Abstract
Background
The study was prompted by a perceived high prevalence of myelographic complications varying in severity and type, and attributed to the contrast material or the procedure.
Hypotheses
1. Any adverse reaction (AAR) is associated with a change in CSF volume induced either by removal of CSF or addition of contrast material. 2. AAR occurs more frequently in horses with higher premyelography neurologic grade. 3. Nonspecific hyperthermia is attenuated by anti‐inflammatory and osmotic agents.
Animals
Horses (n = 278) that underwent myelography between 2000 and 2012 at 5 institutions: A (87), B (68), C (65), D (46), and E (12).
Methods
Multi‐institutional, retrospective, observational cross‐sectional study.
Results
AAR were observed in 95/278 (34%) horses, were associated with longer general anesthesia time (P = .04) and higher contrast‐medium volume (P = .04); euthanasia because of AAR was performed in 5/278 (2%) horses. Adverse neurologic reactions were the most common type of complication observed occurring in 15/278 (5%) and 42/235 (18%) of horses in the intra‐ and postmyelography periods. A relationship between AAR and premyelography neurologic grade was not identified (P = .31). Nonspecific hyperthermia was observed in 25/235 (11%) horses; no relationship was observed with administration of anti‐inflammatory drugs and osmotic agents (P = .30).
Conclusions and clinical importance
The category of AAR occurred in one‐third of the horses generally was mild and self‐limiting. These reactions were associated with increased contrast‐medium volume and longer anesthesia time; but, no specific procedural recommendations could be made because of small odds ratios (OR) of <2 for each 1 mL increase in contrast material and for each 1 minute of additional anesthesia time.
Keywords: Contrast radiology, Neurology, Spinal cord disease
Abbreviations
- ANR
acute nonidiosyncratic reaction
- AAR
any adverse reaction
- CI
95% confidence interval
- CSF
cerebrospinal fluid
- DNR
delayed nonidiosyncratic reaction
- EDM
equine degenerative myeloencephalopathy
- EHM
equine herpes myeloencephalopathy
- EPM
equine protozoal myeloencephalitis
- IR
idiosyncratic reaction
- MAP
mean arterial pressure
- OR
odds ratio
- PSSM
polysaccharide storage myopathy
- WNV
West Nile virus
Horses undergoing cervical myelography may experience adverse reactions associated with general anesthesia or myelographic technique, including removal of cerebrospinal fluid (CSF), administration of iodinated contrast material, or manipulation of the head and neck.1 Horses with higher neurologic grades can be more ataxic during induction and recovery, and therefore at a higher risk of falling and inducing spinal cord trauma.2 Adverse reactions to contrast material are reported infrequently in animals and much of what is known is derived from reports in humans. Adverse reactions to the IV administration of iodinated contrast material are classified broadly into idiosyncratic and nonidiosyncratic reactions.3
In humans, idiosyncratic reactions (IR) resemble anaphylactic reactions but are not true hypersensitivity reactions because immunoglobulin E antibodies are not involved, previous sensitization is not required, and these reactions do not consistently recur in given patients.3, 4 Idiosyncratic reactions generally develop within 20 minutes of contrast medium injection, are independent of dose, and range in severity from mild, self‐limiting reactions to severe, life‐threatening anaphylactoid reactions.4 Nonidiosyncratic reactions are related to the physicochemical properties of the contrast material, the dose, and the speed of injection. They can be acute (ANR) or delayed (DNR) and include cardiovascular reactions, nephropathy, neuropathy, extravasation of contrast material into soft tissues during injection, and aggravation of underlying disease.4, 5 Acute nonidiosyncratic reactions occur within 30 minutes of injection and include cardiac arrhythmias, decreased myocardial contractility, pulmonary edema, and seizures, and are likely related to contrast material‐associated hyperosmolality, calcium binding, or both.4 Delayed nonidiosyncratic reactions occur 30 minutes to 1 week after contrast medium injection and commonly include development of flu‐like signs that frequently resolve with minimal or no treatment.4
The same adverse reactions associated with IV injection of contrast material can occur during myelography after intrathecal injection of contrast material. Additional adverse reactions can arise because of alterations in craniospinal volume causing changes in craniospinal pressure because of the removal of CSF, the addition of contrast medium, or CSF leakage secondary to dural and arachnoid puncture, as a result of direct injury to the spinal cord because of misplacement of the spinal needle, or because of the neurotoxicity of iohexol, which has been linked to its osmolarity, presence of sodium ions, and lipid solubility.6, 7 The most common mild adverse effects after iohexol myelography in people are headache, nausea, vomiting, and dizziness.8 Severe complications reported in people include seizures, aseptic or septic meningitis, meningoencephalitis, intracranial hemorrhage, spinal hematoma, encephalopathy, transient confusion, and paraplegia.9 Aseptic meningitis as a sequel of intrathecal injection of nonionic contrast material also has been reported in dogs.10
The primary aim of this study was to describe the frequency and type of AAR, which can include IR, ANR, DNR, and adverse reactions specifically associated with intrathecal injection of contrast, observed in a clinical population of horses. Because of limitations in sample size, AARs were not classified by severity or type for statistical analysis. We investigated 3 specific hypotheses: The first hypothesis was that AAR are associated with volume alterations of the CSF after CSF removal or injection of contrast material. The second hypothesis was that AAR occur more frequently in horses that had a higher neurologic grade before myelography. The third hypothesis was nonspecific hyperthermia postmyelography occurs more frequently in horses that did not receive pre‐ or intra‐anesthetic, anti‐inflammatory, and osmotic agents.
