Abstract
Osteosarcoma is a malignant mesenchymal neoplasm that accounts for the majority of primary bone tumors in dogs and shares biological and clinical similarities with osteosarcoma in humans. Despite dose intensification with conventional cytotoxic therapies, survival times for dogs and humans diagnosed with high‐grade osteosarcoma have not changed in the past 20 years, with the principal cause of mortality being the development of pulmonary metastases. Given the therapeutic plateau reached for delaying metastatic progression with cytotoxic agents, exploration of alterative adjuvant therapies for improving management of osteosarcoma micrometastases is clinically justified. Evidence suggests that osteosarcoma is an immunogenic tumor, and development of immunotherapies for the treatment of microscopic lung metastases might improve long‐term outcomes. In this review, the history and foundational knowledge of immune interactions to canine osteosarcoma are highlighted. In parallel, immunotherapeutic strategies that have been explored for the treatment of canine osteosarcoma are summarized. With a greater understanding and awareness for how the immune system might be redirected toward combating osteosarcoma metastases, the rational development of diverse immune strategies for managing osteosarcoma holds substantial promise for transforming the therapeutic landscape and improving disease management in both dogs and human beings.
Keywords: Bone sarcoma, Cellular immunity, Immunotherapeutics, Metastases
Abbreviations
- OS
osteosarcoma
- MST
median survival time
- DFI
disease free interval
Dogs are second only to humans in terms of naturally occurring inherited disease, and dogs retain breed homogeneity that often mimics certain human demographics such as race or geographic phenotypes.1 Dogs also acquire similar genetic diseases and cancers as do people and consequently might serve as suitable comparative models for conserved pathologies.1, 2 The accelerated aging of dogs, especially large breeds in comparison with humans, combined with the large numbers of veterinary healthcare dollars spent on dogs (second only to human healthcare dollars spent), provide researchers with a relatively large population of pet dogs that might be available for the study of cancer pathogenesis, as well as for participation in clinical cancer trials.3, 4 Collectively, the shared genetics of specific canine cancers with their human counterparts and the high societal value placed upon dogs as companion animals allow pet dogs to serve as valuable large animal models for translational cancer research.
Osteosarcoma (OS) accounts for 85% of all skeletal tumors in the dog, making it the most common primary bone tumor5, 6, 7, 8, 9 with an estimated 10,000 dogs diagnosed with OS each year.10 Histologically, OS is composed of malignant mesenchymal cells of stem cell or osteoblast lineage that produce osteoid. Different histologic subtypes of OS exist including osteoblastic, fibroblastic, chondroblastic, and telangiectactic phenotypes, and are based upon the morphology and differentiation characteristics of tumor cells.11 Typically, OS is considered as a disease of large and giant breed dogs12 and it has a predilection to arise from the appendicular skeleton.13, 14 Middle‐aged to older dogs (median age of 7 years) are most commonly affected by OS,5, 6, 7, 8, 9, 13, 15, 16, 17, 18, 19, 20, 21, 22 but a bimodal age distribution may occur with a smaller peak of OS at 18–24 months of age.18
Similar to dogs, OS is the most common primary focal skeletal tumor in people and accounts for more than 56% of all bone tumors. In adolescents, OS is the third most frequent cause of cancer and often affects taller adolescents, similar to large breed dogs.2 The diagnosis of OS in people also follows a bimodal age distribution, but unlike dogs where the incidence of OS is highest in older animals, adolescents are affected more frequently in humans.23
The biologic behavior of OS is aggressive, initially restricted to the local bone microenvironment but with distant organ involvement as a result of metastatic disease progression. Although only about 15% of dogs and 20% of people present with detectable lung metastases, the eventual development of distant metastatic foci in the absence of chemotherapy is 90% within 1 year for dogs and 80% within 2 years for people.7, 10, 20, 24 Although adjuvant chemotherapy has increased the cure rate of people and survival time of dogs diagnosed with high‐grade OS, there has been no improvement in long‐term treatment outcomes in the last 20 years for either species, despite the institution of conventional dose intensification strategies.25, 26, 27, 28 Based upon the current therapeutic ceiling reached, the identification of promising and complementary adjuvant treatments for improving the management of micrometastatic disease is clinically warranted.
Focused scientific development and clinical assessment of novel immunotherapeutic strategies are areas that are rapidly gaining momentum in veterinary medicine and in the treatment of canine OS (Fig 1). Given the conserved biology of OS between dogs and people, unique opportunities exist for veterinary researchers and clinical oncologists to adapt immunobiological advances in the human oncology arena for translational purposes in dogs with OS. Reciprocally, novel tumor immunotherapeutic strategies first evaluated in dogs with OS also have the potential to inform and guide development of new treatment regimens for the benefit of human cancer patients.
Figure 1.
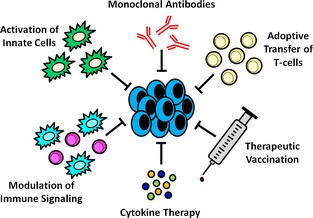
General categories of antitumor immunotherapy (clockwise from top). Monoclonal antibodies (mAbs) can be used directly against tumor cells or targeted towards the tumor microenvironment. Direct killing of tumor cells via mAbs is typically through receptor antagonist or agonist activity, but can also target enzymatic activities within the tumor cells. Conjugation of cytotoxic drugs to mAbs is another mechanism of direct tumor killing. These mechanisms can also be applied to the tumor microenvironment. Enhanced immune‐mediated killing of tumor cells can also be harnessed through mAbs via enhancement of phagocytosis, antibody‐dependent cellular cytotoxicity (ADCC), complement activation, or T‐cell cross‐presentation and activation. Adoptive transfer of T‐cells specific for certain tumor cell antigens can enhance antitumor immunity. T‐cells can be genetically engineered to express T‐cell receptors that recognize specific tumor cell antigens or tumor‐specific autologous T‐cells can be isolated from the tumor itself with subsequent expansion and reinfusion into the host for the exertion of therapeutic activities. Therapeutic vaccination is aimed at redirecting or enhancing immune responses to tumors. Some therapeutic vaccines employ ex vivo expansion of autologous antigen‐presenting cells (APC) with a common tumor antigen or focus on the modification of tumor cells to express or secrete cytokines that enhance APC activation; in both situations these cells are reinfused into the patient. Other therapeutic vaccines focus on delivery of a specific protein that is overexpressed by the tumor of interest or serves a specific immune function, delivery of an infectious agent to enhance general antitumor immunity, or a combination thereof. Cytokine therapies, such as IL‐2 or the interferons, can be used in vivo to enhance immune responses, but can also be employed in the ex vivo expansion of immune cells for cell‐based therapies. Modulation of immune signaling with agents such as BCG and muramyl peptides refers to enhancement of beneficial antitumor immune responses or blockade of immunosuppressive signaling. Manipulation of tumor cells to express costimulatory molecules can enhance immune activation, while blockade of inhibitory immune cells (such as Tregs or MDSCs) or inhibitory immune receptors (such as CTLA‐4) can prevent tumor‐based immunosuppression. Activation of innate immune cells, such as dendritic cells, can be achieved through ex vivo cytokine activation, in vivo treatment with toll‐like receptor (TLR) ligands or targeted antigen‐loaded antibodies, or even occur naturally secondary to tumor cell death from radiation therapy or chemotherapy.
