Abstract
Background
Hypernatremia has been associated with substantial morbidity and death in human patients. The incidence and importance of hypernatremia in dogs and cats has not been determined.
Hypothesis/Objectives
To describe the incidence of and prognosis associated with hypernatremia in dogs and cats at a university teaching hospital.
Animals
A total of 16,691 dogs and 4,211 cats with measured blood or serum sodium concentration.
Methods
Retrospective study. Medical records of animals with a blood or serum sodium concentration measured during a 60‐month period were reviewed to determine the severity of hypernatremia and its associated case fatality rate. Cases with moderate (11–15 mmol/L above the reference range) or severe hypernatremia (≥16 mmol/L above the reference range) were further reviewed.
Results
A total of 957 dogs (5.7%) and 338 cats (8.0%) were diagnosed with hypernatremia. Case fatality rates of dogs and cats with hypernatremia was 20.6 and 28.1%, respectively compared to 4.4 and 4.5% with a normal blood or serum sodium concentration (P < .0001). The magnitude of hypernatremia was linearly associated with a higher case fatality rate (P < .0001). Hypernatremia was associated with a higher case fatality rate than hyponatremia. Among the animals with moderate or severe hypernatremia, 50% of dogs and 38.5% of cats presented with community‐acquired hypernatremia, and 50% of dogs and 61.5% of cats developed hospital‐acquired hypernatremia.
Conclusions and clinical importance
Hypernatremia was found infrequently in this population but was associated with increased case fatality rates in dogs and cats. Presence and severity of hypernatremia might be useful as a prognostic indicator.
Keywords: Dysnatremia, Osmolality, Sodium, Water balance
Abbreviations
- ADH
antidiuretic hormone
- CDI
central diabetes insipidus
- ECF
extracellular fluid
- GI
gastrointestinal
- ICF
intracellular fluid
- ICU
intensive care unit
- NDI
nephrogenic diabetes insipidus
Hypernatremia has been associated with substantial morbidity and death in human patients.1, 2 The incidence of hypernatremia is 1–3% in all hospitalized human patients and 6–26% in patients treated in medical and surgical intensive care units (ICU).3, 4, 5, 6, 7, 8, 9, 10 Among human ICU patients with hypernatremia only 23% had hypernatremia present on admission and 77% of patients developed hypernatremia during the ICU stay.7, 11 Hypernatremia on admission or acquired during hospitalization was found to be an independent risk factor for fatality rate in these studies.7, 11 Even mild increases in serum sodium levels have been associated with increased fatality rate compared with normonatremic human patients.10, 11
To date, no studies have reported the overall incidence, animal characteristics or causes of hypernatremia in veterinary medicine. The objective of this retrospective study was to evaluate the epidemiology of hypernatremia, primarily focusing on the overall incidence of this abnormality, concurrent underlying diseases and potential pathophysiologic factors contributing to development of hypernatremia, and the associated morbidity and fatality rate in dogs and cats.
Materials and Methods
We used computerized medical records to identify all dogs and cats that had blood or serum sodium concentration measured on a blood gas determination or serum chemistry profile during a 60‐month period (January 1, 2008 to December 31, 2012) at the University of California, Davis, William R. Pritchard, Veterinary Medical Teaching Hospital. This study included any dogs and cats that had a blood or serum sodium concentration measured at our institution during the specified time period.
Measurements
Blood or serum samples for sodium concentrations were measured using a point‐of‐care blood gas analyzer1 or 1 of 2 diagnostic laboratory biochemical analyzers.2 , 3 At our institution, heparinized blood samples are measured for sodium as well as acid base parameters and other electrolytes immediately after sample collection using a point‐of‐care blood gas analyzer.1 Alternatively, serum is submitted to the diagnostic laboratory for analysis within 12 hour of sample collection. Using the point‐of‐care blood gas analyzer, the reference range for blood sodium concentration in dogs and cats were 145–153 and 150–155 mmol/L, respectively. Using the diagnostic laboratory, the reference range for the sodium concentration in dogs were 143–151 mmol/L before January 05, 2011 and 145–154 mmol/L thereafter. The reference range for serum sodium concentration in cats using the diagnostic laboratory was 151–158 mmol/L throughout the study period.