Materials and Methods
The design was a retrospective, observational cross‐sectional study. The sample population consisted of all horses admitted to 5 university equine hospitals between January 1, 2000 and December 31, 2012 that underwent cervical myelography using injection of iohexol into the cerebellomedullary cistern under general anesthesia. Horses that underwent myelography for research purposes or immediately after euthanasia were excluded. The medical records were reviewed by professionals at each institution who agreed to participate in a study on the adverse effects of myelography, but who were blinded to the results of the other investigators and the explicit study aims. The medical record of each case was reviewed for variables that occurred before myelography and up to the time of injection of contrast material (premyelography), from injection of contrast material to recovery from general anesthesia (intra‐myelography), and from completion of recovery to 1 week after myelography (post‐myelography). Patient data including the occurrence specified adverse reactions in the intra‐ and postmyelography period were entered into an online survey instrument.1
Premyelography
Premyelography variables were date of birth, date of myelography, sex, breed, body weight, duration of neurologic signs, neurologic grade, administration of anti‐inflammatory medications, and osmotic agents (drug, dosage, time relative to injection of contrast material), and mean arterial pressure (MAP) for the 10 minutes before injection of contrast material measured by indirect or direct blood pressure monitoring. If neurologic grade was not recorded in the medical record, but neurologic examination findings were noted, the grade was assigned by the investigator reviewing the record based upon closest agreement with the grade from a previously described scale.11 For horses that had different grades for forelimbs and hindlimbs, or had asymmetric ataxia, the highest grade assigned was recorded. Adverse reactions during the premyelography period were not recorded.
Intramyelography
Intramyelography variables were the type, concentration, and volume of contrast material injected; duration of injection (≤ or >5 minutes); whether the head was elevated for at least 5 minutes postinjection; volume of CSF removed; whether a simultaneous lumbosacral puncture was performed to facilitate caudal flow of the contrast medium; administration of anti‐inflammatory medications and osmotic agents (drug, dosage, time relative to injection of contrast material); and, MAP. The durations of general anesthesia and recovery were recorded, as were the neck positions during myelography (neutral, extended, flexed, and ventrodorsal). The quality of recovery was based on descriptions in the medical record that most closely aligned with: excellent – calmly in sternal recumbency before standing, 1–2 strong attempts to stand, calm demeanor; very good – calm, sternal or sitting with hind legs to 1 side, 2–3 slightly weak to strong attempts to stand; good – calm to mild excitement, sternal or sitting with hind legs to 1 side, 2–4 attempts to stand; fair – excitement, uncoordinated attempts to stand, attempting to stand before strong enough, multiple attempts to stand; poor – very excited to frantic, rough uncoordinated attempts to stand, multiple weak attempts to stand, needed assistance during recovery; very poor – frantic, rough uncoordinated, weak attempts to stand; did not recover – died or euthanasia.
Adverse reactions recorded during the intramyelography period included hypotension (MAP <60 mmHg for ≥10 minutes) or hypertension (MAP >120 mmHg for ≥10 minutes) within 20 minutes of contrast medium injection, seizures (focal, generalized), blindness, hyperesthesia, peripheral neuropathy, myopathy, and other adverse reactions. Outcome at the end of the recovery period (normal, improved, unchanged, worse, died, euthanized) was recorded. Death was further characterized as spontaneous death as a result of an adverse reaction, euthanasia because of poor prognosis or owner discretion, or euthanasia because of an adverse reaction.
