Abstract
Background
Most information about pharyngeal collapse in dogs is anecdotal and extrapolated from human medicine. A single case report describing dynamic pharyngeal collapse in a cat has been published, but there is no literature describing this disease process in dogs.
Objective
To describe the signalment, clinical presentation, concurrent disease processes, and imaging findings of a population of client‐owned dogs with pharyngeal collapse.
Animals
Twenty‐eight client‐owned dogs with pharyngeal collapse.
Methods
Radiology reports of dogs for which fluoroscopy of the respiratory system was performed were reviewed retrospectively. Patients with a fluoroscopic diagnosis of pharyngeal collapse were included in the study population. Data regarding clinical signs, diagnostic, and pathologic findings were evaluated.
Results
Twenty‐eight dogs met the inclusion criteria. The median age of affected patients was 6.6 years, whereas median body condition score was 7/9. The most common clinical signs were coughing (n = 20) and stertor (n = 5). In 27 of 28 cases, a concurrent or previously diagnosed cardiopulmonary disorder was detected. The most common concurrent disease processes were mainstem bronchi collapse (n = 18), tracheal collapse (n = 17), and brachycephalic airway syndrome (n = 8). Fluoroscopy identified complete pharyngeal collapse in 20 of 28 dogs.
Conclusions
Pharyngeal collapse is a complex disease process that likely is secondary to long‐term negative pressure gradients and anatomic and functional abnormalities. Based on the findings of this study, pharyngeal fluoroscopy may be useful diagnostic test in patients with suspected tracheal and mainstem bronchial collapse to identify concurrent pharyngeal collapse.
Keywords: Anatomy and pathology, Larynx, Pharynx, Physiology, Pulmonary, Respiratory tract, Trachea, Tracheal collapse
Abbreviations
- OSAS
obstructive sleep apnea syndrome
- BCS
body condition score
- CT
computed tomograpy
- MRI
magnetic resonance imaging
Pharyngeal diseases are relatively common in veterinary patients and can include foreign bodies,1 neoplasia,1 inflammatory polyps,1 cysts,1 nasopharyngeal strictures or stenosis,2, 3 excessive nasal turbinates,4 abscesses,1 pharyngitis or tonsillitis1, and dynamic processes such as pharyngeal collapse.5, 6, 7, 8, 9, 10, 11, 12, 13 Pharyngeal collapse refers to complete or partial collapse of the pharynx with dorsal displacement of the soft palate or ventral deviation of the dorsal pharyngeal wall.14 This condition is relatively common and well described in humans and is thought to be a major component of obstructive sleep apnea (OSAS).15, 16 It has diagnosed by a number of different methods including gated computed tomography (CT), magnetic resonance imaging (MRI), endoscopy and fluoroscopy.17, 18 This condition is less well defined in the veterinary literature, with only a few small animal6, 7, 8, 9, 10 and equine11, 12, 13 cases described. Studies in humans suggest that pharyngeal collapse is likely a multifactorial condition that includes anatomic abnormalities and pharyngeal dilator myopathies.15, 16, 17, 19 Unfortunately because of the limited information regarding pharyngeal collapse in small animal veterinary medicine, the clinical incidence and relevance of the condition is not known and the best method of diagnosis has not been elucidated. Although no gold standard has been established in small animal medicine, fluoroscopy has been used as a noninvasive diagnostic tool to evaluate other dynamic processes such as esophageal motility and tracheal collapse.20
Several components of brachycephalic obstructive airway syndrome (BOAS), including an elongated soft palate and everted laryngeal saccules, can affect pharyngeal diameter. Increased inspiratory effort against a fixed airway obstruction, such as stenotic nares, or dynamic airway obstruction, such as airway collapse associated with tracheobronchomalacia, also may result in pharyngeal narrowing. Brachycephalic dogs experiencing long‐term negative pressure gradients associated with increased inspiratory upper airway resistance may develop pathologic changes of the pharyngeal dilator muscles and therefore are predisposed to pharyngeal collapse.6, 8 Although several studies have suggested that pharyngeal collapse occurs in brachycephalic breeds, little is known about its overall incidence in these dogs and in other breeds.6, 7, 8, 9 The objective of the study was to describe the signalment, clinical features, imaging findings, and concurrent disease processes in dogs diagnosed with pharyngeal collapse.
