Abstract
Bovine respiratory disease (BRD) is an economically important disease of cattle and continues to be an intensely studied topic. However, literature summarizing the time between pathogen exposure and clinical signs, shedding, and seroconversion is minimal. A structured literature review of the published literature was performed to determine cattle responses (time from pathogen exposure to clinical signs, shedding, and seroconversion) in challenge models using common BRD viral and bacterial pathogens. After review a descriptive analysis of published studies using common BRD pathogen challenge studies was performed. Inclusion criteria were single pathogen challenge studies with no treatment or vaccination evaluating outcomes of interest: clinical signs, shedding, and seroconversion. Pathogens of interest included: bovine viral diarrhea virus (BVDV), bovine herpesvirus type 1 (BHV‐1), parainfluenza‐3 virus, bovine respiratory syncytial virus, Mannheimia haemolytica, Mycoplasma bovis, Pastuerella multocida, and Histophilus somni. Thirty‐five studies and 64 trials were included for analysis. The median days to the resolution of clinical signs after BVDV challenge was 15 and shedding was not detected on day 12 postchallenge. Resolution of BHV‐1 shedding resolved on day 12 and clinical signs on day 12 postchallenge. Bovine respiratory syncytial virus ceased shedding on day 9 and median time to resolution of clinical signs was on day 12 postchallenge. M. haemolytica resolved clinical signs 8 days postchallenge. This literature review and descriptive analysis can serve as a resource to assist in designing challenge model studies and potentially aid in estimation of duration of clinical disease and shedding after natural pathogen exposure.
Keywords: Bovine respiratory disease, Bovine respiratory syncytial virus, Bovine viral diarrhea, Cattle, Infectious bovine rhinotracheitis, Mannheimia haemolytica, Mycoplasma bovis, Parainfluenza‐3, Virus shedding
Abbreviations
- BRD
bovine respiratory disease
- BRSV
bovine respiratory syncytial virus
- BVDV
bovine viral diarrhea virus
- CS
clinical signs
- BHV‐1
infectious bovine rhinotracheitis
- PI
persistent infection
- PI3
parainfluenza‐3
Bovine respiratory disease (BRD) continues to be an economically important disease of cattle with losses estimated as $23.60 per treated calf.1, 2 Bovine respiratory disease is a multi‐factorial disease involving infectious agents, compromised host immune system, and environmental factors ultimately resulting in bronchopneumonia. The viral pathogens associated with BRD include: bovine herpesvirus type 1 (BHV‐1), parainfluenza‐3virus (PI3), bovine viral diarrhea virus (BVDV), and bovine respiratory syncytial virus (BRSV). Bacterial pathogens associated with BRD include: Mannheimia haemolytica, Mycoplasma bovis, Pasteurella multocida, and Histophilus somni.
Viral pathogens are capable of causing primary infection that is generally associated with mild clinical signs (CS) of BRD.3, 4, 5, 6, 7, 8 An important role for BRD viral pathogens is causing immune suppression which increases susceptibility to secondary bacterial infections.6 Both BVDV and BHV‐1 are spread via aerosolization with BHV‐1 able to persist in a latent state in neural tissues and recrudesce during times of stress.5, 6, 7, 9 Parainfluenza‐3 virus and BRSV are considered to be minor contributors to BRD and are spread via aerosolization.
Similar to the viral pathogens, BRD bacterial pathogens are often present as co‐infections. Mannheimia haemolytica is considered the most common bacterial pathogen in beef cattle BRD and is a normal inhabitant of the nasopharynx, becoming opportunistic during stress or viral infection.5, 6, 10 Mycoplasma bovis can be a primary pathogen or co‐infection, with some studies showing synergism with M. haemolytica.11, 12, 13 Like M. haemolytica, Pastuerella multocida and Histophilus somni are also normal flora of the respiratory tract and become opportunistic colonizers of the lung after viral infection of the respiratory tract.6
As BRD is a syndrome, the specific pathogens involved in individual cases or outbreaks are often unknown. Management and control of BRD outbreaks is influenced by disease risk factors as well as transmission dynamics of the pathogens involved. Understanding the cattle response and infectious period associated with each pathogen can lead to a better understanding of how to mitigate negative impacts of BRD in populations. While there are numerous challenge studies using the common BRD pathogens, a resource summarizing the time from exposure to a viral or bacterial BRD pathogen to exhibition of CS, pathogen shedding, and seroconversion does not exist.
The objective for this study was to perform a structured literature review of the published literature and a descriptive analysis of cattle responses (the minimum time to onset of CS, time to peak outbreak, time to resolution of CS, minimum time to shedding, time to maximum shedding, time to resolution of shedding, time to seroconversion, and time to maximum seroconversion) to challenge with common viral and bacterial BRD pathogens.
Materials and Methods
A structured literature search was performed using PubMed, CAB, and Agricola databases to identify studies published in English that reported cattle BRD experimental challenge models for BHV‐1, BVDV, PI‐3 virus, BRSV, Histophilus, Pasteurella, Mycoplasma, and Mannheimia. The search strategies and keywords are listed in Table 1. Inclusion criteria for each study included: cattle confirmed pathogen‐free before challenge, single pathogen exposure model, utilization a negative control group, and challenge animals receiving no other treatment or vaccination for BVDV, BHV‐1, PI‐3 virus, BRSV, Mannheimia haemolytica, Mycoplasma bovis, Histophilus somni, and Pasteurella multocida. Outcomes of interest included: minimum time to onset of CS, time to peak outbreak, time to resolution of CS, minimum time to rectal temperature exceeding 40°C, time to peak rectal temperature, return of rectal temperature to less than 40°C, minimum time to shedding, time to maximum shedding, time to resolution of shedding, time to seroconversion, and time to maximum antibody titers. Only challenge models were included as time to onset of CS, shedding, and resolution times were important outcomes and challenge models provide data with specific known time of pathogen exposure. The titles and abstracts from the combined search outcomes were evaluated for inclusion and exclusion criteria. Of the pertinent abstracts, the full text was reviewed to determine inclusion or exclusion from the structured literature review based on study criteria (attached Appendix S1 lists the references considered for inclusion). A hand search was performed of included studies to ensure no additional valid studies were omitted from the search results. A study could have multiple trials within the manuscript with multiple treatment group allocation. Therefore, a published manuscript was considered a study, whereas each individual challenge pathogen was considered a trial.