Clinical Evidence of Immunogenicity: Limb‐Spare Infections in Osteosarcoma
The capacity for the immune system to recognize and eliminate cancer has been recognized for over a century with some of the earliest reports including the eradication of bone sarcomas. In the early 1890's, William Coley reported that accidental acquisition or intentional inoculation of the bacterium responsible for erysipelas (Streptococcus pyogenes) could result in regression or delayed recurrence of various cancers.29, 30 These clinical insights eventually led to Coley's development of a vaccine consisting of 2 killed bacteria, Streptococcus pyogenes and Serratia marcescens.31 The vaccine was named “Coley's Toxins” and was efficacious in the treatment of a variety of tumor types, including bone sarcomas.32
Coley's observations that underscored the potency of the immune system against cancer have been long recognized, but not until recently was direct in vivo evidence regarding infection‐enhanced antitumor immunity for OS revisited and reported to the medical community. Although histologic evidence of non‐eptic, chronic inflammation in biopsy specimens of canine OS has not been found to be prognostic,18 spontaneous regression of OS in dogs has been reported33 and dogs that experience acute bacterial infection secondary to limb‐salvage surgery have been found to have increased survival times in several independent studies.34, 35, 36, 37 The initial observation of this finding was reported in a study investigating the use of radiation therapy before cortical allograft limb‐sparing surgery in dogs with high‐grade appendicular OS. Although radiotherapy before limb‐sparing surgery was deemed detrimental for achieving durable fixation of bone allografts, a significant increase in survival time was noted between dogs whose allograft became infected as compared to dogs with allografts that remained uninfected (11 versus 5 mos respectively).34
These initial findings later were corroborated by 2 additional studies that examined the outcome of dogs with OS treated with limb‐sparing surgery and adjuvant chemotherapy.35, 36 Dogs with distal radial OS that developed cortical allograft infection were half as likely to die and half as likely to develop metastatic disease as compared to dogs without infection, which resulted in a significant difference in median survival time (MST) of 18 versus 7.6 mos respectively.35 Similar findings were reported in dogs undergoing either cortical allograft or endoprosthetic limb‐sparing surgery.36 No difference in MST was found between the 2 surgical groups but MST was found to be significantly longer in dogs that experienced construct failure (22.8 versus 10.7 mos) or postoperative infection (22.8 versus 9.6 mos). Dogs with postoperative infection also were 25 times less likely to die, and median metastasis‐free interval (MFI) was increased for dogs with infection (18.5 versus 9.1 mos). All dogs with construct failure also had postoperative infections, and irritation secondary to construct failure might have contributed to infection development. Lastly, in a recent retrospective study, when evaluating only dogs that lived for >1 year after histopathologic diagnosis of OS, increased survival time of dogs that developed postoperative limb‐spare infections also was identified. Dogs with limb‐spare infections had a MST of 6 mos beyond 1 year, whereas dogs that underwent limb‐sparing surgery but did not acquire infection only achieved a MST of 0.9 mos beyond 1 year.37
People with OS who were treated by endoprosthetic replacement and experienced postoperative infection also had increased survival time.38 A later study, however, found no difference in survival time between infected and noninfected patients when matched for type of chemotherapy, histologic response, tumor size and location, and local recurrence.39 No case–control studies for comparison have been performed in dogs to date, but the majority of studies do suggest that nonspecific immune stimulation secondary to infection prevents the recurrence or delays progression of OS in a clinical setting.
The immune mechanisms that contribute to increased survival secondary to limb‐spare infection have not been well‐studied, but evidence for innate system involvement on the suppression of OS growth has been derived from a murine model of chronic bacterial osteomyelitis. In this study, osteomyelitis decreased tumor growth and increased survival time in mice when tumors were established after infection, but this effect was abrogated when tumors were established before induction of osteomyelitis.40 Several different types of infectious agents have been cultured in affected dogs,34, 35, 36 and infection‐associated inhibition of tumor growth in the murine model of chronic bacterial osteomyelitis was not dependent on the specific infectious agent involved. Increased circulating and splenic inflammatory monocytes as well as increased tumor‐associated macrophages (TAM) were observed in infected mice, and depletion of natural killer (NK) cells or monocytes and macrophages was found to reverse the tumor growth inhibition seen with concurrent osteomyelitis. These observations led the authors to conclude that both NK cells and monocytes and macrophages are associated with the innate antitumor response elicited by chronic bacterial osteomyelitis, and they speculated that the increase in inflammatory monocytes was associated with repopulation of activated TAM, which were expected to be tumor‐inhibitory in this setting rather than tumor‐promoting. Furthermore, the finding of increased NK cells might be related to back‐and‐forth activation between NK cells and monocytes, also contributing to tumor inhibition.40
These clinical and preclinical studies strongly suggest that OS is an immunogenic neoplasm, and micrometastatic disease potentially can be controlled or eliminated after recognition by the immune system. Case–control studies in dogs to either confirm or refute these findings39 and mechanistic studies to characterize the specific immune responses against OS cells elicited by limb‐spare infections are lacking.
Humoral Evidence of Immunogenicity
Cell‐Mediated Reactivity and Serum Blocking Activity
Cell‐mediated reactivity (CMR) and serum blocking activity (SBA) experiments were used to investigate interactions of the immune system with canine OS. Simply defined, CMR refers to inhibition of target cell growth whereas SBA refers to promotion of target cell growth in the presence of serum. Autologous serum from dogs with progressively growing OS exhibited SBA effects in vitro, and decreased CMR was observed when cocultures of autologous lymphocytes and tumor cells were incubated in the presence of the patient's serum. In the absence of autologous serum, high numbers of lymphocytes could inhibit tumor growth in vitro, whereas low lymphocyte numbers conversely stimulated growth. Based upon these observations, humoral factors present in the patient's serum (eg blocking antibodies or antigen‐antibody complexes) were surmised to prevent tumor destruction by autologous lymphocytes, but other serum‐derived factors likely potentiated tumor growth. Whether the SBA was mediated by inhibitory cytokines was not considered in these studies.41, 42
Extending these initial findings, changes in SBA were investigated pre‐ and postoperatively in dogs with OS that underwent amputation of the tumor‐bearing limb. Increased presurgical SBA was noted in dogs that eventually developed metastatic disease, and postsurgical SBA increased before development of overt metastatic disease in the majority of dogs (6/8, 75%). For dogs remaining free of metastasis, SBA was unmeasurable.43 In a complementary study, postsurgical SBA in dogs with OS given Bacillus Calmette‐Guérin (BCG) intradermally also was found to increase in conjunction with radiographic appearance of metastasis.44 Based upon these findings, SBA was proposed to be of potential value for determining prognosis, but identification of specific factors mediating SBA (eg antibodies or inhibitory cytokines) was not determined in these studies.43
C1q Binding Levels
The C1q‐binding test is used in immunology to evaluate circulating immune complexes (CIC). A single study evaluated serum C1q‐binding levels in 56 dogs with OS, and demonstrated that a large percentage of dogs (46/56, 82%) had increased C1q‐binding at the time of diagnosis. In a subset of dogs (n = 12) in which serial C1q‐binding levels were available for quantification, divergent trends in C1q‐binding levels were observed based upon disease status. In dogs that survived up to 1 year postdiagnosis (n = 4), the C1q‐binding levels where found to have fallen within normal reference ranges after completion of therapy. Conversely, in dogs that experienced local disease recurrence or distant metastases (n = 8), levels of C1q‐binding either remained increased throughout the entire study duration or only transiently decreased before increasing again. The CIC identified in these dogs had characteristics consistent with IgG, but this conclusion was made cautiously because anomalous fractionation results were observed in normal control dogs.45
TP‐1 and TP‐3 Antibodies
TP‐1 and TP‐3 are murine antihuman monoclonal antibodies (mAbs) created using the hybridoma technique by immunization of mice with human OS cells. These 2 distinct mAbs bind different epitopes of the same unknown antigen and have been shown to be highly sensitive and moderately specific for human OS. Using immunohistochemistry (IHC) on canine tumor tissues, the TP‐1 and TP‐3 antibodies also were shown to have useful specificity for canine OS, although chondrosarcomas and several carcinomas also were cross‐reactive with these antibodies. Staining of normal canine tissues with these antibodies was limited.46 Extending upon the recognition of conserved epitopes in formalin‐fixed tissues, additional studies in OS dogs with 131I‐ or 123I‐labeled F(ab′)2 fragments of TP‐1 or18F‐labeled TP‐3 Fab fragments showed high specificity of these antibodies for primary and metastatic OS lesions using immunoscintigraphy and positron emission tomography (PET) scanning respectively.47, 48 Immunoscintigraphic evaluation of I‐labeled F(ab′)2 fragments of TP‐1 also detected multiple metastatic lesions that were not detectable by conventional radiography.47 Despite the sensitivity and specificity of TP‐1 and TP‐3 antibodies for conserved OS epitopes, use of these antibodies for diagnostic purposes, either for IHC or molecular imaging, is limited in both human and veterinary medicine, and likely stems from a lack of commercial availability. Nonetheless, cross‐reactivity of TP‐1 and TP‐3 antibodies for conserved OS epitopes in canine tumor tissues further supports the capacity for immune recognition of canine OS antigens.