Materials and Methods
Medical Records
Medical records and imaging findings of dogs with a diagnosis of pharyngeal collapse between February 2007 and April 2014 were reviewed retrospectively. All patients were examined at the Matthew J. Ryan Veterinary Hospital at the University of Pennsylvania. Only patients with complete medical records and fluoroscopic imaging results available for review were included. Information concerning age at diagnosis, sex, body weight, body condition, presenting clinical signs, and previous or concurrent respiratory diseases was collected. For this study, the diagnosis of pharyngeal collapse for each animal was made on fluoroscopic evaluation and was defined as wide dorsal to ventral excursions of the pharyngeal soft tissue with narrowing of the nasopharyngeal or laryngopharyngeal luminal diameter during respiration or coughing.14 Pharyngeal collapse was further defined as complete collapse if complete loss of lumen was seen, and partial collapse if lumen diameter subjectively decreased by >50% (Fig 1). Subjects were excluded from the study if results of fluoroscopy were suggestive of pharyngeal collapse but additional endoscopic or CT evaluation indicated other abnormalities (e.g., tumor had caused a mass effect on the pharynx), even if a dynamic component of pharyngeal collapse was present, or definitive nasopharyngeal stenosis was identified.
Figure 1.
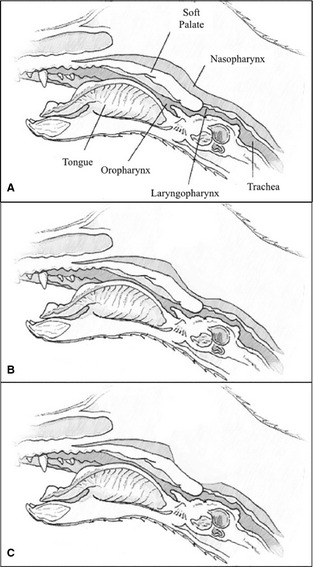
Schematic representation of the pharynx in the dog with (A) normal, (B) partial nasopharyngeal collapse, and (C) complete nasopharyngeal collapse.
Imaging Studies
All procedures were performed or supervised by a board‐certified radiologist, who then was involved in creation of a finalized report for the medical record. When available, standard 3‐view thoracic and single lateral cervical neck radiographs were evaluated in conjunction with fluoroscopic examinations.
Fluoroscopic examinations,1 , 2 were obtained with the dog restrained in right lateral or sternal recumbency at the discretion of the attending radiologist and based on the patient's respiratory stability and tolerance for restraint. Patients were manually restrained in right lateral recumbency or were placed in a plastic box that limited movement for evaluation in sternal recumbency or while standing. All fluoroscopic airway‐imaging studies were performed with the dog awake or under mild sedation utilizing acepromazine or butorphanol at the primary clinician's discretion. Regular respiration was observed and recorded. Tracheal manipulation and compression were performed to allow fluoroscopic evaluation and recording of the trachea and bronchi during coughing. Collapse of the trachea, mainstem bronchi, and pharynx during respiration and coughing were subjectively evaluated and results were recorded as present or absent. All studies were part of the Picture Archiving and Communication System (PACS) and stored in Digital Imaging and Communications in Medicine (DICOM) format (Figs 2, 3).
Figure 2.
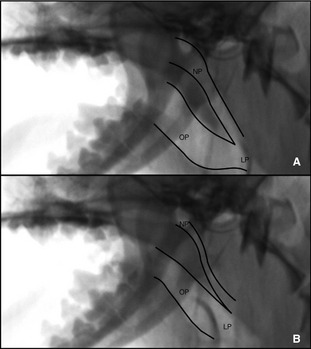
Dynamic complete collapse of the nasopharynx (NP) with static oropharynx (OP) and laryngopharynx (LP). (A) Note the open lumen and then complete obliteration of the nasopharynx (B) noted on inspiration.
Figure 3.
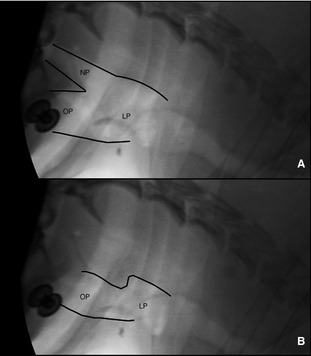
Dynamic partial collapse of the nasopharynx (NP) with static oropharynx (OP) and laryngopharynx (LP). (A) Note the open lumen and then partial collapse (B) noted on inspiration.
Pharyngolaryngoscopy was performed or supervised by a board‐certified surgeon. A soft palate that extended >1–2 mm beyond the dorsal border of the epiglottic cartilage was considered too long. The larynx was examined for collapse, edema, and the presence of ventricular eversion.