Table 1.
Structured literature search results by database. English only results are listed
| Search terms | Pubmed | CAB | Agricola |
|---|---|---|---|
| Baseline searches for individual search terms | |||
| Bovine OR cattle OR calves | 369,041 | 611,304 | 171,371 |
| Respirator* OR BRD* OR shipping fever OR pneumonia | 570,214 | 135,124 | 23, 539 |
| Mannheimia | 1,024 | 855 | 853 |
| Pasteurella | 7,886 | 12,515 | 2,932 |
| Histophilus OR Haemophilus | 34,820 | 8,305 | 1,377 |
| Mycoplasma | 21,499 | 18,379 | 5,967 |
| Infectious Bovine Rhinotracheitis* OR IBR* OR BHV‐1* | 14,375 | 7,758 | 4,329 |
| Parainfluenza‐3* OR PI‐3* | 7,300 | 2,666 | 1,674 |
| Bovine Respiratory Syncytial Virus OR BRSV* | 841 | 1,502 | 411 |
| Bovine Viral Diarrh* OR BVDV* | 3,743 | 6,953 | 1,847 |
| Searches for articles on individual pathogens for analyses | |||
| (Bovine OR cattle OR calves)+(Respirator* OR BRD*)+Mannheimia | 534 | 855 | 269 |
| (Bovine OR cattle OR calves)+(Respirator* OR BRD*)+ Pasteurella | 1,191 | 1, 411 | 311 |
| (Bovine OR cattle OR calves)+(Respirator* OR BRD*)+ (Histophilus OR Haemophilus) | 1,107 | 305 | 69 |
| (Bovine OR cattle OR calves)+(Respirator* OR BRD*)+Mycoplasma | 474 | 862 | 182 |
| (Bovine OR cattle OR calves)+(Respirator* OR BRD*)+(Infectious Bovine Rhinotracheitis* OR IBR* OR BHV‐1*) | 428 | 1, 202 | 77 |
| (Bovine OR cattle OR calves)+(Respirator* OR BRD*)+(Parainfluenza‐3* OR PI‐3*) | 235 | 662 | 88 |
| (Bovine OR cattle OR calves)+(Respirator* OR BRD*)+(Bovine Respiratory Syncytial Virus OR BRSV*) | 841 | 1, 488 | 429 |
| (Bovine OR cattle OR calves)+(Respirator* OR BRD*)+(Bovine Viral Diarrh* OR BVD*) | 327 | 865 | 153 |
| Total number of articles (from articles on individual pathogens; some articles are present for multiple pathogens) | 5,137 | 7,069 | 1,575 |
Other data collected included: study length, challenge inoculum route, number of calves in the trial, frequency of sample collection, and blinding status. Bovine viral diarrhea virus type 1 and 2 were analyzed together and not separated into 2 separate categories. Trial day 0 was defined as the day the pathogen challenge was administered for all included studies. Trials were included for analysis regardless of completion of all outcomes of interest (eg, data only present for CS, or CS, fever, or shedding had not resolved before completion of the trial were still included for data analysis). Seroconversion data only included trials utilizing serum neutralization to test for antibody response. For the viral pathogens, shedding was determined by trials utilizing virus isolation from nasal swabs. For the bacterial pathogens, trials that utilized PCR for determination of shedding from nasal swabs were included in the structured literature review.
Data points were collected for each outcome of interest from each trial. For CS: minimum time until CS was recorded as the day postchallenge calves began showing CS (eg, at least 1 animal) for each trial, time to peak outbreak recorded as the day the highest number of cattle were affected with CS for each trial, and the resolution of CS recorded as the day all cattle were asymptomatic for each trial (with the exception of outliers as reported and determined by the published trial when present). For rectal temperatures: minimum time until rectal temperature exceeded 40°C was recorded as the day postchallenge the mean rectal temperature of challenge calves was equal to or greater than 40°C for each trial, time to peak rectal temperature was the day the calves mean rectal temperature was the highest, time to resolution of rectal temperature less than 40°C was recorded as the day the calves mean rectal temperature was less than 40°C. For pathogen shedding: minimum time to shedding was recorded as the day calves began to shed the pathogen postchallenge (eg, at least 1 animal) for each trial, time to maximum shedding was recorded as the day the most calves with the highest titers obtained, time to resolution of shedding was recorded as the day all calves ceased pathogen shedding for each trial. Time to seroconversion was recorded for each trial as the day at least 1 calf has seroconverted, and time to maximum antibody titers was recorded as the trial day the challenge calves had the highest titer. A weighted mean accounting for the number of calves present in each study was utilized and descriptive statistics were performed to analyze the data. Box and whisker plots were produced summarizing the data points from each trial for each pathogen.
Results
After evaluation of article titles, abstracts, and then complete review of subsequent manuscripts, a total of 35 studies and 64 trials were included in the descriptive analysis. Table 2 shows the number of papers included for each pathogen during each stage of evaluation. No additional study was included after a hand search of references cited in included articles. All included studies were in the PubMed and CAB databases. No single trial contained all the desired areas of interest for structured literature review. Therefore, Table 3 demonstrates the number of trials that had data present and were analyzed for each area of interest. Bacterial shedding data were excluded from the analysis because of insufficient data present (only present for 1 study for M. bovis).
Table 2.
Number of studies present for each pathogen during each stage of evaluation
| BVDV | IBR | PI‐3 | BRSV | M. haemolytica | M. bovis | P. multocida | H. somni | |
|---|---|---|---|---|---|---|---|---|
| Number of relevant abstracts | 17 | 25 | 7 | 44 | 17 | 20 | 19 | 9 |
| Number papers read for analysis inclusion | 8 | 9 | 5 | 31 | 13 | 10 | 12 | 5 |
| Number of papers (studies) included in structured literature review | 8 | 7 | 3 | 15 | 5 | 4 | 1 | 0 |
| Number of trials included in analysis | 12 | 9 | 3 | 22 | 5 | 8 | 4 (all from same study) | 0 |
Table 3.