Postmyelography
Postmyelography variables were: final diagnosis and how it was obtained (myelography, other ante‐mortem diagnostic tests, or postmortem examination), neurologic grade, administration of anti‐inflammatory medications and osmotic agents, and duration of hospitalization postmyelography. Adverse reactions in the postmyelography period were grouped according to body system involved: musculoskeletal, neurologic, respiratory, nonspecific hyperthermia, gastrointestinal, and renal. Outcomes of adverse reactions were recorded as complete recovery by discharge, partial recovery by discharge, no improvement by discharge, or death. Death in the postmyelography period was recorded as spontaneous death as a result of an adverse reaction or euthanasia because of poor prognosis, owner discretion, or because of an adverse reaction.
Statistical Analyses
Statistical analyses were performed by 2 of the investigators (RB, PVS) using commercially available software.2 , 3 , 4 For the descriptive analysis, categorical variables were described as frequency of occurrence and displayed using a contingency table or bar chart. Numerical data were determined to have a normal distribution using the Kolmogorov–Smirnov test. Hypothesis testing for categorical data was performed using the Chi‐Square test. To evaluate the effect of institution, date of birth, date of myelography, age, sex, breed, body weight, duration of neurologic signs, neurologic grade, administration of anti‐inflammatory and osmotic agents, volume of contrast material injected, volume of contrast material per kilogram body weight injected, general anesthesia time, recovery time, recovery grade, and final diagnosis on the odds of AAR, a logistic regression model was fitted to the data using the logistic procedure of SAS.c Independent variables were sequentially removed from the models in decreasing order of P‐value (backward stepwise) until only significant (P‐value <.05) variables were retained.
Results
The sample population consisted of a 278 horses from 5 institutions (Institution A [n = 87], B [68], C [65], D [46], and E [12]). The type of contrast material was reported in 249 cases and was iohexol 240 mg/mL organic iodine in all instances. In 83/85 (98%) cases that had head position reported, the head was elevated for at least 5 minutes after injection and before image acquisition. Lateral radiographs were reported to have been obtained with the neck in neutral (n = 241), extended (207), flexed (239), and ventrodorsal (41) positions. In 91 cases, the medical record stated whether a lumbosacral puncture was performed simultaneously; in 2/91 (2%) cases, a lumbosacral puncture was performed simultaneously. Any adverse reaction was observed in 95/278 (34%) horses: Institution A 37/87 (43%); B 21/68 (31%); C 22/65 (34%); D 8/46 (17%); and, E 7/12 (58%). No horse died spontaneously. After myelography, neurologic grade remained unchanged in 72/102 (71%) and increased 1 or 2 grades in 25/102 (25%; Table 1). The descriptive data by group is presented (Table 2).
Table 1.
Comparison of neurologic grade before and after myelography in 102 horses
| Pre‐Grade | Post‐Grade | |||||
|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | |
| 0 | 5 | 0 | 0 | 0 | 0 | 0 |
| 1 | 1 | 11 | 6 | 0 | 0 | 0 |
| 2 | 0 | 2 | 28 | 9 | 3 | 0 |
| 3 | 0 | 0 | 1 | 17 | 5 | 1 |
| 4 | 0 | 0 | 0 | 0 | 4 | 1 |
| 5 | 0 | 0 | 0 | 0 | 1 | 7 |
Table 2.