Results
Medical records identified 178 dogs that underwent upper airway fluoroscopy during the study time period. Of these, 29 dogs initially were identified with fluoroscopic evidence of dynamic nasopharyngeal or laryngopharyngeal collapse. One case was excluded because nasopharyngoscopy via retroflexed rhinoscopy confirmed a soft palate polyp after a suspected nasopharyngeal mass, as well as dynamic pharyngeal collapse, were diagnosed on fluoroscopy. Twenty‐eight dogs, therefore, met the inclusion criteria for the study.
Signalment
Of the 28 dogs, 17 were male (60.7%) and 11 were female (39.3%). Fourteen dogs were male neutered, 3 male intact, 8 female spayed, and 3 female intact. The age at time of diagnosis varied between 8 months and 15.8 years (median, 6.6 years; mean, 7.3 years; interquartile range [IQR], 4.4 years).
Dogs weighed between 1.9 and 13.3 kg (median, 5.2 kg). On the basis of a body condition score (BCS) scale of 1–9 (1 = cachectic, 9 = obese), 18 of 28 (64.3%) dogs were considered overweight or obese (BCS > 6) and only 1 of 28 (3.6%) dogs was considered thin or cachectic (BCS < 3). The remaining 9 dogs were assessed to be in ideal body condition. Yorkshire Terriers (n = 12) were the most common breed affected with Chihuahuas (3), Pugs (3), Pomeranians (2), and French Bulldogs (2) occurring less frequently. There were 6 other breeds represented, including 1 each of English bulldog, Maltese, mixed breed, Pekingese, Shih Tzu, and toy poodle. Eight dogs were classified as brachycephalic including the English bulldog, French bulldogs, Pekingese, Pugs, and Shih Tzu.
Clinical Signs
The most common presenting clinical signs were coughing (n = 19, 67.8%) and stertorous upper air way noise (n = 5, 17.8%). Other clinical signs included gagging (n = 3, 10.7%), regurgitation (n = 1, 3.6%), and sneezing (n = 1, 3.6%). Twenty‐two of 28 (78.6%) dogs had previous medical treatment including combinations of cough suppressants (hydrocodone, butorphanol, and diphenoxylate [n = 12, 42.8%]), bronchodilators (theophylline, terbutaline, and aminophylline [n = 10, 35.7%]), prednisone (n = 8, 28.5%), and various antibiotics (n = 6, 21.4%). Two dogs had previously been diagnosed with tracheal collapse, both of which were treated with tracheal stents. Four dogs had previous brachycephalic airway surgery including a combination of staphylectomy (n = 4), laryngeal sacculectomy (n = 3), and alar fold resection (n = 3). One dog was identified previously as having epiglottic retroversion, which was corrected with an epiglottic tacking suture.
Imaging Studies
Thoracic and cervical radiographs were reviewed by a board‐certified radiologist in 27 of 28 cases. A single dog had no radiographs available for review. Radiographic abnormalities included findings suggestive of tracheal collapse (n = 16, 57.1%), a diffuse bronchointerstitial pattern (n = 10, 35.7%), left‐sided cardiomegaly (n = 6, 21.4%), pulmonary alveolar pattern (n = 2, 7.1%), bronchiectasis (n = 2, 7.1%), and peri‐bronchial infiltrates (n = 1, 3.6%). Findings suggestive of pharyngeal collapse (e.g. narrowing of the nasopharynx or laryngopharynx) were present in 8 of 28 dogs (28.5%). Radiographs were considered to be normal in 7 dogs (25%).
Fluoroscopic evaluation indicated that complete pharyngeal collapse occurred in 20 dogs (71.4%), whereas 8 dogs had partial collapse. Patients were imaged either in lateral recumbency (n = 13) or in sternal recumbency or standing (n = 12) depending on patient compliance, respiratory stability, and attending radiologist preference. Medical records of 2 patients did not note patient position during evaluation. Only 3 dogs were sedated for imaging studies, with 1 patient being imaged while sedated, and then re‐imaged after recovery. This patient had partial pharyngeal collapse on both studies. Manipulation of the trachea with the goal of inducing coughing was attempted in all dogs, but only 16 dogs had documentation of a cough on fluoroscopic examination. Of the 28 patients that had pharyngeal collapse, 20 patients had evidence of collapse in other areas of their respiratory tract as described in Table 1. Eighteen dogs had concurrent mainstem bronchial collapse, 16 had evidence of intrathoracic tracheal collapse, and 14 had evidence of cervical tracheal collapse. Cranial lung lobe herniation through the thoracic inlet was identified in 8 cases.
Table 1.