Negative Cellular and Soluble Regulators: Evidence for Immune Evasion
Regulatory T‐cells
Regulatory T‐cells (Tregs) are a component of the immune system responsible for controlling and suppressing excessive immune activation. Phenotypic characterization of Tregs includes concurrent expression of CD4 and CD25 surface antigens along with transcription of the FoxP3 gene. Dysregulation of Tregs has been incriminated in the induction of autoimmunity, and conversely promotion of ineffective antitumor immunity. Given the potential role of Tregs in suppressing the immune surveillance of cancer, Tregs have been investigated in dogs with cancer and specifically in dogs with OS. Several studies have documented increased expression of CD4+ FoxP3 + Tregs in dogs with cancer compared to controls, findings that suggest potential participation of Treg‐induced immune suppression and cancer progression. When stratified by tumor histology, no difference in the percentage or absolute number of Tregs was identified between OS‐bearing and normal dogs in either peripheral blood or draining lymph nodes, but the small sample sizes of dogs with OS used for comparison might have limited the power to detect the existence of a true difference between groups.49, 50 A later study examining only dogs diagnosed with OS and free of measurable metastatic disease also confirmed no difference between peripheral blood or lymph node CD4+CD25+ FoxP3 + Treg numbers when compared to healthy controls. In fact, Treg numbers were found to be significantly lower in the tumor‐draining nodes when compared to nondraining nodes of the OS dogs.51
Discordant with the findings derived from other investigations, differences in Tregs were identified in another study that evaluated dogs with OS that had not received chemotherapy within 3 weeks of blood collection. Significantly increased numbers of relative and absolute circulating CD4+ FoxP3 + Tregs were identified in OS dogs versus control dogs. Despite differences in Tregs identified in the blood, similar differences between OS‐bearing and normal dogs were not identified when Tregs were evaluated in draining or nondraining lymph nodes. Additionally, no differences in circulating Tregs were noted between pre‐ and postamputation blood samples. Concordant changes in effector T lymphocyte populations also were examined, and decreased numbers of circulating CD8+ cells (absolute and relative) as well as a decreased CD8+:Treg ratio were observed in dogs with OS. The effector to regulatory T lymphocyte ratio provided prognostic information, with a low CD8+:Treg ratio being associated with decreased survival as compared to a high ratio.52
Myeloid‐Derived Suppressor Cells
Myeloid‐derived suppressor cells (MDSCs) are immature cells of myeloid lineage derived from bone marrow progenitor cells. In diseases such as cancer, MDSCs are increased and contribute to a global immunosuppressive state. In veterinary medicine, the identification of MDSCs as a small percentage of circulating white blood cells in normal dogs and tumor‐bearing dogs has been possible using flow cytometry. In 1 study, the percentages of circulating putative MDSCs identified by CD11blow and CADO48Alow surface expression were quantitatively different between normal healthy dogs and dogs diagnosed with different tumor types including OS.53 A higher percentage of MDSCs was identified in tumor‐bearing dogs (7.9%) compared to normal dogs (3.6%), and in vitro generated MDSCs possessed the capacity to suppress concanavalin A‐induced splenocyte proliferation. In a complementary study, granulocytic MDSCs were identified by CD11b+CD14−MHCII− surface expression in healthy dogs, dogs with early stage nonmetastatic cancers, and dogs with advance stage metastatic cancers.54 In dogs with advance stage metastatic cancers, which included OS and hemangiosarcoma, the percentage of circulating MDSCs was significantly higher than in healthy dogs and those with early stage nonmetastatic cancers. Similarly, isolated MDSCs exhibited immunosuppressive activity as demonstrated by attenuation of concanavalin A and human recombinant IL‐2 (hrIL‐2)‐stimulated T‐cell proliferation. Based on these 2 investigations, evidence was generated to substantiate the existence of MDSCs in dogs, both in health and disease. Importantly, both studies identified increases in the percentage of circulating MDSCs in dogs diagnosed with highly metastatic tumors such as OS.
Despite their proven existence in dogs with cancer, MDSCs have not been definitively identified to play a role in the progression of canine OS. They have been speculated, however, to exert some form of negative immunomodulation in dogs with OS, because dogs with monocyte counts >0.4 × 103 cells/μL have a shorter median disease‐free interval (DFI) (6.7 versus 15.5 mos). In the majority of dogs evaluated (59/69, 86%) this monocyte count did not represent a monocytosis, and monocyte numbers were still within the reference interval.55 In a study investigating prognostic factors in dogs with OS of the maxilla, mandible, or calvarium, there was also a significantly increased hazard of death with increasing monocyte count.56
Tumor‐Derived Soluble Factors
Tumor‐derived soluble factors (TDSFs) produced from 2 immortalized canine OS cell lines (OSA8 and OSA16) have been shown to suppress the function of cultured canine myeloid cells. Coculture of canine dendritic cells, macrophages, or both with tumor‐conditioned media (TCM) containing TDSFs suppressed activation of these antigen‐presenting cells by decreased expression of MHC Class II and CD80 (B7.1), decreased phagocytic activity, and decreased capability to induce splenic effector cell proliferation.57
Immunotherapeutics: Historical and Current Strategies
Bacillus Calmette‐ Guérin
Although utilized for vaccination against tuberculosis since the 1920's,58 Bacillus Calmette‐Guérin (BCG) immunotherapy also has been investigated since the 1930's as an antitumor immune modulator after the observation that people who died from tuberculosis coincidently also had a decreased incidence of cancer. Broad antitumor immune activity elicited by BCG has been demonstrated in several murine tumor model systems, as well as in various naturally occurring tumor types including stomach cancer, melanoma, and leukemia. Today, BCG's principal anticancer immunotherapeutic role is for the treatment of nonmuscle invasive bladder cancer in people.59 In the setting of bladder cancer, BCG is believed to exert its immunobiologic effects by upregulation of MHC II molecules in malignant transitional epithelial cells, along with the induction of CD4+ Th1 and cytotoxic T lymphocyte responses.60
The exploration of BCG immunotherapy for canine OS began in the early 1970's. Initially, injection of BCG either IV, intraperitoneal, or intrathoracic into normal dogs was performed to investigate the capacity of BCG to create pathologic lesions. Histologically, BCG injections generated granuloma formation within the liver and lung parenchyma, along with lymphoid hyperplasia of the tonsils and bronchial lymph nodes. Positive tuberculin test reactions were noted most often in dogs receiving intrathoracic BCG. Subsequently, dogs with spontaneous OS, the majority of which did not have evidence of metastatic disease, were given IV BCG at variable intervals, with or without concurrent vaccination with irradiated autologous tumor cells. Findings derived from these OS‐bearing dogs receiving BCG indicated no enhancement of metastasis, but instead a possible delay in metastatic development and progression, conclusions that were substantiated by longer survival times in dogs that received BCG as compared with a historical control group.61 Another study reported findings derived only from dogs with OS that underwent amputation (n = 12) and that received an identical BCG IV injection scheme, with or without irradiated autologous tumor cells.62 This study noted a significant increase in survival compared to a previously published historical control group (51 versus 14 weeks).62 Interestingly, improvements in survival times were not restricted by the route (IV) of BCG administration, and intradermal delivery of BCG to dogs with OS after amputation also significantly extended survival to 40 weeks versus 13 weeks for controls.44
After documented clinical activity in dogs with OS, mechanistic studies were conducted to characterize the potential mode of action of BCG. Studies conducted in normal and tumor‐bearing dogs demonstrated that BCG administration could stimulate alveolar macrophage activity and promote lymphocyte cytotoxicity ex vivo. Not surprisingly, the antitumor cytotoxicity induced by BCG was observed to be nonspecific with lysis of several tumor types in addition to OS, including melanoma and mammary carcinomas.63, 64, 65 Nonspecific cytotoxicity exerted by BCG‐primed alveolar macrophages and lymphocytes against multiple cancer cell lines was suspected to be the result of shared tumor cell antigens or polyclonal lymphocyte activation.63 Given its capacity to induce granuloma formation, BCG's immunobiologic effects could be associated with activation of circulating monocytes or tissue macrophages in reticuloendothelial organs. Specifically, CCR2+ inflammatory monocytes that expand within the marginal zone of the spleen have been associated with cross‐presentation of tumor antigens and subsequent tolerance of CD8+ memory T‐cells, whereby splenectomy restores lymphocyte and antitumor function.66 As such, the effect of splenectomy was evaluated in dogs with OS treated with amputation and administration of an intradermal methanol‐extracted residue of BCG (MER).67 Unexpectedly, splenectomy led to decreased survival in the OS dogs, whereas dogs treated with amputation and MER without splenectomy had similar survival times to historical controls.67