Descriptive statistics for numerical data in 278 horses by group
| Group | Variable | n | Mean | CI | SD | Min‐Max |
|---|---|---|---|---|---|---|
| No adverse reaction | Age (years) | 182 | 4.4 | 3.9–5.0 | 3.6 | 0.1–22.3 |
| Body weight (kg) | 181 | 471 | 455–487 | 112 | 146–700 | |
| Neurologic signs (days) | 141 | 168 | 104–232 | 387 | 1–4015 | |
| CSF volume removed (mL) | 46 | 44 | 40–48 | 13 | 0–60 | |
| Contrast volume (mL) | 152 | 52 | 50–54 | 12 | 15–80 | |
| Contrast dose (mL/kg) | 151 | 0.12 | 0.11–0.12 | 0.03 | 0.03–0.28 | |
| Precontrast MAP (mmHg) | 159 | 80 | 78–83 | 14 | 45–125 | |
| Postcontrast MAP (mmHg) | 176 | 78 | 76–79 | 9.5 | 58–112 | |
| Myelography (minutes) | 175 | 64 | 60–67 | 25 | 10–160 | |
| Anesthesia (minutes) | 180 | 96 | 91–100 | 31 | 45–205 | |
| Recovery (minutes) | 122 | 46 | 42–50 | 23 | 10–159 | |
| Hospitalization (days) | 146 | 4 | 3–6 | 7 | 0–49 | |
| Any adverse reaction | Age (years) | 94 | 4.9 | 4.2–5.6 | 3.4 | 0.1–13.7 |
| Body weight (kg) | 95 | 487 | 463–510 | 116 | 109–739 | |
| Neurologic signs (days) | 75 | 155 | 105–205 | 218 | 1–900 | |
| CSF volume removed (mL) | 14 | 49 | 45–54 | 8 | 30–60 | |
| Contrast volume (mL) | 69 | 56 | 53–60 | 15 | 30–110 | |
| Contrast dose (mL/kg) | 69 | 0.12 | 0.11–0.14 | 0.05 | 0.07–0.37 | |
| Precontrast MAP (mmHg) | 80 | 82 | 79–85 | 15 | 53–137 | |
| Postcontrast MAP (mmHg) | 95 | 79 | 77–82 | 13 | 55–128 | |
| Myelography (minutes) | 92 | 71 | 65–76 | 27 | 30–150 | |
| Anesthesia (minutes) | 94 | 101 | 95–107 | 30 | 50–190 | |
| Recovery (minutes) | 78 | 55 | 49–62 | 27 | 19–205 | |
| Hospitalization (days) | 91 | 8 | 5–10 | 12 | 0–60 |
n, number; CI, 95% confidence interval; SD, standard deviation; Min, minimum; Max, maximum; MAP, mean arterial pressure.
Precontrast MAP is the average for the 10 minutes before contrast‐medium injection. Postcontrast MAP is the average for 0–20 minutes following contrast‐medium injection.
Horses with No Adverse Reaction [183/278 (66%)]
There were 54 stallions or colts, 87 geldings, and 42 mares or fillies. Breeds included were American Saddlebred (n = 3), Arabian (2), Draft (2), Friesian (1) Irish Draught (1), mixed (10), not specified (2), Paint (8), Palomino (1), Pony (4), Quarter Horse (26), Standardbred (17), Tennessee Walking Horse (5), Thoroughbred (65), and Warmblood (36). Final diagnoses were made in 112/183 (61%) horses and included cervical compressive myelopathy alone (n = 82), equine protozoal myeloencephalitis (EPM) alone (16), cervical compressive myelopathy and EPM (3), cervical compressive myelopathy and neuroborreliosis (1), equine degenerative myeloencephalopathy (EDM) (2), equine motor neuron disease (1), other (5), polysaccharide storage myopathy (PSSM) (1), and rabies (1) in a horse that was euthanized 3 days postmyelography because of a lack of improvement in premyelography neurologic signs. In 178 horses with no adverse reaction, the neurologic grade before myelography was 0 (n = 10), 1 (36), 2 (67), 3 (45) 4 (14), and 5 (6; Fig 1). In 46/152 (30%) horses that did not experience AAR, the volume of contrast material administered exceeded 50 mL (Fig 2). Euthanasia was performed in 38/183 (21%) horses at the owners' discretion.
Figure 1.
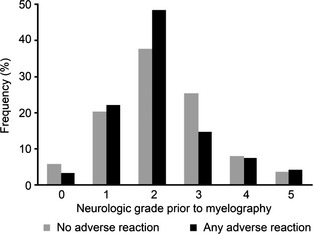
Percentages of neurologic grades prior to myelography by group. There was no significant difference in premyelography grade by group (P = .31).
Figure 2.
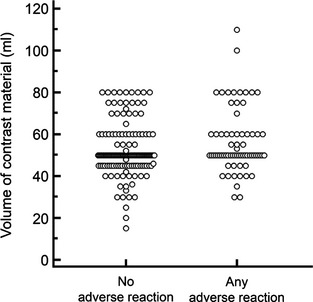
Dot plots of volume of contrast material by group. Horses with AAR received a larger volume of contrast material than horses with no adverse reaction (P = .042).