Localizations of concurrent respiratory tract collapse in cases of pharyngeal collapse. Number cases with complete collapse noted in parenthesis. The respiratory tract is defined as the cervical trachea, intrathoracic trachea, and mainstem bronchi
| Concurrent Respiratory Tract Collapse | Number of Cases (Complete Collapse) |
|---|---|
| No respiratory tract collapse | 8 (6) |
| Cervical tracheal collapse | 1 (1) |
| Intrathoracic tracheal collapse | 0 |
| Cervical and intrathoracic collapse | 1 (1) |
| Mainstem bronchial collapse | 3 (3) |
| Cervical and mainstem bronchial collapse | 0 |
| Intrathoracic and mainstem bronchial collapse | 3 (2) |
| Cervical, intrathoracic and mainstem bronchial collapse | 12 (7) |
Pharyngolaryngoscopy Findings
Fourteen of 28 dogs had pharyngolaryngoscopy examination performed at the time of tracheal stent placement, surgical correction of BOAS, or as a stand‐alone procedure. Abnormal findings included elongated soft palate (n = 4), everted laryngeal saccules (n = 1), laryngeal edema (n = 1), and epiglottic retroversion (n = 1). Eight dogs had normal pharyngolaryngoscopy examinations.
Previous and Concurrent Respiratory Diseases
In 27 of 28 cases, a concurrent or previously diagnosed respiratory or cardiac disorder was detected. In dogs with concurrent disease, it is unknown whether the signs were attributed to the pharyngeal collapse, to the concurrent respiratory disease, or a combination of both. These concurrent disorders included mainstem bronchial collapse (n = 18), tracheal collapse (n = 17), brachycephalic airway syndrome (n = 8) mitral or tricuspid valve regurgitation (n = 7), pneumonia (n = 5), epiglottic retroversion (n = 1), and pulmonary hypertension (n = 1). None of the dogs diagnosed with mitral or tricuspid valve regurgitation were in congestive heart failure at the time of fluoroscopic evaluation.
Discussion
Pharyngeal collapse is a well‐documented disease process in humans and is considered a major part of OSAS,14 but it is not a well‐recognized syndrome in small animal veterinary medicine.
The pathophysiology of pharyngeal collapse has not been completely elucidated, but it is likely multifactorial in nature.15, 16, 17, 19 Pharyngeal anatomy and pharyngeal dilator muscle responsiveness are probable important components.16 The major pharyngeal dilator muscles in dogs are the sternohyoideus and genohyoideus muscles, both of which pull the hyoid apparatus caudally and allow for a larger pharyngeal diameter. The genoglossius is a secondary pharyngeal dilator muscle, which retracts the tongue cranially and ventrally allowing for greater airflow.21 These 3 muscles all are innervated either directly or by a branch of the hypoglossal nerve.21 Experimental studies in horses illustrate that selective blocking of the hypoglossal nerve results in a marked increase in nasopharyngeal collapse under exercise conditions.13 Clinical studies in humans have identified neural dysfunction and injury in adults with obstructive sleep apnea. Sensory nerve action potential amplitudes are decreased in individuals with OSA, and treatment of sleep apnea partially reverses these decreased nerve action potential amplitudes.22 Assessment of hypoglossal nerve dysfunction in dogs affected with airway collapse, brachycephalic obstructive airway syndrome, and pharyngeal collapse is warranted to determine if hypoglossal pathology exists similar to that described in people and horses.