Number of trials that reported data for each outcome of interest included for structured literature review and descriptive analysis
| Minimum | Peak | Resolve | |
|---|---|---|---|
| BVDV | |||
| Clinical Signs | 8 | 7 | 2 |
| Antibody titers | 2 | 1 | NA |
| Rectal Temperature | 10 | 8 | 8 |
| Virus Isolation | 10 | 9 | 2 |
| IBR | |||
| Clinical Signs | 9 | 5 | 8 |
| Antibody titers | 4 | 3 | NA |
| Rectal Temperature | 8 | 6 | 8 |
| Virus Isolation | 9 | 9 | 6 |
| PI‐3 | |||
| Clinical Signs | 3 | 0 | 0 |
| Virus Isolation | 3 | 3 | 3 |
| BRSV | |||
| Clinical Signs | 17 | 15 | 7 |
| Serum Neutralization | 8 | 4 | NA |
| Temperature | 8 | 6 | 4 |
| Virus Isolation | 17 | 14 | 12 |
| Mannheimia haemolytica | |||
| Clinical Signs | 4 | 4 | 1 |
| Rectal Temperature | 4 | 4 | 4 |
| Mycoplasma bovis | |||
| Clinical Signs | 3 | 3 | NA |
| Serum Neutralization | 3 | 3 | NA |
| Temperature | 5 | 2 | 5 |
| Pasteurella multocida | |||
| Clinical Signs | 4 | 4 | 4 |
| Temperature | 2 | 2 | 2 |
Bovine Viral Diarrhea Virus
We identified 12 BVDV trials from 8 studies for inclusion in the analysis.3, 14, 15, 16, 17, 18, 19 Table S1 summarizes the studies that were reviewed and analyzed. Blinding was reported for 9 of the trials. The mean trial duration was 15.5 days (range 9–27 days) with a mean of 10.2 calves (range 4–16 calves) included in each trial. Type 1 and type 2 BVDV were included in the same category for analysis. Type 1 BVDV was used as the challenge pathogen for 6 trials. Two trials utilized BVDV type 2 and 4 trials did not specify the BVDV type. Eight trials challenged the calves intranasal with the BVDV challenge pathogen. Three trials challenged via aerosolized administration and 1 trial used a combination of intranasal and aerosolized for challenge exposure.
The median for the minimum number of days until BVDV shedding was 2 days (range 1–5 days) for the 8 trials that reported time until CS started postchallenge (Fig 1). The median day for peak of BVDV shedding occurred at 7 days (range 4–9 days) postchallenge with resolution at 12 days (range 11–12 days) postchallenge. Median day that rectal temperatures began exceeding 40°C was 4 days (range 2–8 days) postchallenge. Rectal temperature peaks occurred with a median at 7 days (range 7–8 days) postchallenge with median time to resolution occurring 10 days (range 7–11 days) after challenge. Median time to onset of CS was 2 days (range 1–6 days) postchallenge. Median time to peak outbreak occurred 8 days (range 5–12 days) postchallenge with median days to resolution being 15 days (range 6–23 days). Median time to seroconversion occurred 17 days (range 15–19 days) postchallenge with peak seroconversion occurring at 27 days postBVDV challenge in 1 study.
Figure 1.
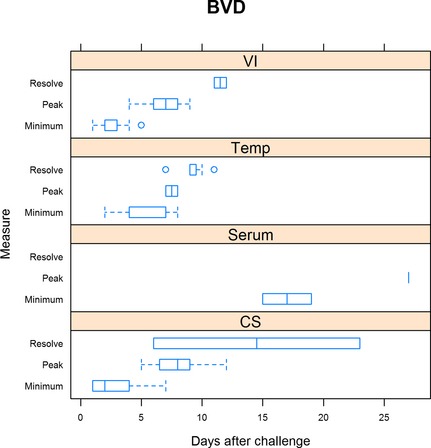
Summary of bovine viral diarrhea trials. For virus isolation, minimum is defined as the day when shedding was first detected, peak is when shedding was at the maximum, and resolution when shedding ceased. For rectal temperature (Temp), minimum is defined as the day when rectal temperature first exceeded 40°C, peak when rectal temperature was highest, and resolution defined as when rectal temperature was less than 40°C. For serum neutralization (Serum), minimum is defined as the day when seroconversion was first detected and peak when serum neutralization was highest. For clinical signs, minimum is defined as the day when clinical signs were first detected, peak being when clinical signs were the most severe, and resolution when clinical signs resumed normal limits.
Bovine Herpesvirus Type 1
Nine trials and 7 studies were included for BHV‐1 analysis.7, 9, 14, 17, 20, 21, 22 Of the 9 trials, 3 reported using blinding with the other 6 trials either not being blinded or blinding status was not reported. The mean study duration was 33.1 days (range 14–55 days) with a mean of 6.2 study calves (range 3–14) for each trial. All trials utilized BHV‐1 for the challenge model. Five trials utilized intranasal administration for the BHV‐1 challenge and 4 trails challenged with aerosolization. Table S2 summarizes the studies that were reviewed and analyzed.
The median time until BHV‐1 began shedding was 2 days (range 1–3 days) (Fig 2). Median time for peak shedding occurred on day 4 (range 2–6 days) postchallenge for BHV‐1. The median time until shedding of BHV‐1 ceased was 14 days, but spanned a time frame of as early as 7 days and as long as 17 days. Median time to rectal temperatures exceeding 40°C occurred 2 days (range 1–10) postchallenge with median time to maximal rectal temperature on day 4 (range 3–10 days) postchallenge. Median time to rectal temperatures returning to less than 40°C occurred on day 8 (range 3–10 days) postchallenge. Median time to BHV‐1 seroconversion occurred on day 17.5 (range 7–28 days) postchallenge with median time to peak antibody response on day 40 (range 28–56 days). The median time until CS began on day 2 after BHV‐1 exposure with a range extending from 2 to 5 days after challenge. The median time to peak outbreak occurred on day 7 (range 2–8 days) with median time to resolution of CS on day 14 (range 10–15 days).
Figure 2.