Horses with AAR [95/278 (34%)]
There were 25 stallions or colts, 48 geldings, and 22 mares or fillies. Breeds included were American Saddlebred (n = 2), Arabian (2), Donkey (1), Gypsy Vanner (1), mixed (4), Morgan (1), not specified (2), National Show Horse (1), Paint (3), Quarter Horse (13), Rocky Mountain horse (1), Standardbred (6), Tennessee Walking horse (2), Thoroughbred (31), and Warmblood (25). A final diagnosis was made in 64/95 (67%) horses and included cervical compressive myelopathy (n = 44), cervical compressive myelopathy and EPM (1), EDM (1), EHM (1), EPM (13), other (2), PSSM (1), and West Nile virus (WNV) (1). In the 95 horses with AAR, the neurologic grade before myelography was 0 (n = 3), 1 (21), 2 (46), 3 (14) 4 (7), and 5 (4; Fig 1). In 29/69 (42%) horses, the volume of contrast material administered exceeded 50 mL (Fig 2).
Adverse reactions reported in the intramyelography period (from injection of contrast material until recovery from general anesthesia; n = 36) were neurological adverse events (15) that included: focal seizures (4), peripheral neuropathy (3), generalized seizures (2), blindness (2), hyperesthesia (1), dull mentation (1), bruxism and star‐gazing (1), and temporary increase in ataxia (1). Among the 3 horses reported to have peripheral neuropathies, 1 horse had difficulty placing the left pelvic limb and 1 horse displayed generalized weakness; no details were provided for the third horse. In the 2 horses reported to have blindness, the origin (central or peripheral) was not reported. Non‐neurologic adverse events in the intramyelography period included hypertension or hypotension (n = 11) as well as mild abrasions from recovery (3), hypothermia (2), intermittent extensor rigidity and pulmonary edema (1), carpal bone fractures (1), myopathy (1), corneal ulceration (1), and intermittent second degree atrioventricular block (1).
Adverse reactions reported in the postmyelography period (recovery to 1 week after myelography; n = 92) were neurologic adverse events (n = 42) including increased neurologic grade (n = 25), generalized seizures (2), partial seizures (4), seizures (type not specified; 3), neck pain or stiffness (4), somnolence (1), excitement (1), head shaking (1), and mild hyperesthesia (1). Nonspecific hyperthermia was recorded in 25 cases. Respiratory adverse reactions (12) included pneumonia (6), increased respiratory rate, nasal discharge or cough (4), and details not specified (2). Gastrointestinal adverse reactions (9) included diarrhea/colitis (4), decreased manure production (2), mild colic (1), impaction colic (1), and impaction with signs of endotoxemia (1). Reactions categorized as musculoskeletal (4) included myopathy (1), increased heat in hooves (1), and details not specified (2). Multiple types of adverse reactions were observed in 15/278 (5%) horses. In 10 horses with nonspecific hyperthermia, 4 horses later developed respiratory complications, gastrointestinal complications, or both, 3 horses had concurrent neurologic adverse reactions, 1 horse had intramyelography hypotension, 1 horse had distal limb edema, and 1 horse had myopathy. Four horses that had intra‐myelography hypotension had neurologic adverse reactions consisting of increased neurologic grade (3) and neurologic adverse reactions type not specified (1). One horse had increased neurologic grade and severe impaction colic. Multiple neurologic adverse reactions were observed in 6/278 (2%) horses.
In horses with AAR, euthanasia was performed in 3/95 (3%) horses at the owners' discretion, and in 5/95 (5%) horses as a consequence of the adverse reaction. The latter included fracture of the left carpus (n = 1), generalized seizures refractory to medical treatment (1), inability to rise 1 day postmyelography (2), and acute, severe colitis 3 days postmyelography (1). The diagnoses of the neurologic lesions in these horses included cervical compressive myelopathy and EPM in the horse with fracture of the carpus, cervical compressive myelopathy in the horse with seizures and in the 2 horses that were unable to rise, and equine herpes myeloencephalopathy (EHM) in the horse with colitis. Except for 1 horse that was unable to rise, all diagnoses were based on postmortem examination findings.