The medical records of all identified dogs and cats with at least 1 blood or serum sodium concentration measured were reviewed to determine the overall incidence and outcome of those animals with hypernatremia. Animals with a blood or serum sodium concentration greater than the reference range were identified and categorized as borderline (≤5 mmol/L higher than the highest reference concentration), mild (6–10 mmol/L higher than the highest reference concentration), moderate (11–15 mmol/L higher than the highest reference concentration), or severe (≥16 mmol/L higher than the highest reference concentration) hypernatremia. The categorization used for severity of hypernatremia was chosen based on previous studies in human patients with some minor modifications.10, 11 Animals with a blood or serum sodium concentration lower than the reference range were categorized as hyponatremia in a similar manner and these results are presented in a companion article.12 Animals could only be enrolled in the study once and only the first occurrence of hypernatremia identified in hospital was included. The medical records of identified animals with moderate or severe hypernatremia were further reviewed to determine the primary disease processes, potential pathophysiologic factors contributing to the development of hypernatremia, time of onset (community versus hospital‐acquired), presence or absence of treatment given by referring veterinarian before presentation, hydration status, intravascular volume status, and clinical signs noted when the animals developed hypernatremia. The primary disease process was determined by the primary clinicians and categorized based on major organ system affected (respiratory, cardiovascular, neurological, musculoskeletal, gastrointestinal, hepatobilliary, urological, pancreatic, and reproductive systems). The specific diseases of neoplasia, diabetes mellitus and sepsis were included separately either because they have multi‐organ effects and could not be simply categorized to an organ system or because they have specific relevance to hypernatremia. In addition, efforts were made to keep the categories used for primary disease processes consistent for both hypernatremia and hyponatremia12 studies to facilitate comparison between the 2 groups of animals.
If hypernatremia was detected on the first blood sample in an admission period and no medical treatment was given by a referring veterinarian before presentation, it was categorized as community‐acquired. If hypernatremia was not evident on admission blood sampling but was detected at a later point during hospitalization, it was categorized as hospital‐acquired. In animals that had hypernatremia on admission but had received medical treatment by a referring veterinarian before presentation, the time of onset of hypernatremia was classified unknown. Hydration and intravascular volume status were based on the clinicians’ description of the animals in the medical records on the day hypernatremia was first noted. The medical record also was searched for evidence of any predefined pathophysiologic factors considered to be possible contributors to the development of hypernatremia. Clinical signs noted when hypernatremia was detected were retrieved from the history and physical examination findings when the animals developed hypernatremia.
Statistical Analysis
The proportion of animals that either died or were euthanized that had hypernatremia at least once upon admission or during hospitalization, was compared to animals with a normal sodium concentration by Chi‐square analysis using commercially available software.4 Animals with hyponatremia were excluded from this analysis. Animals then were stratified into either severe, moderate, mild, borderline hypernatremia, or normal sodium concentration. The degree of sodium abnormality and outcome were compared by Chi‐square analysis for trend. In post hoc analysis, case fatality rate for borderline hypernatremia was compared to case fatality rate of animals with a normal sodium concentration by Chi‐square analysis. P < .05 was considered significant.
Results
During the 60‐month study period, 16,691 dogs and 4,211 cats were identified in which ≥1 blood samples were analyzed for blood or serum sodium concentration. Of these 957 (5.7%) dogs and 338 (8.0%) cats were classified as having hypernatremia. The animal distributions in the categories of hypernatremia are listed in Table 1. The highest sodium concentration measured was 188 mmol/L in dogs and 214 mmol/L in cats.
Table 1.
Incidence of abnormal blood or serum sodium concentration in dogs and cats at a veterinary medical teaching hospital.
| Dogs N (%) | Cats N (%) | |
|---|---|---|
| Total | 16,691 | 4,211 |
| Normal sodium | 11,480 (68.8) | 1,792 (42.6) |
| All hypernatremia | 957 (5.7) | 338 (8) |
| Severe | 27 (0.2) | 10 (0.2) |
| Moderate | 29 (0.2) | 10 (0.2) |
| Mild | 105 (0.6) | 45 (1) |
| Borderline | 796 (4.8) | 273 (6.5) |
| All hyponatremia | 4,254 (25.5) | 2,081 (49.4) |
Borderline hypernatremia, ≤5 mmol/L higher than the highest reference concentration; mild hypernatremia, 6–10 mmol/L higher than the highest reference concentration; moderate hypernatremia, 11–15 mmol/L higher than the highest reference concentration; severe hypernatremia, ≥16 mmol/L higher than the highest reference concentration.
