predicted by perioperative measurements of plasma ACTH levels. J Clin Endocrinol Metab 2013;98:1458–1465. [DOI] [PubMed] [Google Scholar]
- 23. Srinivasan L, Laws ER, Dodd RL, et al. The dynamics of post‐operative plasma ACTH values following transsphenoidal surgery for Cushing's disease. Pituitary 2011;14:312–317. [DOI] [PubMed] [Google Scholar]
- 24. Lindsay JR, Oldfield EH, Stratakis CA, Nieman LK. The postoperative basal cortisol and CRH tests for prediction of long‐term remission from Cushing's disease after transsphenoidal surgery. J Clin Endocrinol Metab 2011;96:2057–2064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Barbot M, Albiger N, Koutroumpi S, et al. Predicting late recurrence in surgically treated patients with Cushing's disease. Clin Endocrinol (Oxf) 2013;79:394–401. [DOI] [PubMed] [Google Scholar]
- 26. Losa M, Mortini P, Dylgjeri S, et al. Desmopressin stimulation test before and after pituitary surgery in patients with Cushing's disease. Clin Endocrinol (Oxf) 2001;55:61–68. [DOI] [PubMed] [Google Scholar]
- 27. Valero R, Vallette‐Kasic S, Conte‐Devolx B, et al. The desmopressin test as a predictive factor of outcome after pituitary surgery for Cushing's disease. Eur J Endocrinol 2004;151:727–733. [DOI] [PubMed] [Google Scholar]
- 28. Arnaldi G, Angeli A, Atkinson A, et al. Diagnosis and complications of Cushing's syndrome: A consensus statement. J Clin Endocrinol Metab 2003;88:5593–5602. [DOI] [PubMed] [Google Scholar]
- 29. van den Berg G, Frölich M, Veldhuis JD, Roelfsema F. Combined amplification of the pulsatile and basal modes of adrenocorticotropin and cortisol secretion in patients with Cushing's disease: Evidence for decreased responsiveness of the adrenal glands. J Clin Endocrinol Metab 1995;80:3750–3757. [DOI] [PubMed] [Google Scholar]
- 30. Greco D, Behrend E, Brown S, et al. Pharmacokinetics of exogenous corticotropin in normal dogs, hospitalized dogs with non adrenal illness and adrenopathic dogs. J Vet Pharmacol Ther 1998;21:369–374. [DOI] [PubMed] [Google Scholar]
- 31. Meij B, Mol J, Bevers M, Rijnberk A. Residual pituitary function after transsphenoidal hypophysectomy in dogs with pituitary‐dependent hyperadrenocorticism. J Endocrinol 1997;155:531–539. [DOI] [PubMed] [Google Scholar]
- 32. Vonderen IK, Kooistra HS, Rijnberk A. Intra‐and interindividual variation in urine osmolality and urine specific gravity in healthy pet dogs of various ages. J Vet Intern Med 1997;11:30–35. [DOI] [PubMed] [Google Scholar]
- 33. Galac S, Kooistra H, Teske E, Rijnberk A. Urinary corticoid/creatinine ratios in the differentiation between pituitary‐dependent hyperadrenocorticism and hyperadrenocorticism due to adrenocortical tumour in the dog. Vet Q 1997;19:17–20. [DOI] [PubMed] [Google Scholar]
- 34. Bosje J, Rijnberk A, Mol J, et al. Plasma concentrations of ACTH precursors correlate with pituitary size and resistance to dexamethasone in dogs with pituitary‐dependent hyperadrenocorticism. Domest Anim Endocrinol 2002;22:201–210. [DOI] [PubMed] [Google Scholar]
- 35. van der Vlugt‐Meijer RH, Voorhout G, Meij BP. Imaging of the pituitary gland in dogs with pituitary‐dependent hyperadrenocorticism. Mol Cell Endocrinol 2002;197:81–87. [DOI] [PubMed] [Google Scholar]
- 36. Kooistra H, Voorhout G, Mol J, Rijnberk A. Correlation between impairment of glucocorticoid feedback and the size of the pituitary gland in dogs with pituitary‐dependent hyperadrenocorticism. J Endocrinol 1997;152:387–394. [DOI] [PubMed] [Google Scholar]
- 37. Meij BP, Voorhout G, van den Ingh TSGAM, et al. Transsphenoidal hypophysectomy in beagle dogs: Evaluation of a microsurgical technique. Vet Surg 1997;26:295–309. [DOI] [PubMed] [Google Scholar]
- 38. Meij BP, Voorhout G, van den Ingh TSGAM, et al. Results of transsphenoidal hypophysectomy in 52 dogs with pituitary‐dependent hyperadrenocorticism. Vet Surg 1998;27:246–261. [DOI] [PubMed] [Google Scholar]
- 39. Hanson J, Kooistra H, Mol J, et al. Plasma profiles of adrenocorticotropic hormone, cortisol, α‐melanocyte‐stimulating hormone, and growth hormone in dogs with pituitary‐dependent hyperadrenocorticism before and after hypophysectomy. J Endocrinol 2006;190:601–609. [DOI] [PubMed] [Google Scholar]
- 40. Dohoo I, Martin W, Stryhn H. Screening and diagnostic tests In: Dohoo I, Martin W, Stryhn H, ed. Veterinary Epidemiologic Research, 2nd ed Prince Edward Island, AVC Inc; 2009:91–134. [Google Scholar]
- 41. Keenan DM, Roelfsema F, Veldhuis JD. Endogenous ACTH concentration‐dependent drive of pulsatile cortisol secretion in the human. Horm Res 2004;287:E652–E661. [DOI] [PubMed] [Google Scholar]