Factors Associated with Adverse Reactions
Any adverse reaction was significantly associated with use of a larger volume of contrast material (P = .04) and longer general anesthesia time (P = .04) when compared to horses with no adverse reaction. No other variables were significant. In this study, the volume of CSF removed before injection of contrast material was only reported in 60/278 (22%) horses and was isovolumetric in 51/60 (85%) horses. There were too few cases to evaluate the relationship between volume of CSF removed and AAR or seizures. The mean ± SD volume of contrast material in the AAR group (56 ± 15 mL) was higher than that used in the no adverse reaction group (52 ± 12 mL; P = .042; Fig 2 and Table 2). For every 1 mL increase in contrast material, horses had an OR of 1.024 (CI, 1.001–1.049) of having AAR. The duration of anesthesia by group is depicted in Figure 3 and Table 2. The mean ± SD duration of anesthesia in the AAR group was significantly longer (101 ± 30 minutes) compared to that of the no adverse reaction group (96 ± 31 minutes; P = .042). For every 1 minute increase in anesthesia time, the OR of having AAR increased by 1.010 (CI, 1.000–1.021). Horses with AAR did not have a significantly higher neurologic grade before myelography than horses with no adverse reaction (P = .31; Fig 1).
Figure 3.
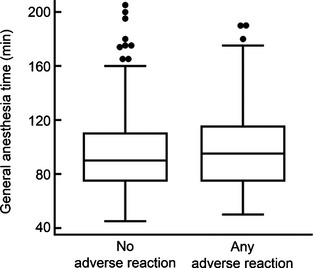
Box and whiskers plots of general anesthesia time in by group. Horses with AAR underwent general anesthesia for a longer time than horses with no adverse reaction (P = .042).
Administration of anti‐inflammatory or osmotic agents was not associated with decreased occurrence of nonspecific hyperthermia (P = .30). In the 25/235 horses with nonspecific hyperthermia, 18/25 (72%) horses received anti‐inflammatory or osmotic agents in the pre‐ or intramyelography periods. In the 210/235 horses without nonspecific hyperthermia, 128/210 (61%) horses received anti‐inflammatory or osmotic agents in the pre‐ or intramyelography period.
Discussion
This study documented the frequency and type of adverse reactions in horses that underwent cervical myelography under general anesthesia at 5 veterinary teaching hospitals. Approximately one‐third of horses had adverse reactions that varied in severity and could be attributed to various aspects of the procedure (eg, general anesthesia, myelography, recovery). Adverse reactions that necessitated euthanasia were observed in 5/278 (2%) horses. This mortality rate is similar to the results of a prospective, multicenter, randomized, controlled trial in horses placed under general anesthesia for any type of surgery.12 In that study, however, limb fracture in recovery, myopathy, and cardiac arrest were the most common perioperative complications resulting in euthanasia or death.12 Some of the complications observed in our study likely were specifically attributable to myelography.
Relating the observed complications in this study to the classification of iodinated contrast medium adverse reactions in people, intramyelography hypertension orhypotension might be an IR.4 Seizures might be nonidiosyncratic reactions.5 Alternatively, seizures could be caused by changes in craniospinal volume, neurotoxicity of iohexol, or could be a sequel of aseptic or septic meningitis.6, 7, 9 Measurement of CSF opening pressure or invasive intracranial pressure monitoring would be necessary to correlate the incidence of adverse reactions with changes in craniospinal pressure. However, to our knowledge, these monitoring procedures have not been performed in horses during myelography. Nonspecific hyperthermia and somnolence postmyelography might be a DNR, similar to what is reported in people who experience flu‐like clinical signs 30 minutes to 7 days after IV injection of contrast medium.4
Neurologic adverse reactions were the most common type of complications observed. The duration of the postprocedural increase in neurologic grade could not be obtained from the medical records. Seizures are a known complication of myelography with iohexol in horses and dogs.1, 13 In a prospective study comparing the type and frequency of adverse effects of metrizamide versus iohexol cervical myelography in horses, seizures and total adverse effects were significantly less frequent with iohexol.1 In a retrospective case series of 503 dogs undergoing myelography with iohexol, 15 (3%) had seizures postmyelography with risk factors including larger total volume of contrast material and shorter duration of time from injection of contrast to anesthetic recovery.13 In the current study, the incidence of seizures in horses was similar to that reported in dogs13 and we also observed a modest relationship between AAR and larger volume of contrast material administered. Unlike in the study of dogs, we found horses with AAR had longer duration of general anesthesia than did horses without reactions. Long duration of surgery under general anesthesia is known to be associated with perioperative complications in horses.14, 15 Anesthesia >90 minutes and lateral recumbency are associated with increased risk of myopathy in horses.12 Because of sample size limitations, we were unable to investigate the relationship between seizures and contrast volume or anesthesia time.