Muramyl Peptides
The muramyl peptides include muramyl dipeptide phosphatidylethanolamine (MDP), a synthetic analog of the peptidoglycan cell wall of mycobacteria that is the smallest part of mycobacteria that is immunostimulatory, and muramyl tripeptide phosphatidylethanolamine (MTP‐PE), a lipophilic derivative of MDP.68, 69 The liposomal form of MTP (L‐MTP‐PE) can be used for the in vivo stimulation of macrophages and monocytes rendering them cytotoxic against tumor cells, but L‐MTP‐PE itself does not exert any direct tumor cytotoxic properties.70, 71 These unique features make L‐MTP‐PE an ideal candidate for the immunotherapy of cancer, and in people, L‐MTP‐PE has resulted in prolonged survival in patients with OS in both initial and relapsed settings.72, 73
In the context of canine OS, the in vitro incubation of canine peripheral blood mononuclear cells (PBMCs) with MDP was shown to elicit cytostasis against the D‐17 cell line, and this cytostatic effect was maximized by a combination of MDP and lipopolysaccharide (LPS) compared to either agent used alone.74 This maximal effect of MDP and LPS was identified to be secondary to TNFα secretion and not because of direct toxicity induced by MDP or the combination of MDP and LPS.75 Correlating with cell culture experiments, PBMCs collected from healthy dogs after IV L‐MTP‐PE administration also had significantly more cytostatic activity against the D‐17 line, and serum harvested from the same dogs had increased TNFα activity within 2 hours after L‐MTP‐PE injection.74, 75
In addition to evaluating the anticancer effects of L‐MTP‐PE as a single agent, a combination of L‐MTP‐PE and doxorubicin chemotherapy also was investigated in vitro and in vivo, and indicated that a combination of doxorubicin and L‐MTP‐PE enhanced PBMC activation and cytotoxicity compared to either drug alone. Again, the observed biologic response was mediated by TNFα.76 Given the predilection of OS to metastasize to the pulmonary parenchyma, a similar combination platform was investigated for the activation of canine pulmonary alveolar macrophages against D‐17 cells. Similar to the results generated by PBMCs, pulmonary alveolar macrophages harvested from dogs receiving combination therapy with doxorubicin and L‐MTP‐PE exerted the greatest ex vivo cytotoxicity against canine OS target cells.77
The translational relevance of the observed in vitro and ex vivo activity of L‐MTP‐PE against canine OS cells has been investigated in dogs with spontaneously occurring OS. In the first reported study of L‐MTP‐PE in dogs with OS, the anticancer activity induced by IV L‐MTP‐PE as a single agent was evaluated by a randomized, placebo‐controlled trial. In comparison with placebo controls, dogs treated with amputation and twice weekly IV L‐MTP‐PE had significant extensions of MST (222 versus 77 days) and median MFI (168 versus 58 days).78 Despite the substantial improvement in survival times induced by single agent L‐MTP‐PE, over 50% of the dogs studied ultimately experienced metastatic progression and were dead by 8 months after therapy. In an attempt to improve long‐term survival in dogs with OS treated with L‐MTP‐PE, a subsequent study evaluated the use of L‐MTP‐PE in combination with adjuvant cisplatin chemotherapy in dogs without macroscopic metastatic disease. When used in combination serially, administration of L‐MTP‐PE after amputation and cisplatin chemotherapy significantly prolonged MST (14.4 versus 11.2 mos) and median DFI (9.8 versus 7.6 mos). However, if L‐MTP‐PE was given concurrently with cisplatin chemotherapy in the adjuvant setting, no survival benefit was identified beyond that achieved with cisplatin chemotherapy alone.79 Given that L‐MTP‐PE failed to be approved by the US Food and Drug Administration in 2007 for the initial treatment of nonmetastatic OS in children, clinical research with muramyl peptides for the treatment of OS in dogs has not advanced during the past decade, and L‐MTP‐PE will not likely be available in the United States for the management of OS in dogs. In contrast, L‐MTP‐PE is approved for use in the European Union for nonmetastatic OS in children, as mifamurtide (Mepact®).
Interleukin‐2 Cytokine Therapies
Interleukin‐2 (IL‐2) is a pleiotropic cytokine responsible for several key immune responses including differentiation and subsequent expansion of activated T‐cells into effector and memory T‐cells after stimulation by immunogenic antigens. Given the potent role of IL‐2 for establishing cell‐mediated immune responses, IL‐2 has been investigated both ex vivo and in vivo for the management of canine OS. In an early study, the ability of hrIL‐2 to activate peripheral blood leukocytes (PBL) derived from either normal dogs or dogs with lung tumors was investigated in vitro. When derived from dogs with lung tumors, autologous stimulated lymphocytes (ASL) generated by culture with hrIL‐2 and phytohemagglutinin (PHA) were significantly more cytotoxic against autologous tumor cells when compared with ASL produced from healthy dogs. These findings suggested that hrIL‐2 could preferentially reactivate ASL collected from tumor‐bearing dogs to exert cytotoxicity against naturally occurring pulmonary tumors, including metastatic OS. In addition, increased ASL cytotoxicity in tumor‐bearing dogs compared to healthy dogs could indicate that tumor‐bearing dogs had tumor‐specific lymphocytes.80 In a separate study in which healthy beagle dogs were given continuous IV IL‐2 via the splenic artery or inferior vena cava, the generation and cytotoxicity of lymphokine‐activated killer (LAK) cells derived from PBL against the canine D‐17 OS cell line was evaluated. Splenic artery infusion of IL‐2 was consistently observed to stimulate LAK cell activity in PBL, whereas inferior cava infusion did not. The spleen also underwent “lymphoblastic change” with splenic artery infusion, which consisted of marked lymphoid proliferation with loss of normal splenic architecture, supporting that the more robust LAK activity seen in these dogs was directly related to splenic immunostimulation.81 Although this study evaluated D‐17 OS cells as the target cells of LAK activity, it was not further determined if the LAK activity generated was specific for OS or a nonspecific tumor response.
Several studies evaluating the immunobiologic activities of IL‐2 administration in vivo have been conducted and focused on direct delivery of IL‐2 or the transgene expression of IL‐2 in the lungs of healthy dogs and in dogs with metastatic OS. Nebulization of liposomal hrIL‐2 in healthy dogs was shown to activate cytolytic and cytostatic activity of bronchoalveolar lavage (BAL) leukocytes, as well as increase total BAL leukocyte numbers with concurrent increases in the lymphocyte and eosinophil percentages. Additionally, nebulized liposomal hrIL‐2 was determined to be safe with no life‐threatening adverse systemic reactions noted.82 When evaluated in a clinical trial of dogs with primary lung tumors or lung metastases, treatment with liposomal hrIL‐2 nebulization resulted in 2 of 4 dogs with OS lung metastases experiencing complete regression of all metastatic lesions (lung or lung and lymph node) for >1 year. Interestingly, liposomal hrIL‐2 nebulization failed to generate uniform responses in dogs with metastatic OS, with the other 50% of dogs experiencing progressive disease. Correlating with the observed clinical responses, immunobiologic activity of liposomal hrIL‐2 nebulization was supported in tumor‐bearing dogs by augmentation in BAL leukocyte numbers, percentages, and cytolytic activities. Additionally, an increase in T lymphocytes and a shift in the CD4:CD8 ratio because of increased CD4+ cells was observed in dogs receiving hrIL‐2 nebulization. Ultimately, all dogs developed neutralizing antibodies against hrIL‐2, limiting application of this immunostimulatory strategy to short‐term use in a clinical setting.83
In an effort to localize IL‐2 preferentially to the lung parenchyma without the need for aerosolization as well as to avoid neutralizing antibody formation, IV administration of cationic liposome‐DNA complexes (LDCs) containing canine IL‐2 cDNA also has been explored in dogs with metastatic lung OS (n = 20), with the intent to induce lung‐specific IL‐2 transgene expression and immunomodulation. Increased NK cell activation, along with increased monocyte expression of MHC Class II and B7.2 (CD86) were observed in this study, indicating the enhancement of immunobiologic activities by LDCs. The LDCs were primarily taken up by CD11b+ monocytes rather than lymphocytes, suggesting that LDCs along with transcription of IL‐2 itself both contributed to a systemic, innate immune response. In 3/20 (15%) dogs treated with LDCs, complete or partial regression of lung metastases was observed and 4/20 (20%) dogs experienced disease stabilization. Overall survival time compared to historical controls matched for age and tumor stage also was marginally increased (MST of 2.7 versus 2 mos).84
Although these 2 in vivo studies exploring IL‐2 cytokine strategies identified only modest clinical benefits, the data do provide evidence supporting the feasibility of targeted, lung‐specific immunotherapy for the treatment of macroscopic OS pulmonary metastases. Presumably, IL‐2 cytokine therapy would be expected to exert greater therapeutic benefit in a microscopic residual disease setting.