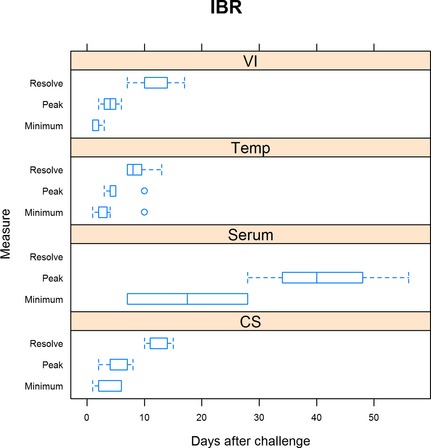
Summary of IBR trials. For virus isolation (VI), minimum is defined as the day when shedding was first detected, peak is when shedding was at the maximum, and resolution when shedding ceased. For rectal temperature (Temp), minimum is defined as the day when rectal temperature first exceeded 40°C, peak when rectal temperature was highest, and resolution defined as when rectal temperature was less than 40°C. For serum neutralization (Serum), minimum is defined as the day when seroconversion was first detected and peak when serum neutralization was highest. For clinical signs (CS), minimum is defined as the day when clinical signs were first detected, peak being when clinical signs were the most severe, and resolution when clinical signs resumed normal limits.
Parainfluenza‐3
Three trials from 3 studies investigating PI‐3 virus were included for analysis.3, 14, 17 Table S3 summarizes the studies that were reviewed and analyzed. The study length was 14 days for all trials with all 3 being blinded. The mean number of calves included for each trial was 14.6 (range 13–16 calves). One trial had the PI‐3 virus challenge administered via aerosolization and the other 2 trials had both intranasal and intratracheal administration.
The median time shedding of PI‐3 virus began 1 day (range 1–2 days) after challenge (Fig 3). Median time to peak nasal shedding occurred 4 days (range 3–5 days) after challenge and median time until shedding resolved was day 10 (range 10–13 days). Literature review data for CS onset were only available for the minimum (when CS first appeared after challenge) which the median time occurred on day 2 (range 2–3 days) postchallenge. Only 1 trial had resolution of CS by the end of the trial (day 14). The other 2 trials did not have resolution of CS by the end of the trial and the study length was 14 days after PI‐3 virus challenge for both trials. Rectal temperature and serum neutralization data were only available for 1 trial; therefore, these outcomes were excluded for the structured literature review for PI‐3 virus.
Figure 3.
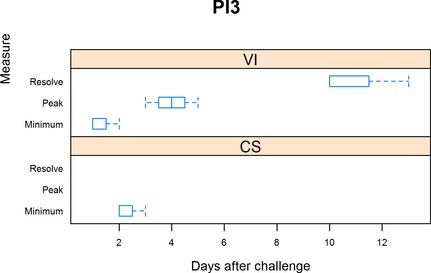
Summary of PI‐3 trials. For virus isolation (VI), minimum is defined as the day when shedding was first detected, peak is when shedding was at the maximum, and resolution when shedding ceased. For clinical signs (CS), minimum is defined as the day when clinical signs were first detected, peak being when clinical signs were the most severe, and resolution when clinical signs resumed normal limits.
Bovine Respiratory Syncytial Virus
Investigations of BRSV for this review included 22 trials with 15 studies.3, 4, 14, 17, 23, 24, 25, 26, 27, 28, 29, 30, 31, 32, 33 Table S4 summarizes the studies that were reviewed and analyzed. The mean study length was 15 days (range 6–42 days) with 12 trials being blinded and 10 trials either not blinded or blinding was not reported. The mean number of calves in each trial was 8 (range 4–15). Nine trials challenged with BRSV via aerosolization, 7 with intranasal challenge, and 6 with a combined intranasal and intratracheal method.
Bovine respiratory syncytial virus median time to CS began on day 3 (range 1–6 days) postinoculation with time to peak median outbreak occurring on day 6 (range 2–11 days). Median time to resolution did not occur until day 12 (range 7–17 days) postinoculation. Median time rectal temperatures exceeded 40°C was day 5 (range 1–7 days) and median time to maximum rectal temperature occurred on day 6 (range 5–8 days). The median time rectal temperatures returned to less than 40°C was trial day 8 (range 7–10 days) postchallenge. Median time to seroconverison for BRSV was day 9 (range 5–21 days) postchallenge using serum neutralization. Time to maximum median antibody response occurred on postchallenge day 23 (range 9–32 days). Median time to BRSV shedding began 3 (range 1–5 days) days after challenge with median time to peak shedding on day 5 (range 3–8 days) and median time to resolution on day 9 (range 7–14 days). Figure 4 summarizes this data.
Figure 4.
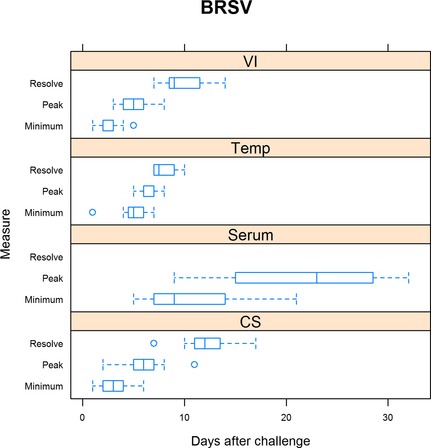
Summary of BRSV trials. For virus isolation (VI), minimum is defined as the day when shedding was first detected, peak is when shedding was at the maximum, and resolution when shedding ceased. For rectal temperature (Temp), minimum is defined as the day when rectal temperature first exceeded 40°C, peak when rectal temperature was highest, and resolution defined as when rectal temperature was less than 40°C. For serum neutralization (Serum), minimum is defined as the day when seroconversion was first detected and peak when serum neutralization was highest. For clinical signs (CS), minimum is defined as the day when clinical signs were first detected, peak being when clinical signs were the most severe, and resolution when clinical signs resumed normal limits.
Mannheimia haemolytica
Five trials from 5 studies investigating Mannheimia haemolytica met inclusion criteria for this structured literature review with the mean trial length being 23 days (3–84 days).34, 35, 36, 37, 38 Table S5 summarizes the studies that were reviewed and analyzed. Of the trials, 4 used Mannheima haemolytica type A1 for challenge induction. One trial used Mannheimia haemolytica, the type was not reported. Two of the trials were blinded, 1 trial was not blinded and 1 trial did not report if the study was blinded. Four trials had the Mannheimia haemolytica challenge administered endoscopically and 1 trial administered the challenge intratracheally. The mean number of calves in each trial was 10.4 (range 3–19 calves). Area of interest data were only present for CS and rectal temperatures. Only 1 trial reported seroconversion.