The classification of AAR was not associated with higher neurologic grade before myelography, compared with a previous study.2 The discrepancy might be because of differences in the type of contrast material (metrizamide versus iohexol), patient population, diagnosis, or myelographic, anesthetic and recovery procedures. In the previous study,2 64% of the horses were diagnosed with cervical compression whereas in our study only 45% of horses were diagnosed with extradural cervical compression. Horses with extradural cervical compressive lesions could be more susceptible to injury caused by manipulations during myelography than horses with other diagnoses.
Nonspecific hyperthermia postmyelography was not attenuated by administration of pre‐ or intramyelography anti‐inflammatory and osmotic agents. Short‐term corticosteroid premedication in humans remains controversial and has not been shown unequivocally to decrease contrast medium reactions.4 In addition, pre‐anesthetic phenylbutazone was not associated with a significant difference in survival in horses undergoing cervical myelography with metrizamide.2 In that study, 18/131 (14%) horses had fevers post‐myelography.2 In addition, nonspecific hyperthermia, neck pain and stiffness, and changes in mentation observed in the postmyelography period reported in our study could be signs of aseptic meningitis or meningoencephalitis. Development of aseptic meningitis or meningoencephalitis after intrathecal administration of iodinated contrast medium in humans is thought to be related to changes in intrathecal osmolarity, direct toxicity, or an immune‐mediated process, but the pathophysiology is poorly understood.9 Several horses with nonspecific hyperthermia in the postmyelography period were diagnosed with respiratory or gastrointestinal adverse effects, highlighting the importance of careful examination of horses postmyelography.
Respiratory adverse effects were observed in 12/235 (5%) horses that had recovered from general anesthesia. These included 6 horses that were diagnosed with pneumonia. A case of pleuropneumonia occurred in a 3‐year‐old Thoroughbred 5 days after general anesthesia and cervical myelography.16 The colt was euthanized and a necropsy confirmed pleuropneumonia and identified severe erosions over the middle third of the trachea. Overinflation of the endotracheal tube cuff and manipulation of the neck are risk factors for damage to the tracheal mucosa and could have predisposed the colt to development of pleuropneumonia.16 Regardless of the mechanism of the development of pneumonia after cervical myelography, horses should be positioned carefully during the procedure to minimize injury and should be monitored closely postmyelography.
The multi‐institutional, retrospective study design is associated with limitations including missing data and institutional differences in anesthetic protocols and myelographic procedures. However, inclusion of cases from several universities allowed for a larger sample population of horses. Results of myelography, ante‐mortem EPM testing, and postmortem examination are known to differ depending on examiner, interpreter, and methodology.17, 18, 19 Final diagnoses therefore should be interpreted cautiously. Myelograms were not reviewed by the authors, and no associations could be made between recognition of contrast material surrounding the brain and in the subarachnoid space and adverse effects, or contrast material volume and image quality. The relationship between diagnoses based on standing radiographs and myelograms was not evaluated.
The major findings of this study are that approximately one‐third of horses that undergo general anesthesia and cervical myelography develop AAR. Most AAR are mild and self‐limiting, but a small percentage (2%) of these horses required euthanasia because of adverse reactions. The AAR were statistically associated with larger volumes of contrast material and longer duration of general anesthesia. However, the actual differences were nominal and might not have clinical relevance. The lack of association between higher premyelography neurologic grade and increased odds of adverse reactions was unexpected and could have occurred because no relationship exists or because sample size was too small to detect a relationship. Twenty‐five percent of cases experienced increased neurologic grade after the procedure. Based on the modest OR observed in this study, no specific procedural recommendations can be made, but attention to anesthesia time and volume of contrast material utilized may be prudent. Our findings provide evidence to assist in the decision to perform myelography and in communicating with clients as to the likelihood and type of adverse reactions.
Acknowledgments
The study design and data analysis and manuscript preparation were performed by investigators at Cornell University. Co‐investigators at the other institutions contributed cases.
The study was not supported by a grant.
A research abstract was presented at the 2014 ACVIM Forum, Indianapolis, IN.
The authors acknowledge the assistance of Jennifer Haber, Stefanie Lang, Blake Shessel and Samantha Tandle with data entry.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Vet‐Investigate 2.0™, Cornell University, Ithaca, NY.
MedCalc Version 12.7.4.0, Ostend, Belgium;
Statistix 9 Version 9.1, Tallahassee, FL USA.
SAS 5.1, Cary, NC USA.