Adoptive Transfer of T‐cells
Only a few studies have investigated the use of adoptive T‐cell transfer in dogs with OS. One investigation evaluated the feasibility, toxicity, and therapeutic outcome associated with IV infusion of the human cytotoxic T‐cell line, TALL‐104, to dogs with OS. In this study, dogs with appendicular OS were treated with surgery and adjuvant cisplatin chemotherapy, and if remaining free of pulmonary metastases at the completion of chemotherapy, subsequently were given IV infusions of TALL‐104 monthly for up to 9 consecutive months. Collectively, combination therapy inclusive of surgery, cisplatin chemotherapy, and adjuvant TALL‐104 was tolerable and allowed OS‐bearing dogs to achieve MSTs and DFIs of 11.5 and 9.8 mos, respectively, which is comparable to dogs treated with combined surgery and chemotherapy. As expected, the xenogeneic nature of TALL‐104 caused dogs to develop antibodies and a cellular immune response against TALL‐104 with generation of a long‐lived, neutralizing antibody response in some dogs. Mechanistically, anticancer activities exerted by TALL‐104 were believed to be principally mediated by endogenous antitumor immunity and partially by MHC‐independent NK cell cytotoxicity, as supported by an ex vivo51Cr‐release assay conducted in dogs before relapse.85
With unprecedented advances in immunobiological techniques, it recently has become possible to create engineered T‐cells that express stable chimeric antigen receptors (CARs) with specificity against a myriad of targetable epitopes, thereby allowing for the immunologic recognition and treatment of different types of cancer. Recently, protocols have been optimized to allow for the generation of canine T‐cells expressing CARs for HER2, a membrane protein expressed in canine OS. Preliminary results indicate that canine T‐cells with CARs specific for HER2 possess the capacity to kill HER2+ canine OS cells in an antigen‐dependent manner.86 Based upon these promising early results, additional studies utilizing molecular CAR technologies for the treatment of dogs with OS are anticipated.
Fas Receptor and Fas Ligand Signaling
Induction of apoptosis is one way the immune system can eliminate cancer cells. Mechanistically, cytotoxic T‐cells or NK cells can trigger programmed cell death in targeted cells by the coordinated release of perforin and granzyme after appropriate cellular recognition cues. However, all too often, this form of direct apoptosis induction by the immune system can be evaded by cancer cells through their acquisition of genetic mutations. Rather than relying directly on immune cell induction of apoptosis by cytotoxic T‐cells, NK activation, or both, investigators have evaluated the ability to induce apoptosis in cancer by indirect mechanisms. One strategy for augmenting immune‐mediated apoptosis in cancer cells has been through manipulation of the Fas receptor (Fas) and Fas ligand (FasL) signaling pathway.
Fas‐dependent apoptosis is mediated through the binding of FasL, expressed by immune effector cells, with cognate Fas receptor expressed on the surface of target cancer cells. Binding of FasL with the Fas receptor results in clustering of intracytoplasmic death domains and consequent cleavage of initiator procaspases by proximity‐mediated activation. In the context of clonal evolution, tumor cells would favor the loss of Fas receptor expression with consequent enrichment of tumor cell populations inherently resistant to Fas‐mediated cell death. In support of such a resistance mechanism, lung metastases in people with OS often fail to express the Fas receptor, in contrast with the primary tumors which are Fas+.87 Additionally, preclinical murine models of OS recapitulate this immune evasive phenomena, with the expression of Fas receptor being lost during OS metastasis, which allows metastatic lesions to circumvent the induction of apoptosis by FasL‐expressing effector immune cells, as well as the constitutive cellular expression of FasL within lung tissue.87, 88
Given the apparent importance of Fas‐mediated signaling in the immune surveillance of OS metastases, strategies that modulate the expression of Fas or FasL might augment antitumor immune responses. In one study, neoadjuvant FasL gene therapy using an adenovirus vector (Ad‐FasL) was delivered to primary bone tumors in dogs with appendicular OS to augment intratumoral apoptosis, inflammation, and consequent innate immune responses. In this study, dogs were given a single dose of Ad‐FasL, which was followed by a 10‐day waiting period after which the dogs underwent amputation of the affected limb. Improved survival was appreciated in dogs whose tumors had inflammation or lymphocyte‐infiltration scores of >1 and in dogs with apoptosis scores (via cleaved caspase‐3 IHC) in the upper 50th percentile. Additionally, decreased tumor Fas expression was associated with increased inflammation, DFI, and MST.89
Derived from preclinical murine studies indicating that gemcitabine (a deoxycytidine analogue) could cause apoptosis of OS cells through Fas/FasL interactions, a study evaluating the tolerability and anticancer activity of aerosol gemcitabine in dogs with OS was conducted. In this study, gemcitabine caused increased apoptosis as measured by terminal deoxynucleotidyl transferase‐mediated dUTP nick end‐labeling (TUNEL) and marked necrosis within lung metastatic lesions. Metastatic foci of gemcitabine‐treated dogs also had increased Fas expression compared to the primary tumor and also when compared to controls, suggesting gemcitabine therapy reverses the down regulation of Fas that is often recognized in metastatic OS lesions. Despite evidence for beneficial immunomodulatory changes induced by gemcitabine aerosolization, little clinical benefit was detected in treated dogs, with median DFI and overall survival time being comparable with historical controls.90
Therapeutic Tumor Vaccines
Recently, various tumor vaccine platforms have been investigated as novel treatment strategies for improving the management of diverse tumor types including canine OS. In one study intended to augment innate immune responses generated within the tumor microenvironment, an IV, attenuated Salmonella typhimurium (VNP20009) that preferentially localizes and proliferates within tumor tissues, was evaluated in a limited number of dogs with OS (n = 4) and produced modest anticancer activities as indicated by a partial response achieved in one dog. Despite some evidence of antitumor immune activation, numerous adverse effects also were noted in this study, limiting the use of VNP20009 to primarily a research setting.91 In addition to a bacterial agent for enhancing immune responses to OS cells, an oncolytic vaccinia virus (strain LIVP6.1.1) also was successfully tested in vitro for its ability to lyse D‐17 cells. Although the oncolytic vaccinia virus strategy produced potent localized innate immune responses in murine xenograft models,92 the translational evaluation of such oncolytic viral strategies has yet to be reported in dogs with OS.
Sophisticated combination cytokine vaccine strategies have been evaluated in dogs with OS, and have produced early evidence of activity. In one study, the effects of vaccination with irradiated autologous or allogeneic OS tumor cells with xenogeneic cytokine‐producing cells (hGM‐CSF and hIL‐2) administered SC plus suicide‐gene therapy with ganciclovir (GCV) delivered either intratumorally or peritumorally was evaluated in dogs with appendicular or axial OS (n = 5). To stimulate a robust innate immune response, the combination vaccine strategy incorporated a herpes simplex virus thymidine kinase (HSVtk) suicide gene that had been shown to sensitize transfected cells to GCV. Clinically, the combination cytokine vaccine strategy exerted modest antitumor activities, with 1 dog with appendicular OS achieving a partial response, and 2 dogs with axial OS maintaining stable disease. Survival time for the 5 OS dogs ranged from 96 to >386 days, with most dogs living <1 year.93
A recent pilot study in healthy beagle dogs investigated the ability of a dual vaccination platform comprised of DNA electroporation and adenovirus serotype 6 (Ad6) for the induction of an immune response against 2 potential targetable tumor‐associated antigens, telomerase (TERT), or HER2/neu. Both vaccines were found to induce polyspecific T‐cell responses, supporting the ability of the vaccines to elicit quantifiable immune activation, and the TERT vaccine was found to significantly increase the number of CD8+ cells. These immune responses were induced with just one injection but could be maintained over time with repeated injections.94
With the demonstration that immune responses could be generated in healthy dogs against HER2/Neu, another group has adopted an innovative approach to test if clinically relevant immune responses can be evoked against HER2/Neu‐expressing canine OS cells, with a resultant delay in micrometastatic disease progression in dogs with OS. Through the use of a recombinant HER2/neu‐expressing Listeria monocytogenes vaccine, preliminary results have been promising with increased survival times in dogs receiving Listeria monocytogenes vaccination in comparison with historical controls (N. M., personal communication). Although early in its clinical assessment in dogs with micrometastatic OS, the reported findings generated by the investigational Listeria monocytogenes vaccine raise exciting possibilities for the future of therapeutic vaccination as a transformative and complementary strategy for improving long‐term treatment outcomes in dogs with OS.