All trials reported the onset of CS occurred 1 day after challenge inoculation (Fig 5). Median time to peak CS occurred 1 day (range 1–2 days) after challenge. All trials reported resolution 8 days after inoculation. Time until rectal temperatures exceeded 40°C was reported as 1 day after challenge by all trials included for the structured literature review. Peak rectal temperatures also occurred 1 day after challenge reported by all trial with the median time until rectal temperatures returned to less than 40°C on day 2 (2–6) postchallenge.
Figure 5.
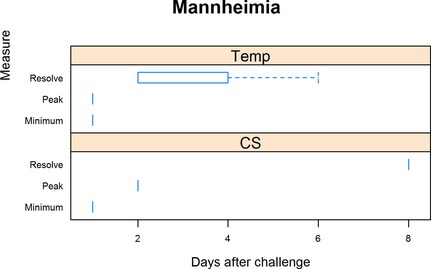
Summary of Mannheimia haemolytica trials. For rectal temperature (Temp), minimum is defined as the day when rectal temperature first exceeded 40°C, peak when rectal temperature was highest, and resolution defined as when rectal temperature was less than 40°C. For clinical signs (CS), minimum is defined as the day when clinical signs were first detected, peak being when clinical signs were the most severe, and resolution when clinical signs resumed normal limits.
Mycoplasma bovis
Investigations of Mycoplasma bovis for this review included 8 trials and 4 studies.12, 39, 40, 41 Table S6 summarizes the studies that were reviewed and analyzed. The mean number of calves included in each trial was 15.6 (range 8–20 calves) with a mean study length of 23.8 days (range 14–28 days). Two studies were blinded, 1 study was not blinded, and 5 did not state blinding status. Seven trials performed intratracheal inoculation and 1 trial challenged intranasally.
The median time to onset of CS was 1 day (range 1–4 days) postchallenge with median time to peak CS occurring on day 2 (range 2–6 days). All trials either still had ongoing CS at the end of the trial or the time to resolution of CS was not reported (Fig 6). Median time to rectal temperatures exceeding 40°C occurred 1 day (range 1–8 days) after challenge with median time to peak CS on 4.5 days (range 1–8 days). Median time to rectal temperature resolving to less than 40°C occurred on day 8 (range 5–13 days). Median time to seroconversion was 21 days (range 14–28 days) postchallenge and median time to peak antibody titers on day 28 (range 21–28 days).
Figure 6.
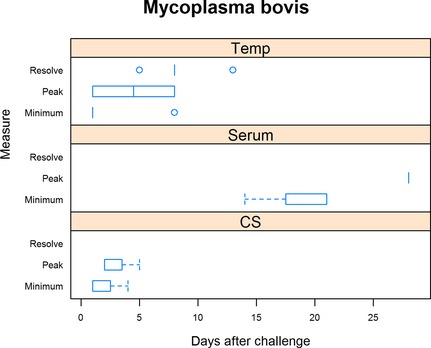
Summary of Mycoplasma bovis trials. For rectal temperature (Temp), minimum is defined as the day when rectal temperature first exceeded 40°C, peak when rectal temperature was highest, and resolution defined as when rectal temperature was less than 40°C. For serum neutralization (Serum), minimum is defined as the day when seroconversion was first detected and peak when serum neutralization was highest. For clinical signs (CS), minimum is defined as the day when clinical signs were first detected, peak being when clinical signs were the most severe, and resolution when clinical signs resumed normal limits.
Pasteurella multocida
One study reporting 4 trials investigating Pasteurella multocida met inclusion criteria for the structured literature review.42 Table S7 summarizes the study that was reviewed and analyzed. Each trial had 4 calves and all calves were challenged intratracheally. Blinding was not reported for the study. The study length was 4 days for all trials.
Onset of CS for Pasteurella multocida occurred 1 day after challenge for all reported trials (Fig 7). Peak CS also occurred 1 day after challenge for all reported trials with median resolution on day 2 (range 2–4 days). Rectal temperatures also exceeded 40°C on day 1 postchallenge for all trials. Maximum rectal temperatures also occurred on day 1 postchallenge for all trials with resolution on day 2 postchallenge for all trials.
Figure 7.
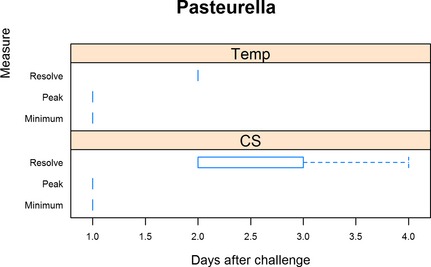
Summary of Pasteurella multocida trials. For rectal temperature (Temp), minimum is defined as the day when rectal temperature first exceeded 40°C, peak when rectal temperature was highest, and resolution defined as when rectal temperature was less than 40°C. For clinical signs (CS), minimum is defined as the day when clinical signs were first detected, peak being when clinical signs were the most severe, and resolution when clinical signs resumed normal limits.
Histophilus somni was excluded from the structured literature review because no trials were identified that coincided with met our inclusion criteria.
Discussion
This structured literature review serves as a resource and summary for the common BRD pathogens with regard to expected times for CS, high rectal temperature, shedding, and seroconversion after pathogen exposure. For the viruses, the relationship between resolution of CS and shedding is interesting. For BVDV, median time for CS persisted 3 days after the resolution of shedding on day 15. However, 1 trial did not have resolution of CS until 23 days postchallenge. Unfortunately, this trial did not report virus isolation data. Both median time to BHV‐1 resolution of shedding and CS occurred on day 14 after challenge. These results correlate with other summaries reporting BHV‐1 shedding resolution between 10 and 17 days and peak CS occurring between 4 and 6 days.43 Our study found peak CS at 5 days postinoculation. For BRSV, median time CS resolved 3 days after shedding ceased on day 12. Sacco summarized that viral detection is expected until 7–10 days after infection with viral detection beginning at day 2–3 which correlates with our results of resolution at 9 days and shedding beginning at day 3.44 Besides the outlier for BVDV, CS for the viral pathogens (BVDV, BHV‐1, BRSV) resolved near the time of shedding cessation or up to 3 days after shedding ceased. This information could be vital to know in regard to instituting proper quarantine periods in association with onset of BRD CS. Unfortunately, investigations of PI‐3 virus, M. haemolytica, M. bovis, and P. multocida did not report complete data sets to make comparisons between shedding and CS.