The most common clinical sign seen in this study was coughing, which is a relatively nonspecific manifestation of respiratory and cardiac disease. Coughing can be classified as reflex‐mediated, which is often the cause for coughing with tracheobronchial disease, or voluntary mediated.23 Multiple studies in humans have noted an association with chronic coughing and OSAS, and although the current recommendation is to eliminate other causes of cough, a small population of chronic coughing may be caused by OSAS.24, 25, 26 In these individuals, treatment for OSAS with continuous positive airway pressure has been shown to decrease the severity and frequency of chronic cough.26 Twenty‐seven cases were identified in which a primary respiratory disease was found that could explain the dog's clinical signs of coughing. Twenty of these dogs had an additional area of the respiratory tract collapse (18 had mainstem bronchial collapse, 1 had cervical tracheal collapse, 3 had intrathoracic tracheal collapse, and 13 had combined cervical and intrathoracic tracheal collapse). In healthy animals, the intratracheal pressure is subatmospheric, allowing air to flow from the upper respiratory tract into the lower airways.27 During inspiration, subatmospheric intrathoracic pressure is created from contraction of the diaphragm and intercostal muscles, resulting in increased volume and decreased pressure within the thoracic cavity which facilitates respiration.28 With tracheal collapse, redundancy of the dorsal tracheal membrane and loss of rigidity of the tracheal cartilages creates a predisposition of the trachea to collapse secondary to changes in airway pressures. The combined normal subatmospheric intratracheal pressure and increased negative pressure generated on inspiration contributes to cervical tracheal collapse on inspiration. The same pathologic changes to the tracheal membrane and cartilaginous rings of the intrathoracic segment of the trachea and mainstem bronchi cause collapse of these portions of the airway upon expiration, when intrapleural pressure is increased.28 The increased airway resistance caused by a collapsed trachea or collapsed mainstem bronchi causes a substantial increase in this negative pressure gradient between the upper and lower airways, leading to progressively increased work of breathing over time.29, 30, 31 This chronically increased negative pressure gradient likely contributes to changes in the pharyngeal dilator muscles thus predisposing dogs to pharyngeal collapse.8 However, in normal animals, large changes in negative pressure in the isolated upper airway triggers a dual protective reflex, which restores airway patency by both activating airway dilators while inhibiting diaphragm electrical activity as detected by electromyography (EMG).19 Therefore, negative airway pressure alone is likely not the sole cause of pharyngeal collapse, even in a chronic setting.6, 7, 8, 9
Fluoroscopy with dogs in lateral or sternal recumbency or while standing was used as the diagnostic test to confirm pharyngeal collapse in this study because it allows real‐time assessment of dynamic changes in the airway during awake or lightly sedated respiration. Doing so is important because generation of negative pressure upon inspiration is proposed to contribute to collapse of the weakened pharynx. In intubated anesthetized patients breathing spontaneously, most airflow generated by muscular contraction to create negative pressure bypasses the nasal passage in favor of the larger diameter endotracheal tube. Therefore, lack of negative pressure on the nasopharynx during respiration could mean that the pharynx may not collapse as it would during normal respiration. Intubation and positive pressure ventilation would alter normal airway pressure changes associated with respiration and may preclude thorough assessment of nasopharyngeal changes. Fluoroscopy also is useful to confirm the location and nature of tracheal and mainstem bronchial collapse in patients with clinical signs and physical examination findings consistent with airway collapse. One of the main benefits is that it can be performed without general anesthesia and permits dynamic assessment of respiratory changes with the phase of respiration and coughing. The gold standard for diagnosing tracheal collapse involves the use of tracheobronchoscopy because it allows intraluminal assessment of the dorsal tracheal membrane and tracheal cartilaginous ring shape along the entire trachea and bronchi.29, 32 Fluoroscopy, however, is a noninvasive imaging modality that supports a diagnosis of tracheal collapse when appropriate clinical signs and examination findings are present and the risk of difficult anesthetic recovery is judged to be high in dogs with advanced disease. In patients with left atrial enlargement causing bronchial compression and coughing, tracheobronchoscopy is a useful diagnostic procedure to confirm bronchial collapse as the sole respiratory pathology. Awake transnasal nasopharyngoscopy utilizing flexible endoscopy is possible in horses, but because of size restrictions of the nasal passages of small animal patients, it can only be performed in dogs weighing >20 kg.33, 34 Awake dynamic CT could be considered for 3‐dimensional assessment of nasopharyngeal changes during respiration, especially if a previously described patient positioning device is used to limit patient movement during the study.35
In humans, craniofacial morphology influences airflow and the apnea hypopnea index, such that these individuals have an increased risk of OSAS.18, 36 The apnea hypopnea index is an objective scoring system that is utilized to quantitate the length of pauses in breathing and the oxygen saturation during these apneic episodes.37 The combination of these scores allows determination of a sleep apnea severity score, which predicts the likelihood of developing OSAS.37 Equivalent studies and scoring systems have not been established in dogs, but; given the craniofacial morphology of brachycephalic breeds, this conformation may be a strong contributory factor to pharyngeal collapse. Eight of the 28 dogs affected in this study were classified as a brachycephalic breed, which is consistent with previous reports that these breeds may be more predisposed to this syndrome.6, 7, 8, 9 During the study time period, 178 dogs had fluoroscopy of their respiratory tract, with 28 of these animals identified as brachycephalic breeds. When evaluating brachycephalic breeds as a whole, 8 of 28 dogs (28.6%) had evidence of pharyngeal collapse compared to 20 of 150 dogs (13.3%) that were classified as either mesocephalic and dolichocephalic. Although this may appear as a marked difference, the exact incidence in each population from this institution is not known because not all fluoroscopic imaging studies performed during the study period included the pharyngeal region.