Conclusion
A large body of scientific and clinical evidence exists supporting the immunogenicity of canine OS. Given the therapeutic plateau reached with conventional cytotoxic therapies for the management of bone sarcomas in both dogs and people, substantive impetus exists for the focused development and validation of innovative immunotherapeutic platforms for improving long‐term disease management. Although new immunotherapeutic platforms potentially could emerge as potent single‐agent therapies for canine OS, adjuvant or combination therapies employing both immunotherapy and cytotoxic chemotherapy also could create substantial impact in the therapeutic management of canine OS. Many of the immunotherapies currently investigated have indicated only limited capacity to substantially extend survival time compared to standard treatment or are still in preliminary phases of testing. Nonetheless, continued research in how to best harness the immune system to combat OS micrometastatic disease remains a highly desirable treatment strategy that holds promise to transform the management of metastatic bone sarcomas in dogs and human beings.
Acknowledgments
Graduate student (KLW) support was provided by Morris Animal Foundation, D14CA‐035: Expression and functionality of toll‐like receptors in canine osteosarcoma: a double‐edged sword between immunomodulation and pro‐inflammatory tumorigenesis.
Conflict of Interest Declaration: Dr. Timothy M. Fan serves as Associate Editor for the Journal of Veterinary Internal Medicine.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
References
- 1. Starkey MP, Scase TJ, Mellersh CS, et al. Dogs really are man's best friend ‐ canine genomics has applications in veterinary and human medicine!. Brief Funct Genomic Proteomic 2005;4:112–128. [DOI] [PubMed] [Google Scholar]
- 2. Rowell JL, McCarthy DO, Alvarez CE. Dog models of naturally occurring cancer. Trends Mol Med 2011;17:380–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kraus C, Pavard S, Promislow DEL. The size‐life span trade‐off decomposed: Why large dogs die young. Am Nat 2013;181:492–505. [DOI] [PubMed] [Google Scholar]
- 4. Patterson DF. Companion animal medicine in the age of medical genetics. J Vet Intern Med 2000;14:1–9. [PubMed] [Google Scholar]
- 5. Brodey RS, McGrath JT, Reynolds H. A clinical and radiological study of canine bone neoplasms I. J Am Vet Med Assoc 1959;134:53–71. [PubMed] [Google Scholar]
- 6. Brodey RS, Sauer RM, Medway W. Canine bone neoplasms. J Am Vet Med Assoc 1963;143:471–495. [PubMed] [Google Scholar]
- 7. Brodey RS, Riser WH. Canine osteosarcoma: A clinicopathologic study of 194 cases. Clin Orthop Relat Res 1969;62:54–64. [PubMed] [Google Scholar]
- 8. Dorfman SK, Hurvitz AI, Patnaik AK. Primary and secondary bone tumours in the dog. J Small Anim Pract 1977;18:313–326. [DOI] [PubMed] [Google Scholar]
- 9. Ling GV, Morgan JP, Pool RR. Primary bone tumors in the dog: A combined clinical, radiographic, and histologic approach to early diagnosis. J Am Vet Med Assoc 1974;165:55–67. [PubMed] [Google Scholar]
- 10. Withrow SJ, Powers BE, Straw RC, et al. Comparative aspects of osteosarcoma: Dog versus man. Clin Orthop Relat Res 1991;270:159–168. [PubMed] [Google Scholar]
- 11. Thompson KG, Pool RR. Tumors of bones In: Meuten DJ, ed. Tumors in Domestic Animals. Ames, IA: Iowa State Press; 2008:245–318. [Google Scholar]
- 12. Ru G, Terracini B, Glickman LT. Host related risk factors for canine osteosarcoma. Vet J 1998;156:31–39. [DOI] [PubMed] [Google Scholar]
- 13. Straw RC, Withrow SJ, Richter SL, et al. Amputation and cisplatin for treatment of canine osteosarcoma. J Vet Intern Med 1991;5:205–210. [DOI] [PubMed] [Google Scholar]
- 14. Heyman SJ, Diefenderfer DL, Goldschmidt MH, et al. Canine axial skeletal osteosarcoma a retrospective study of 116 cases (1986 to 1989). Vet Surg 1992;21:304–310. [DOI] [PubMed] [Google Scholar]
- 15. Brodey RS, Abt DA. Results of surgical treatment in 65 dogs with osteosarcoma. J Am Vet Med Assoc 1976;168:1032–1035. [PubMed] [Google Scholar]
- 16. Knecht CD, Priester WA. Musculoskeletal tumors in dogs. J Am Vet Med Assoc 1978;172:72–74. [PubMed] [Google Scholar]
- 17. Misdorp W. Skeletal osteosarcoma. Animal model: Canine osteosarcoma. Am J Path 1980;98:285–288. [PMC free article] [PubMed] [Google Scholar]
- 18. Misdorp W, Hart A. Some prognostic and epidemiologic factors in canine osteosarcoma. J Natl Cancer Inst 1979;62:537–545. [DOI] [PubMed] [Google Scholar]
- 19. Nielsen SW, Schroder JD, Smith DL. The pathology of osteogenic sarcoma in dogs. J Am Vet Med Assoc 1954;124:28–35. [PubMed] [Google Scholar]
- 20. Spodnick GJ, Berg J, Rand WM, et al. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978‐1988). J Am Vet Med Assoc 1992;200:995–999. [PubMed] [Google Scholar]
- 21. Tjalma RA. Canine bone sarcoma: Estimation of relative risk as a function of body size. J Natl Cancer Inst 1966;36:1137–1150. [PubMed] [Google Scholar]
- 22. Wolke RE, Nielsen SW. Site incidence of canine osteosarcoma. J Small Anim Pract 1966;7:489–492. [DOI] [PubMed] [Google Scholar]
- 23. Kansara M, Thomas DM. Molecular pathogenesis of osteosarcoma. DNA Cell Biol 2007;26:1–18. [DOI] [PubMed] [Google Scholar]
- 24. Janeway KA, Walkley CR. Modeling human osteosarcoma in the mouse: From bedside to bench. Bone 2010;47:859–865. [DOI] [PubMed] [Google Scholar]
- 25. Rainusso N, Wang LL, Yustein JT. The adolescent and young adult with cancer: State of the art ‐ bone tumors. Curr Oncol Rep 2013;15:296–307. [DOI] [PubMed] [Google Scholar]
- 26. Ando K, Heymann MF, Stresing V, et al. Current therapeutic strategies and novel approaches in osteosarcoma. Cancers 2013;5:591–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Chun R, Kurzman ID, Couto CG, et al. Cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma: A pilot study. J Vet Intern Med 2000;14:495–498. [DOI] [PubMed] [Google Scholar]
- 28. Chun R, Garrett LD, Henry C, et al. Toxicity and efficacy of cisplatin and doxorubicin combination chemotherapy for the treatment of canine osteosarcoma. J Am Anim Hosp Assoc 2005;41:382–387. [DOI] [PubMed] [Google Scholar]
- 29. Coley WB. Contribution to the knowledge of sarcoma. Ann Surg 1891;14:199–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Coley WB. The treatment of malignat tumors by repeated inoculations of erysipelas: With a report of ten original cases. Am J Med Sci 1893;105:487–511. [PubMed] [Google Scholar]
- 31. Coley WB. The treatment of inoperable sarcoma by bacterial toxins (the mixed toxins of the Streptococcus erysipelas and the Bacillus prodigiosus). Proc R Soc Med 1910;3:1–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cann SAH, van Netten JP, van Netten C. Dr William Coley and tumour regression: A place in history or in the future. Postgrad Med J 2003;79:672–680. [PMC free article] [PubMed] [Google Scholar]