Generally, most induced infections whether viral or bacterial in origin reported resolution of pyrexia before all CS resolve. For BVDV, high rectal temperature resolution occurred 6 days before the cessation of CS on day 15. Median time BHV‐1 and BRSV resolved high rectal temperatures was 5 and 4 days before the resolution of CS. Median time M. haemolytica resolved high rectal temperature was day 2 which was 6 days before resolution of CS. M. bovis had median time to resolution of high rectal temperatures on day 8 postchallenge. However, we have no data regarding time to resolution of CS since all the trials included concluded before resolution of CS. This could be a result of short trial durations or it could be in conjunction with the known long, often chronic disease course associated with M. bovis. Parainfluenza‐3 did not have data for time to resolution of CS and rectal temperatures. P. multocida was the only outlier of the common BRD pathogens with high rectal temperatures and CS resolving around the same time (high rectal temperature resolution on day 2, clinical sign resolution on day 2). Knowledge of high rectal temperature resolution with regard to time to resolution of CS could be an important disease progression indicator for producers, veterinarians, and researchers. For the most BRD pathogens, we can expect clinical sign resolution 4–6 days after rectal temperatures have returned to less than 40°C.
Seroconversion is defined as the time at which antibodies are first detected in the serum. Median time to seroconversion occurred between 9 and 21 days for the pathogens in this study, with median time to seroconversion occurring on day 17 for BVDV, day 17.5 for BHV‐1, day 9 for BRSV, and day 21 for M. bovis. Data were not available for PI‐3 virus and M. haemolytica. Median time to peak seroconversion occurred on day 27 for BVDV, day 40 for IBR, day 23 for BRSV, and day 28 for M. bovis. For M. bovis, this partially concurs with 1 study evaluating response of naïve calves being exposed to a herd endemically infected with M. bovis with antibodies first detected by day 29–35; however, peak antibody response did not occur until day 60 postintroduction.45 The time to seroconversion or peak seroconversion could have been affected simply by the animals' ability to respond to the antigen and produce appropriate antibody or simply confounded by the sampling time selected by the researchers for each trial. For BVDV, BHV‐1, BRSV, and M. bovis, seroconversion can be expected to occur in a range of 9 days to 21 with peaks between 23 days to 40.
There are certainly limitations associated with this structured literature review and descriptive analysis. The biggest limitation would be the low number of trials for each pathogen. Unfortunately, the limited number of trials and the heterogeneity of the dataset limited any substantial statistics beyond descriptive. Thus, preventing any interpretation sample size has on outcome variables. For example, a larger study group might have an increase number of days to peak CS and resolution of CS over a smaller group. However, sample size may have no effect on time peak CS and resolution of CS since only challenge models were included. The answer to this question is beyond the ability of this manuscript. Ideally, the structured literature review would have been limited to studies that were blinded. Nonblinded studies were included because the number of trials included would have been severely limited. Additionally, nonblinding is not as likely to affect objective areas of interest such as: rectal temperature, seroconversion, and viral shedding. Another limitation is the lack of shedding data for the bacterial pathogens. However, interpreting shedding data (culture or PCR) for bacterial pathogens is difficult as bacterial pathogens are often normal flora of the nasopharynx of cattle. Ideally, a researcher would collect a deep nasopharyngeal swab for analysis before pathogen challenge ensuring the individual is negative for the pathogen challenge strain and thus correlating bacterial shedding postchallenge matches the appropriate strain. With this review, only 1 study looked at bacterial shedding through utilization of PCR without determining the resolution of shedding. Other factors to consider are the effects of pathogen strain, dose, and route of inoculation of the disease severity and disease course. Unfortunately, the limited dataset prevents utilization of any statistical analysis to determine if the study ranges are because of chance or trial variables (dose, strain, route of inoculation). One must be careful in extrapolating these data to clinical scenarios, as challenge studies may not represent a valid model for natural disease. However, the information in this structured literature review provides a resource when designing clinical trials for the specific pathogens of interest.
This structured literature review serves as a valuable summary and resource for veterinary researchers, veterinarians, and producers interested in the duration of time between exposure to common BRD pathogens until expected time to resolution of CS, high rectal temperature, shedding, and seroconversion. Important conclusions are that CS resolved near the time of shedding cessation or up to 3 days after shedding ceased for BVDV, BHV‐1, and BRSV; and high rectal temperatures resolved approximately 4–6 days before resolution of CS for BVDV, BHV‐1, BRSV, and M. haemolytica.
Supporting information
Appendix S1. Articles reviewed for analysis inclusion.
Table S1. References reviewed for BVDV challenge studies. NR‐not reported
Table S2. References reviewed for BHV‐1 challenge studies. NR‐not reported
Table S3. References reviewed for PI‐3 challenge studies. NR‐not reported
Table S4. References reviewed for BRSV challenge studies. NR‐not reported
Table S5. References reviewed for Mannheimia haemolytica challenge studies, NR‐not reported
Table S6. References reviewed for Mycoplasma bovis challenge studies. NR‐not reported
Table S7. References reviewed for Pasteurella multocida challenge studies. NR‐not reported
Acknowledgments
The authors thank Miles Theurer for technical assistance.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This manuscript represents a portion of a thesis by the first author to the Kansas State University Department of Clinical Sciences as a partial fulfillment of the requirements for a Master's degree.