The median BCS was 7/9 for dogs included in this study, with 18 of 28 (64.2%) patients considered overweight or obese. Obesity has been identified as the main risk factor for sleep apnea in humans, and increases in adipose tissue and neck circumference are strong predictors of the disease in people.19 Although not compared statistically to the population of animals seen at this hospital, the rate in this study is considerably higher than the national average for obesity, which is 34.1% for all dogs seen in private practice.38 Therefore, increased body condition scores and obesity in affected dog breeds may be possible risk factors for development of pharyngeal collapse.
One dog in this study (an English bulldog) had no respiratory signs, and pharyngeal collapse was identified as an incidental finding on an esophagram performed to evaluate regurgitation. In the absence of other current respiratory anatomic abnormalities, the collapse may have been mild enough so as not to cause any detectable signs or simply was diagnosed early in the disease course whereas investigating another problem commonly associated with brachycephalic airway syndrome.9, 39 Because up to 73% of brachycephalic dogs with even mild respiratory clinical signs had frequent ptyalism, regurgitation, and vomiting in 1 study, it is not surprising that gastrointestinal diagnostic evaluation of a brachycephalic dog could identify concurrent respiratory pathologies.39 Gastrointestinal lesions confirmed in brachycephalic dogs include hiatal hernia, pyloric mucosal hyperplasia, esophagitis, gastritis and duodenitis, pyloric stenosis, and gastric atony. Surgical correction of obstructive airway anatomic abnormalities, combined with medical management of gastrointestinal signs, decreases the complication rate and improves prognosis for brachycephalic dogs undergoing corrective airway surgery.9
The major limitations of this study are associated with its retrospective nature. Unfortunately, although many patients did return for follow‐up examination, limited information was available in the record to determine if treating the other respiratory diseases improved or lessened the severity of the pharyngeal collapse as well as which clinical signs could be attributed solely to pharyngeal collapse by treating concurrent respiratory or cardiac disorders. Two dogs did undergo tracheal stenting before to a diagnosis of pharyngeal collapse, and it was only on follow‐up examination for persistent respiratory effort that pharyngeal collapse was identified. One of these dogs was not stable enough for awake fluoroscopy on initial examination because of the severity of its airway obstruction from tracheal collapse. Therefore, this dog may have had pharyngeal collapse before stenting that was not diagnosed. The other dog had a normal nasopharynx on initial fluoroscopy to evaluate for tracheal collapse, and developed pharyngeal collapse 6 months later. This observation suggests that even after treating 1 of the primary causes of increased negative pressure gradient, pharyngeal collapse still can occur and might represent a progressive disease process or 1 that is irreversible even after a primary disease is treated. Another explanation could be the relative insensitivity of fluoroscopy for the evaluation of pharyngeal collapse or marked variability in findings for a given patient when assessed under different conditions. Unfortunately, there is no current gold standard for diagnosing pharyngeal collapse in small animal veterinary medicine against which to compare the results of this study. Patients underwent direct pharyngolaryngoscopy at the discretion of the attending clinician and as such the number of dogs with concurrent upper airway abnormalities (e.g., elongated soft palate, everted saccules, laryngeal edema) may be falsely lower than if every patient underwent evaluation. One of the other major limitations of this study is the lack of a control group, because normal dogs rarely have upper airway fluoroscopy performed. This situation likely led to a biased population consisting of primarily small breed dogs, because they are more predisposed to chondrobronchomalacia, and therefore, more often have tracheal fluoroscopy performed as part of their respiratory tract evaluation.20
The underlying causes of pharyngeal collapse likely vary considerably among affected dogs, but most dogs in this study had an identifiable primary disease process that could account for their clinical signs. Based on the results of this study, pharyngeal collapse is unlikely to be a primary disease in many dogs, but instead a complication of other forms of airway pathology especially those that contribute to increased negative intrathoracic pressure. Additionally, many of the patients affected with pharyngeal collapse also were overweight or obese, which may be a predisposing factor to the development of the disease or may contribute to worsening of the disease. However, these observations need to be further investigated using case–controls to understand the relationship between obesity and pharyngeal collapse.
Future studies are warranted to prospectively investigate whether treating concurrent disease processes, especially early in their course, can eliminate or reverse fluoroscopic evidence of pharyngeal collapse, or whether it is an irreversible condition once the primary disease process is severe or long‐standing enough to cause permanent pathology. In addition, based on the findings of this study, pharyngeal fluoroscopy may be a useful diagnostic test in patients with suspected tracheal and mainstem bronchial collapse to identify other areas of pathologic collapse along the respiratory tract.