- 33. Mehl ML, Withrow SJ, Sequin B, et al. Spontaneous regression of osteosarcoma in four dogs. J Am Vet Med Assoc 2001;219:614–617. [DOI] [PubMed] [Google Scholar]
- 34. Thrall DE, Withrow SJ, Powers PE, et al. Radiotherapy prior to cortical allograft limb sparing in dogs with osteosarcoma: A dose response assay. Int J Radiat Oncol Biol Phys 1990;18:1351–1357. [DOI] [PubMed] [Google Scholar]
- 35. Lascelles BDX, Dernell WS, Correa MT, et al. Improved survival associated with postoperative wound infection in dogs treated with limb‐salvage surgery for osteosarcoma. Ann Surg Oncol 2005;12:1073–1083. [DOI] [PubMed] [Google Scholar]
- 36. Liptak JM, Dernell WS, Ehrhart N, et al. Cortical allograft and endoprosthesis for limb‐sparing surgery in dogs with distal radial osteosarcoma: A prospective clinical comparison of two different limb‐sparing techniques. Vet Surg 2006;35:518–533. [DOI] [PubMed] [Google Scholar]
- 37. Culp WTN, Olea‐Popelka F, Sefton J, et al. Evaluation of outcome and prognostic factors for dogs living greater than one year after diagnosis of osteosarcoma: 90 cases (1997–2008). J Am Vet Med Assoc 2014;245:1141–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Jeys LM, Grimer RJ, Carter SR, et al. Post operative infection and increased survival in osteosarcoma patients: Are they associated? Ann Surg Oncol 2007;14:2887–2895. [DOI] [PubMed] [Google Scholar]
- 39. Lee JA, Kim MS, Kim DH, et al. Postoperative infection and survival in osteosarcoma patients. Ann Surg Oncol 2009;16:147–151. [DOI] [PubMed] [Google Scholar]
- 40. Sottnik JL, U'Ren LW, Thamm DH, et al. Chronic bacterial osteomyelitis suppression of tumor growth requires innate immune responses. Cancer Immunol Immunother 2010;59:367–378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Brodey RS, Fidler IJ, Bech‐Nielsen S. Correlation of in vitro immune response with clinical course of malignant neoplasia in dogs. Am J Vet Res 1975;36:75–80. [PubMed] [Google Scholar]
- 42. Fidler IJ, Brodey RS, Bech‐Nielsen S. In vitro immune stimulation‐inhibition to spontaneous canine tumors of various histologic types. J Immunol 1974;112:1051–1060. [PubMed] [Google Scholar]
- 43. Bech‐Nielsen S, Reif JS, Brodey RS. Pre‐and postoperative studies of in vitro cell‐mediated reactivity in canine osteosarcoma. Am J Vet Res 1978;39:87–93. [PubMed] [Google Scholar]
- 44. Bech‐Nielsen S, Brodey RS, Fidler IJ, et al. The effect of BCG on in vitro immune reactivity and clinical course in dogs treated surgically for osteosarcoma. Eur J Cancer 1977;13:33–41. [DOI] [PubMed] [Google Scholar]
- 45. Segal‐Eiras A, Robins RA, Hannant D, et al. Circulating immune complexes in dogs with osteosarcoma. Br J Cancer 1982;46:444–447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Haines DM, Bruland OS. Immunohistochemical detection of osteosarcoma‐associated antigen in canine osteosarcoma. Anticancer Res 1989;9:903–908. [PubMed] [Google Scholar]
- 47. Haines DM, Bruland OS, Matte G, et al. Immunoscintigraphic detection of primary and metastatic spontaneous canine osteosarcoma with F(ab')2 fragments of osteosarcoma‐associated monoclonal antibody TP‐1. Anticancer Res 1992;12:2151–2158. [PubMed] [Google Scholar]
- 48. Page RL, Garg PK, Garg S, et al. PET imaging of osteosarcoma in dogs using a fluorine‐18‐labeled monoclonal antibody Fab fragment. J Nucl Med 1994;35:1506–1513. [PubMed] [Google Scholar]
- 49. Biller BJ, Elmslie RE, Burnett RC, et al. Use of FoxP3 expression to identify regulatory T cells in healthy dogs and dogs with cancer. Vet Immunol Immunopathol 2007;116:69–78. [DOI] [PubMed] [Google Scholar]
- 50. O'Neill K, Guth A, Biller B, et al. Changes in regulatory T cells in dogs with cancer and associations with tumor type. J Vet Intern Med 2009;23:875–881. [DOI] [PubMed] [Google Scholar]
- 51. Rissetto KC, Rindt H, Selting KA, et al. Cloning and expression of canine CD25 for validation of an anti‐human CD25 antibody to compare T regulatory lymphocytes in healthy dogs and dogs with osteosarcoma. Vet Immunol Immunopathol 2010;135:137–145. [DOI] [PubMed] [Google Scholar]
- 52. Biller BJ, Guth A, Burton JH, et al. Decreased ratio of CD8+ T cells to regulatory T cells associated with decreased survival in dogs with osteosarcoma. J Vet Intern Med 2010;24:1118–1123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Sherger M, Kisseberth W, London C, et al. Identification of myeloid derived suppressor cells in the peripheral blood of tumor bearing dogs. BMC Vet Res 2012;8:209–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Goulart MR, Pluhar GE, Ohlfest JR. Identification of myeloid derived suppressor cells in dogs with naturally occurring cancer. PLoS One 2012;7:e33274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Sottnik JL, Rao S, Lafferty MH, et al. Association of blood monocyte and lymphocyte count and disease‐free interval in dogs with osteosarcoma. J Vet Intern Med 2010;24:1439–1444. [DOI] [PubMed] [Google Scholar]
- 56. Selmic LE, Lafferty MH, Kamstock DA, et al. Outcome and prognostic factors for osteosarcoma of the maxilla, mandible, or calvarium in dogs: 183 cases (1986‐2012). J Am Vet Med Assoc 2014;245:930–938. [DOI] [PubMed] [Google Scholar]
- 57. Wasserman J, Diese L, VanGundy Z, et al. Suppression of canine myeloid cells by soluble factors from cultured canine tumor cells. Vet Immunol Immunopathol 2010;145:420–430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Luca S, Mihaescu T. History of BCG vaccine. Maedica 2013;8:53–58. [PMC free article] [PubMed] [Google Scholar]
- 59. Gandhi NM, Morales A, Lamm DL. Bacillus Calmette‐Guérin immunotherapy for genitourinary cancer. BJU Int 2013;112:288–297. [DOI] [PubMed] [Google Scholar]
- 60. Kawai K, Miyazaki J, Joraku A, et al. Bacillus Calmette‐Guerin (BCG) immunotherapy for bladder cancer: Current understanding and perspectives on engineered BCG vaccine. Cancer Sci 2013;104:22–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Owen LN, Bostock DE. Effects of intravenous BCG in normal dogs and in dogs with spontaneous osteosarcoma. Eur J Cancer 1974;10:775–780. [DOI] [PubMed] [Google Scholar]
- 62. Owen LN, Bostock DE, Lavelle RB. Studies on chemotherapy and immunotherapy in canine lymphosarcoma and osteosarcoma. Bibl Haematol 1975;43:522–523. [DOI] [PubMed] [Google Scholar]
- 63. Betton GR, Gorman NT, Owen LN. Cell mediated cytotoxicity in dogs following systemic or local BCG treatment alone or in combination with allogeneic tumour cell lines. Eur J Cancer 1979;15:745–754. [DOI] [PubMed] [Google Scholar]
- 64. Betton GR, Gorman NT. Cell‐mediated responses in dogs with spontaneous neoplasms. I. Detection of cell‐mediated cytotoxicity by the chromium‐51 release assay. J Natl Cancer Inst 1978;61:1085–1093. [PubMed] [Google Scholar]
- 65. Gorman NT. Alveolar macrophage cytotoxicity in dogs following intravenous BCG. Eur J Cancer 1979;15:1051–1059. [DOI] [PubMed] [Google Scholar]