References
- 1. Widmer WR, Blevins WE, Jakovljevic S, et al. A prospective clinical trial comparing metrizamide and iohexol for equine myelography. Vet Radiol Ultrasound 1998;39:106–109. [DOI] [PubMed] [Google Scholar]
- 2. Hubbell JA, Reed SM, Myer CW, et al. Sequelae of myelography in the horse. Equine Vet J 1988;20:438–440. [DOI] [PubMed] [Google Scholar]
- 3. Namasivayam S, Kalra MK, Torres WE, et al. Adverse reactions to intravenous iodinated contrast media: A primer for radiologists. Emerg Radiol 2006;12:210–215. [DOI] [PubMed] [Google Scholar]
- 4. American College of Radiology . ACR Manual on Contrast Media 2012 – Version 8 Contrastmedia/Contrastmedia 2012.
- 5. Singh J, Daftary A. Iodinated contrast media and their adverse reactions. J Nucl Med Technol 2008;36:69–74; quiz 76‐7. [DOI] [PubMed] [Google Scholar]
- 6. Yuh EL, Dillon WP. Intracranial hypotension and intracranial hypertension. Neuroimaging Clin N Am 2010;20:597–617. [DOI] [PubMed] [Google Scholar]
- 7. Caillé JM, Allard M. Neurotoxicity of hydrosoluble iodine contrast media. Invest Radiol 1988;23(Suppl 1):S210–12. [DOI] [PubMed] [Google Scholar]
- 8. Kieffer SA, Binet EF, Davis DO, et al. Lumbar myelography with iohexol and metrizamide: A comparative multicenter prospective study. Radiology 1984;151:665–670. [DOI] [PubMed] [Google Scholar]
- 9. Romesburg J, Ragozzino M. Aseptic meningoencephalitis after iohexol CT myelography. Am J Neuroradiol 2009;30:1074–1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Spencer CP, Chrisman CL, Mayhew IG, et al. Neurotoxicologic effects of the nonionic contrast agent iopamidol on the leptomeninges of the dog. Am J Vet Res 1982;43:1958–1962. [PubMed] [Google Scholar]
- 11. de Lahunta A, Glass EN. Veterinary Neuroanatomy and Clinical Neurology. St. Louis, MO: Saunders Elsevier; 2009. [Google Scholar]
- 12. Johnston G, Eastment J, Taylor P, et al. Is isoflurane safer than halothane in equine anaesthesia? Results from a prospective multicentre randomised controlled trial. Equine Vet J 2004;36:64–71. [DOI] [PubMed] [Google Scholar]
- 13. da Costa RC, Parent JM, Dobson H. Incidence of and risk factors for seizures after myelography performed with iohexol in dogs: 503 cases (2002‐2004). J Am Vet Med Assoc 2011;238:1296–1300. [DOI] [PubMed] [Google Scholar]
- 14. Johnston GM, Steffey E. Confidential enquiry into perioperative equine fatalities (CEPEF). Vet Surg 1995;24:518–519. [DOI] [PubMed] [Google Scholar]
- 15. Johnston G, Eastment J, Wood J, et al. The confidential enquiry into perioperative equine fatalities (CEPEF): Mortality results of Phases 1 and 2. Vet Anaesth Analg 2002;29:159–170. [DOI] [PubMed] [Google Scholar]
- 16. Rainger JE, Hughes KJ, Kessell A, et al. Pleuropneumonia as a sequela of myelography and general anaesthesia in a Thoroughbred colt. Aust Vet J 2006;84:138–140. [DOI] [PubMed] [Google Scholar]
- 17. Levine JM, Scrivani PV, Divers TJ, et al. Multicenter case‐control study of signalment, diagnostic features, and outcome associated with cervical vertebral malformation‐malarticulation in horses. J Am Vet Med Assoc 2010;237:812–822. [DOI] [PubMed] [Google Scholar]
- 18. Reed SM, Howe DK, Morrow JK, et al. Accurate antemortem diagnosis of equine protozoal myeloencephalitis (EPM) based on detecting intrathecal antibodies against Sarcocystis neurona using the SnSAG2 and SnSAG4/3 ELISAs. J Vet Intern Med 2013;27:1193–1200. [DOI] [PubMed] [Google Scholar]
- 19. Johnson AL, Morrow JK, Sweeney RW. Indirect fluorescent antibody test and surface antigen ELISAs for antemortem diagnosis of equine protozoal myeloencephalitis. J Vet Intern Med 2013;27:596–599. [DOI] [PubMed] [Google Scholar]