Acknowledgment
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
GE Advantx high frequency, multi‐pulse 65 kW generator, General Electric, Fairfield, CT, USA
Philips Veradius C‐arm, Koninklijke Philips Electronics NV, Amsterdam, Netherlands
References
- 1. Hunt GM, Perkins MC, Foster SF, et al. Nasopharyngeal disorders of dogs and cats: A review and retrospective study. Compend Contin Educ Vet 2002;24:184–199. [Google Scholar]
- 2. Griffon DJ, Tasker S. Use of a mucosal advancement flap for the treatment of nasopharyngeal stenosis in a cat. J Small Anim Pract 2000;41:71–73. [DOI] [PubMed] [Google Scholar]
- 3. Berent A, Weisse C, Todd K, et al. Use of a balloon‐expandable metallic stent for treatment of nasopharyngeal stenosis in dogs and cats: Six cases (2005–2007). J Am Vet Med Assoc 2008;233:1432–1440. [DOI] [PubMed] [Google Scholar]
- 4. Ginn JA, Kumar MSA, McKiernan BC, Powers BE. Nasopharyngeal turbinates in Brachycephalic dogs and cats. J Am Anim Hosp Assoc 2008;44:243–249. [DOI] [PubMed] [Google Scholar]
- 5. Cehak A, Rohn K, Barton AK, et al. Effect of head and neck position on pharyngeal diameter in horses. Vet Radiol Ultrasound 2010;51:491–497. [DOI] [PubMed] [Google Scholar]
- 6. Hendricks JC, Kline LR, Kovalski RJ, et al. The English bulldog: A natural model of sleep‐disordered breathing. J Appl Physiol 1987;63:1344–1350. [DOI] [PubMed] [Google Scholar]
- 7. Hendricks JC, Petrof BJ, Panckeri K, Pack AI. Compensatory hyperactivity of an upper airway dilator in bulldogs. Am Rev Respir Dis 1993;148:185–194. [DOI] [PubMed] [Google Scholar]
- 8. Petrof BJ, Pack AI, Kelly AM, et al. Pharyngeal myopathy of loaded upper airway in dogs with sleep apnea. J Appl Physiol 1994;76:1746–1752. [DOI] [PubMed] [Google Scholar]
- 9. Poncet CM, Dupre GP, Freiche VG, Bouvy BM. Long‐term results of upper respiratory syndrome surgery and gastrointestinal tract medical treatment in 51 brachycephalic dogs. J Small Anim Pract 2006;47:137–142. [DOI] [PubMed] [Google Scholar]
- 10. Zaid MS, Porat‐Mosenco Y, Mosenco AS. Dynamic collapse of the common pharynx in a cat. J Vet Intern Med 2011;25:1458–1460. [DOI] [PubMed] [Google Scholar]
- 11. Dart AJ, Dowling BA, Hodgson DR, Rose RJ. Evaluation of high‐speed treadmill videoendoscopy for diagnosis of upper respiratory tract dysfunction in horses. Aust Vet J 2001;79:109–112. [DOI] [PubMed] [Google Scholar]
- 12. Tessier C, Holcombe SJ, Derksen FJ, et al. Effects of stylopharyngeus muscle dysfunction on the nasopharynx in exercising horses. Equine Vet J 2004;36:318–323. [DOI] [PubMed] [Google Scholar]
- 13. Cheetham J, Pigott JH, Hermanson JW, et al. Role of the hypoglossal nerve in equine nasopharyngeal stability. J Appl Physiol 2009;107:471–477. [DOI] [PubMed] [Google Scholar]
- 14. Rodriguez‐Lozano FJ, Saez‐Yuguero MR, Linares‐Tovar E, Bermejo‐Fenoll A. Sleep apnea and mandibular advancement device. Revision of the literature. Med Oral Patol Oral Cir Bucal 2008;13:E549–E554. [PubMed] [Google Scholar]
- 15. Malhotra A, Huang Y, Fogel R, et al. Aging influences on pharyngeal anatomy and physiology: The predisposition to pharyngeal collapse. Am J Med 2006;119:72.e9–72.e14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Campana L, Eckert DJ, Patel SR, Malhotra A. Pathophysiology and genetics of obstructive sleep apnoea. Indian J Med Res 2010;131:176–187. [PMC free article] [PubMed] [Google Scholar]
- 17. Togeiro SM, Chaves CM Jr, Palombini L, et al. Evaluation of the upper airway in obstructive sleep apnoea. Indian J Med Res 2010;131:230–235. [PubMed] [Google Scholar]
- 18. Suratt PM, Dee P, Atkinson RL, et al. Fluoroscopic and computed tomographic features of the pharyngeal airway in obstructive sleep apnea. Am Rev Respir Dis 1983;127:487–492. [DOI] [PubMed] [Google Scholar]