References
- 1. USDA . Part IV: Health and Health Management on U.S. Feedlots With a Capacity of 1,000 or More Head. In: APHIS , ed. Fort Collins, CO: National Animal Health Monitoring System; 2011. [Google Scholar]
- 2. Broderson B, Kelling CL. Effect of concurrent experimentally induced bovine respiratory syncytial virus and bovine viral diarrhea virus infection on respiratory tract and enteric disease in calves. Am J Vet Res 1998;59:1423–1430. [PubMed] [Google Scholar]
- 3. Salt JS, Thevasagayam SJ, Wiseman A, et al. Efficacy of a quadrivalent vaccine against respiratory diseases caused by BHV‐1, PI3V, BVDV and BRSV in experimentally infected calves. Vet J 2007;174:616–626. [DOI] [PubMed] [Google Scholar]
- 4. Van der Poel W, Schrijver R, Middel W, et al. Experimental reproduction of respiratory disease in calves with non‐cell‐culture passaged bovine respiratory syncytial virus. Vet Quart 1996;18:81–86. [DOI] [PubMed] [Google Scholar]
- 5. Smith RA, Stokka GL, Radostits OM, Griffin DD. Health and production management in beef feedlots In: Radostits O, ed. Herd Health: Food Animal Production Medicine, 3rd ed Philadelphia: W.B. Saunders Company; 2001:581–633. [Google Scholar]
- 6. Woolums AR. The bronchopneumonias (respiratory disease complex of cattle, sheep, and goats) In: Smith BL, ed. Large Animal Internal Medicine, 5th ed St. Louis, MO: Mosby Elsevier; 2015:584–603. [Google Scholar]
- 7. Gilliam SE, Thackray AM, Brown GA, et al. The pathogenesis of wild type and drug resistant mutant strains of bovine herpesvirus‐1 (BHV‐1) in the natural host. Arch Virol 1993;128:43–54. [DOI] [PubMed] [Google Scholar]
- 8. Martin SW, Bateman KG, Shewen PE, et al. The frequency, distribution and effects of antibodies, to seven putative respiratory pathogens, on respiratory disease and weight gain in feedlot calves in Ontario. Can J Vet Res 1989;53:355–362. [PMC free article] [PubMed] [Google Scholar]
- 9. Meyer G, Lemaire M, Ros C, et al. Comparative pathogenesis of acute and latent infections of calves with bovine herpesvirus types 1 and 5. Arch Virol 2001;146:633–652. [DOI] [PubMed] [Google Scholar]
- 10. Purdy CW, Raleigh RH, Collins JK, et al. Serotyping and enzyme characterization of Pasteurella haemolytica and Pasteurella multocida isolates recovered from pneumonic lungs of stressed feeder calves. Curr Microbiol 1997;34:244–249. [DOI] [PubMed] [Google Scholar]
- 11. Nicholas RA, Ayling RD. Mycoplasma bovis: Disease, diagnosis, and control. Res Vet Sci 2003;4:105–112. [DOI] [PubMed] [Google Scholar]
- 12. Dudek K, Bednarek D, Ayling R, et al. Immunomodulatory effect of Mycoplasma bovis in experimentally infected calves. Bull Vet Inst Pulawy 2013;57:499–506. [Google Scholar]
- 13. Houghton SB, Gourlay RN. Synergism between Mycoplasma bovis and Pasteurella haemolytica in calf pneumonia. Vet Rec 1983;113:41–42. [DOI] [PubMed] [Google Scholar]
- 14. Xue W, Ellis J, Mattick D, et al. Immunogenicity of a modified‐live virus vaccine against bovine viral diarrhea virus types 1 and 2, infectious bovine rhinotracheitis virus, bovine parainfluenza‐3 virus, and bovine respiratory syncytial virus when administered intranasally in young calves. Vaccine 2010;28:3784–3792. [DOI] [PubMed] [Google Scholar]
- 15. Xue W, Mattick D, Smith L, et al. Vaccination with a modified‐live bovine viral diarrhea virus (BVDV) type 1a vaccine completely protected calves against challenge with BVDV type 1b strains. Vaccine 2011;29:70–76. [DOI] [PubMed] [Google Scholar]
- 16. Kelling CL, Hunsaker BD, Steffen DJ, et al. Characterization of protection against systemic infection and disease from experimental bovine viral diarrhea virus type 2 infection by use of a modified‐live noncytopathic type 1 vaccine in calves. Am J Vet Res 2007;68:788–796. [DOI] [PubMed] [Google Scholar]
- 17. Peters AR, Thevasagayam SJ, Wiseman A, et al. Duration of immunity of a quadrivalent vaccine against respiratory diseases caused by BHV‐1, PI3V, BVDV, and BRSV in experimentally infected calves. Prev Vet Med 2004;66:63–77. [DOI] [PubMed] [Google Scholar]
- 18. Galav V, Mishra N, Dubey R, et al. Pathogenicity of an Indian isolate of bovine viral diarrhea virus 1b in experimentally infected calves. Res Vet Sci 2007;83:364–368. [DOI] [PubMed] [Google Scholar]
- 19. Ganheim C, Hulten C, Carlsson U, et al. The acute phase response in calves experimentally infected with bovine viral diarrhoea virus and/or Mannheimia haemolytica . J Vet Med B Infect Dis Vet Public Health 2003;50:183–190. [DOI] [PubMed] [Google Scholar]
- 20. Castrucci G, Frigeri F, Osburn BI, et al. Further investigations on the efficacy of a non‐specific defence inducer evaluated in calves exposed to infectious bovine rhinotracheitis virus. Comp Immunol Microbiol Infect Dis 1998;21:155–163. [DOI] [PubMed] [Google Scholar]
- 21. Castrucci G, Ferrari M, Osburn BI, et al. The use of a non‐specific defence mechanism inducer in calves exposed to bovine herpesvirus‐1 infection: Preliminary trials. Comp Immunol Microbiol Infect Dis 1995;18:85–91. [DOI] [PubMed] [Google Scholar]
- 22. Castrucci G, Ferrari M, Osburn BI, et al. A non‐specific defence inducer in preventing clinical signs of infectious bovine rhinotracheitis in calves. Comp Immunol Microbiol Infect Dis 1996;19:163–169. [DOI] [PubMed] [Google Scholar]