- 66. Ugel S, Peranzoni E, Desantis G, et al. Immune tolerance to tumor antigens occurs in a specialized environment of the spleen. Cell Rep 2012;2:628–639. [DOI] [PubMed] [Google Scholar]
- 67. Meyer JA, Dueland RT, MacEwen EG, et al. Canine osteogenic sarcoma treated by amputation and MER: An adverse effect of splenectomy on survival. Cancer 1982;49:1613–1616. [DOI] [PubMed] [Google Scholar]
- 68. Schroit AJ, Fidler IJ. Effects of liposome structure and lipid composition on the activation of the tumoricidal properties of macrophages by liposomes containing muramyl dipeptide. Cancer Res 1982;42:161–167. [PubMed] [Google Scholar]
- 69. Kleinerman ES, Jia SF, Griffin J, et al. Phase II study of liposomal muramyl tripeptide in osteosarcoma: The cytokine cascade and monocyte activation following administration. J Clin Oncol 1992;10:1310–1316. [DOI] [PubMed] [Google Scholar]
- 70. Kleinerman ES, Murray JL, Snyder JS, et al. Activation of tumoricidal properties in monocytes from cancer patients following intravenous administration of liposomes containing muramyl tripeptide phosphatidylethanolamine. Cancer Res 1989;49:4665–4670. [PubMed] [Google Scholar]
- 71. Asano T, McWatters A, An T, et al. Liposomal muramyl tripeptide up‐regulates interleukin‐1 alpha, interleukin‐1 beta, tumor necrosis factor‐alpha, interleukin‐6 and interleukin‐8 gene expression in human monocytes. J Pharmacol Exp Ther 1994;268:1032–1039. [PubMed] [Google Scholar]
- 72. Kleinerman ES, Gano JB, Johnston DA, et al. Efficacy of liposomal muramyl tripeptide (CGP 19835A) in the treatment of relapsed osteosarcoma. Am J Clin Oncol 1995;18:93–99. [DOI] [PubMed] [Google Scholar]
- 73. Meyers PA, Schwartz CL, Krailo M, et al. Osteosarcoma: A randomized, prospective trial of the addition of ifosfamide and/or muramyl tripeptide to cisplatin, doxorubicin, and high‐dose methotrexate. J Clin Oncol 2005;23:2004–2011. [DOI] [PubMed] [Google Scholar]
- 74. Smith BW, Kurzman ID, Schultz KT, et al. Muramyl peptides augment the in vitro and in vivo cytostatic activity of canine plastic‐adherent mononuclear cells against canine osteosarcoma cells. Cancer Biother Radiopharm 1993;8:137–144. [DOI] [PubMed] [Google Scholar]
- 75. Kurzman ID, Shi F, MacEwen EG. In vitro and in vivo canine mononuclear cell production of tumor necrosis factor induced by muramyl peptides and lipopolysaccharide. Vet Immunol Immunopathol 1993;38:45–56. [DOI] [PubMed] [Google Scholar]
- 76. Shi F, MacEwen EG, Kurzman ID. In vitro and in vivo effect of doxorubicin combined with liposome‐encapsulated muramyl tripeptide on canine monocyte activation. Cancer Res 1993;53:3986–3991. [PubMed] [Google Scholar]
- 77. Kurzman ID, Shi F, Vail DM, MacEwen EG. In vitro and in vivo enhancement of canine pulmonary alveolar macrophage cytotoxic activity against canine osteosarcoma cells. Cancer Biother Radiopharm 1999;14:121–128. [DOI] [PubMed] [Google Scholar]
- 78. MacEwen EG, Kurzman ID, Rosenthal RC, et al. Therapy for osteosarcoma in dogs with intravenous injection of liposome‐encapsulated muramyl tripeptide. J Natl Cancer Inst 1989;81:935–938. [DOI] [PubMed] [Google Scholar]
- 79. Kurzman ID, MacEwen EG, Rosenthal RC, et al. Adjuvant therapy for osteosarcoma in dogs: Results of randomized clinical trials using combined liposome‐encapsulated muramyl tripeptide and cisplatin. Clin Cancer Res 1995;1:1595–1601. [PubMed] [Google Scholar]
- 80. Mitchell DH, Withrow SJ, Johnston MR, et al. Cytotoxicity against autologous, allogeneic, and xenogeneic tumor targets by human recombinant interleukin‐2‐activated lymphocytes from healthy dogs and dogs with lung tumors. Am J Vet Res 1991;52:1132–1136. [PubMed] [Google Scholar]
- 81. Ohnishi H, Okuno K, Yasutomi M. Successful in vivo generation of canine lymphokine‐activated killer cells by continuous recombinant interleukin‐2 infusion through the splenic artery. Cancer Biother Radiopharm 1993;8:213–222. [DOI] [PubMed] [Google Scholar]
- 82. Khanna C, Hasz DE, Klausner JS, et al. Aerosol delivery of interleukin 2 liposomes is nontoxic and biologically effective: Canine studies. Clin Cancer Res 1996;2:721–734. [PubMed] [Google Scholar]
- 83. Khanna C, Anderson PM, Hasz DE, et al. Interleukin‐2 liposome inhalation therapy is safe and effective for dogs with spontaneous pulmonary metastases. Cancer 1997;79:1409–1421. [DOI] [PubMed] [Google Scholar]
- 84. Dow S, Elmslie R, Kurzman I, et al. Phase I study of liposome‐DNA complexes encoding the interleukin‐2 gene in dogs with osteosarcoma lung metastases. Hum Gene Ther 2005;16:937–946. [DOI] [PubMed] [Google Scholar]
- 85. Visonneau S, Cesano A, Jeglum KA, et al. Adjuvant treatment of canine osteosarcoma with the human cytotoxic T‐cell line TALL‐104. Clin Cancer Res 1999;5:1868–1875. [PubMed] [Google Scholar]
- 86. Mata M, Vera JF, Gerken C, et al. Toward immunotherapy with redirected T cells in a large animal model: Ex vivo activation, expansion, and genetic modification of canine T cells. J Immunother 2014;37:407–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Koshkina NV, Khanna C, Mendoza A, et al. Fas‐negative osteosarcoma tumor cells are selected during metastasis to the lungs: The role of the Fas pathway in the metastatic process of osteosarcoma. Mol Cancer Res 2007;5:991–999. [DOI] [PubMed] [Google Scholar]
- 88. Gordon N, Koshkina NV, Jia SF, et al. Corruption of the Fas pathway delays the pulmonary clearance of murine osteosarcoma cells, enhances their metastatic potential, and reduces the effect of aerosol gemcitabine. Clin Cancer Res 2007;13:4503–4510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Modiano JF, Bellgrau D, Cutter GR, et al. Inflammation, apoptosis, and necrosis induced by neoadjuvant Fas ligand gene therapy improves survival of dogs with spontaneous bone cancer. Mol Ther 2012;20:2234–2243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rodriguez CO Jr, Crabbs TA, Wilson DW, et al. Aerosol gemcitabine: Preclinical safety and in vivo antitumor activity in osteosarcoma‐bearing dogs. J Aerosol Med Pulm Drug Deliv 2010;23:197–206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Thamm DH, Kurzman ID, King I, et al. Systemic administration of an attenuated, tumor‐targeting Salmonella typhimurium to dogs with spontaneous neoplasia: Phase I evaluation. Clin Cancer Res 2005;11:4827–4834. [DOI] [PubMed] [Google Scholar]
- 92. Gentschev I, Patil SS, Adelfinger M, et al. Characterization and evaluation of a new oncolytic vaccinia virus strain LIVP6.1.1 for canine cancer therapy. Bioengineered 2013;4:84–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Finocchiaro LME, Villaverde MS, Gil‐Cardeza ML, et al. Cytokine‐enhanced vaccine and interferon‐beta plus suicide gene as combined therapy for spontaneous canine sarcomas. Res Vet Sci 2001;91:230–234. [DOI] [PubMed] [Google Scholar]
- 94. Peruzzi D, Mesiti G, Ciliberto G, et al. Telomerase and HER‐2/neu as targets of genetic cancer vaccines in dogs. Vaccine 2010;28:1201–1208. [DOI] [PubMed] [Google Scholar]