- 19. Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev 2010;90:47–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Johnson LR, Pollard RE. Tracheal collapse and bronchomalacia in dogs: 58 cases (2001–2008). J Vet Intern Med 2010;24:298–305. [DOI] [PubMed] [Google Scholar]
- 21. Evans HE. The muscular system In: Evans HE, ed. Miller's Anatomy of the Dog, 3rd ed Toronto, ON: WB Saunders Co; 1993:279–285. [Google Scholar]
- 22. Dziewas R, Schilling M, Engel P, et al. Treatment for obstructive sleep apnoea: Effect on peripheral nerve function. J Neurol Neurosurg Psychiatry 2007;78:295–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Magni C, Chellini E, Lavorini F, et al. Voluntary and reflex cough: Similarities and differences. Pulm Pharmacol Ther 2011;24:308–311. [DOI] [PubMed] [Google Scholar]
- 24. Birring SS, Ing AJ, Chan K, et al. Obstructive sleep apnoea: A cause of chronic cough. Cough 2007;3:1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Faruqi S, Fahim A, Morice AH. Chronic cough and obstructive sleep apnoea: Reflux‐associated cough hypersensitivity? Eur Respir J 2012;40:1049–1050. [DOI] [PubMed] [Google Scholar]
- 26. Sundar KM, Daly SE, Willis AM. A longitudinal study of CPAP therapy for patients with chronic cough and obstructive sleep apnoea. Cough 2013;9:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Opdyke DF, Brecher GA. Effect of normal and abnormal changes of intrathoracic pressure on effective right and left atrial pressures. Am J Physiol 1950;160:556–566. [DOI] [PubMed] [Google Scholar]
- 28. Guyton AC, Hall JE. Pulmonary ventilation In: Guyton AC, Hall JE, eds. Textbook of Medical Physiology, 11th ed Philadelphia, PA: Elsevier Saunders Co; 2006:471–482. [Google Scholar]
- 29. White RAS, Williams JM. Tracheal collapse in the dog – is there really a role for surgery? A survey of 100 cases. J Small Anim Pract 1994;35:191–196. [Google Scholar]
- 30. Maggiore AD. Tracheal and airway collapse in dogs. Vet Clin North Am Small Anim Pract 2014;44:117–127. [DOI] [PubMed] [Google Scholar]
- 31. Herrtage ME, White RAS. Management of tracheal collapse In: Bonagura JD, ed. Kirk's Current Veterinary Therapy, XIII. Philadelphia: WB Saunders; 2000:796–801. [Google Scholar]
- 32. Tangner CH, Hobson HP. A retrospective study of 20 surgically managed cases of collapsed trachea. Vet Surg 1982;11:146–149. [Google Scholar]
- 33. Radlinsky MG, Mason DE, Hodgson D. Transnasal laryngoscopy for the diagnosis of laryngeal paralysis in dogs. J Am Anim Hosp Assoc 2004;40:211–215. [DOI] [PubMed] [Google Scholar]
- 34. Radlinsky MG, Williams J, Frank PM, et al. Comparison of three clinical techniques for the diagnosis of laryngeal paralysis in dogs. Vet Surg 2009;38:434–438. [DOI] [PubMed] [Google Scholar]
- 35. Stadler K, Hartman S, Matheson J, O'Brien R. Computed tomography imaging of dogs with primary laryngeal or tracheal airway obstruction. Vet Radiol Ultrasound 2011;52:377–384. [DOI] [PubMed] [Google Scholar]
- 36. Cakirer B, Hans MG, Graham G, et al. The relationship between craniofacial morphology and obstructive sleep apnea in whites and in African‐Americans. Am J Respir Crit Care Med 2001;163:947–950. [DOI] [PubMed] [Google Scholar]
- 37. Ruehland WR, Rochford PD, O'Donoghue FJ, et al. The new AASM criteria for scoring hypopneas: Impact on the apnea hypopnea index. Sleep 2009;32:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Lund EM, Armstrong PJ, Kirk CA, Klausner JS. Prevalence and risk factors for obesity in adult dogs from private US veterinary practices. Int J Appl Res Vet Med 2006;4:177–186. [Google Scholar]
- 39. Poncet CM, Dupre GP, Freiche VG, et al. Prevalence of gastrointestinal tract lesions in 73 brachycephalic dogs with upper respiratory syndrome. J Small Anim Pract 2005;46:273–279. [DOI] [PubMed] [Google Scholar]