- 23. Otto P, Elschner M, Reinhold P, et al. A model for respiratory syncytial virus (RSV) infection based on experimental aerosol exposure with bovine RSV in calves. Comp Immunol Microbiol Infect Dis 1996;19:85–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Elvander M, Baule C, Persson M, et al. An experimental study of a concurrent primary infection with bovine respiratory syncytial virus (BRSV) and bovine viral diarrhoea virus (BVDV) in calves. Acta Vet Scand 1998;39:251–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gershwin LJ, Gunther RA, Anderson ML, et al. Bovine respiratory syncytial virus‐specific IgE is associated with interleukin‐2 and ‐4, and interferon‐gamma expression in pulmonary lymph of experimentally infected calves. Am J Vet Res 2000;61:291–298. [DOI] [PubMed] [Google Scholar]
- 26. LeBlanc PH, Baker JC, Gray PR, et al. Effects of bovine respiratory syncytial virus on airway function in neonatal calves. Am J Vet Res 1991;52:1401–1406. [PubMed] [Google Scholar]
- 27. Ellis J, West K, Konoby C, et al. Efficacy of an inactivated respiratory syncytial virus vaccine in calves. J Am Vet Med Assoc 2001;218:1973–1980. [DOI] [PubMed] [Google Scholar]
- 28. Vangeel I, Antonis A, Fluess M, et al. Efficacy of a modified live intranasal bovine respiratory syncytical virus vaccine in a 3‐week old calves experimentally challenged with BRSV. Vet J 2007;174:627–635. [DOI] [PubMed] [Google Scholar]
- 29. Woolums AR, Anderson ML, Gunther RA, et al. Evaluation of severe disease induced by aerosol inoculation of calves with bovine respiratory syncytial virus. Am J Vet Res 1999;60:473–480. [PubMed] [Google Scholar]
- 30. West K, Petrie L, Konoby C, et al. The efficacy of modified‐live bovine respiratory syncytial virus vaccines in experimentally infected calves. Vaccine 2000;18:907–919. [DOI] [PubMed] [Google Scholar]
- 31. Ellis J, Gow S, West K, et al. Response of calves to challenge exposure with virulent bovine respiratory syncytial virus following intranasal administration of vaccines formulated for parenteral administration. J Am Vet Med Assoc 2007;230:233–243. [DOI] [PubMed] [Google Scholar]
- 32. Mawhinney IC, Burrows MR. Protection against bovine respiratory syncytial virus challenge following a single dose of vaccine in young calves with maternal antibody. Vet Rec 2005;156:139–143. [DOI] [PubMed] [Google Scholar]
- 33. Ciszewski DK, Baker JC, Slocombe RF, et al. Experimental reproduction of respiratory tract disease with bovine respiratory syncytial virus. Vet Microbiol 1991;28:39–60. [DOI] [PubMed] [Google Scholar]
- 34. Amrine DE, White BJ, Larson RL, et al. Pulmonary lesions and clinical disease response to Mannheimia haemolytica challenge 10 days following administration of tildipirosin or tulathromycin. J Anim Sci 2014;92:311–319. [DOI] [PubMed] [Google Scholar]
- 35. Fajt VR, Apley MD, Roth JA, et al. The effects of danofloxacin and tilmicosin on neutrophil function and lung consolidation in beef heifer calves with induced Pasteurella (Mannheimia) haemolytica pneumonia. J Vet Pharmacol Ther 2003;26:173–179. [DOI] [PubMed] [Google Scholar]
- 36. Olchowy TW, TerHune TN, Herrick RL. Efficacy of difloxacin in calves experimentally infected with Mannheimia haemolytica . Am J Vet Res 2000;61:710–713. [DOI] [PubMed] [Google Scholar]
- 37. Theurer ME, Anderson DE, White BJ, et al. Effect of Mannheimia haemolytica pneumonia on behavior and physiologic responses of calves during high ambient environmental temperatures. J Anim Sci 2013;91:3917–3929. [DOI] [PubMed] [Google Scholar]
- 38. Hewson J, Viel L, Caswell JL, et al. Impact of isoflupredone acetate treatment on clinical signs and weight gain in weanling heifers with experimentally induced Mannheimia haemolytica bronchopneumonia. Am J Vet Res 2011;72:1613–1621. [DOI] [PubMed] [Google Scholar]
- 39. White BJ, Anderson DE, Renter DG, et al. Clinical, behavioral, and pulmonary changes in calves following inoculation with Mycoplasma bovis . Am J Vet Res 2012;73:490–497. [DOI] [PubMed] [Google Scholar]
- 40. Howard CJ, Gourlay RN. Immune response of calves following the inoculation of Mycoplasma dispar and Mycoplasma bovis . Vet Microbiol 1983;8:45–56. [DOI] [PubMed] [Google Scholar]
- 41. Godinho KS, Wolf RM, Sherington J, et al. Efficacy of tulathromycin in the treatment and prevention of natural outbreaks of bovine respiratory disease in European cattle. Vet Ther 2005;6:122–135. [PubMed] [Google Scholar]
- 42. Dowling A, Hodgson JC, Schock A, et al. Experimental induction of pneumonic pasteurellosis in calves by intratracheal infection with Pasteurella multocida biotype A:3. Res Vet Sci 2002;73:37–44. [DOI] [PubMed] [Google Scholar]
- 43. Straub O. Infectious bovine rhinotracheitis virus In: Dinter Z, Morein B, eds. Virus Infections of Ruminants. Amsterdam: Elsevier; 1990:71–108. [Google Scholar]
- 44. Sacco RE, McGill JL, Pillatzki AE, et al. Respiratory syncytial virus infection in cattle. Vet Pathol 2014;51:427–436. [DOI] [PubMed] [Google Scholar]
- 45. Nagatomo H, Shimizu T, Higashiyama Y, et al. Antibody response to Mycoplasma bovis of calves introduced to a farm contaminated with the organism. J Vet Med Sci 1996;58:919–920. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Articles reviewed for analysis inclusion.
Table S1. References reviewed for BVDV challenge studies. NR‐not reported
Table S2. References reviewed for BHV‐1 challenge studies. NR‐not reported
Table S3. References reviewed for PI‐3 challenge studies. NR‐not reported
Table S4. References reviewed for BRSV challenge studies. NR‐not reported
Table S5. References reviewed for Mannheimia haemolytica challenge studies, NR‐not reported
Table S6. References reviewed for Mycoplasma bovis challenge studies. NR‐not reported
Table S7. References reviewed for Pasteurella multocida challenge studies. NR‐not reported