Case fatality rates in dogs and cats with hypernatremia were 20.6% (197/957) and 28.1% (95/338) respectively. This is in comparison to case fatality rates of 4.4% (500/11,480) in dogs and 4.5% (81/1,792) in cats with a normal sodium concentration, and case fatality rates associated with hyponatremia of 13.7% (581/4,254) in dogs and 11.9% (248/2,081) in cats from the same population.12 Hypernatremia was associated with a higher case fatality rate than having a normal sodium concentration or hyponatremia in both dogs and cats (P < .0001). The odds ratio (95% CI) of nonsurvival with all degrees of hypernatremia was 5.7 (4.8–6.8) in dogs and 8.3 (6.0–11.4) in cats. In addition, the magnitude of hypernatremia was linearly associated with higher case fatality rate in both dogs and cats (P < .0001; Fig 1). Borderline hypernatremia in both dogs and cats was associated with nonsurvival (P < .0001).
Figure 1.
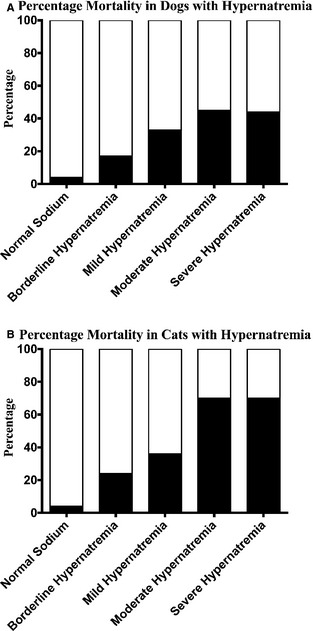
Percent death among various degrees of hypernatremia in dogs (A) and cats (B). There is a linear association of increasing case fatality rate with greater hypernatremia P < .0001. Hypernatremia was categorized as borderline (≤5 mmol/L higher than the highest reference concentration), mild (6–10 mmol/L higher than the highest reference concentration), moderate (11–15 mmol/L higher than the highest reference concentration), or severe (≥16 mmol/L higher than the highest reference concentration). The white area of each column represents the percentage of survivors and the black area represents the percentage of death.
Among the animals with moderate or severe hypernatremia, 71.4% (40/56) dogs could be classified according to the timing of development of hypernatremia. Of these dogs, 50% (20/40) presented with community‐acquired whereas 50% (20/40) of dogs developed hospital‐acquired hypernatremia. Twenty eight percent of dogs (16/56) had received medical treatment by a referring veterinarian before presentation to our hospital and the time of onset of hypernatremia was considered unknown. In cats with moderate or severe hypernatremia, 65% (13/20) could be classified according to the timing of development of hypernatremia. Of these, 38.5% (5/13) presented with community‐acquired and 61.5% (8/13) developed hospital‐acquired hypernatremia. Thirty‐five percent of cats (7/20) had received medical treatment by a referring veterinarian before presentation to our hospital and the time of onset of hypernatremia was considered unknown.
The primary disease processes of the animals with moderate or severe hypernatremia are shown in Table 2. The most frequent disease processes identified in dogs were neurological (14/56, 21.4%), neoplastic (12/56, 21.4%), and respiratory (11/56, 19.6%). In cats with moderate or severe hypernatremia, the most frequent disease processes were urological (11/20, 55%), neurological (5/20, 25%), and hyperthyroidism (4/20, 20%). In both dogs and cats, many animals had ≥1 concurrent disease process.
Table 2.
Primary disease processes of dogs and cats identified with moderate or severe hypernatremia. Note, individual animals may have >1 condition
| Primary Disease Processes | Dogs with Hypernatremia N (%) | Cats with Hypernatremia N (%) | ||||
|---|---|---|---|---|---|---|
| Moderate | Severe | Total | Moderate | Severe | Total | |
| Total | 29 | 27 | 56 | 10 | 10 | 20 |
| Neurological | 5 (17.2) | 9 (33.3) | 14 (25) | 2 (20) | 3 (30) | 5 (25) |
| Neoplasia | 6 (20.7) | 6 (22.2) | 12 (21.4) | 1 (10) | 0 | 1 (5) |
| Respiratory | 7 (24.1) | 4 (14.8) | 11 (19.6) | 0 | 2 (20) | 2 (10) |
| Hepatobiliary | 6 (20.7) | 4 (14.8) | 10 (17.9) | 2 (20) | 1 (10) | 3 (15) |
| Gastrointestinal | 5 (17.2) | 2 (7.4) | 7 (12.5) | 0 (6) | 1 (10) | 1 (5) |
| Urological | 5 (17.2) | 2 (7.4) | 7 (12.5) | 5 (50) | 6 (60) | 11 (55) |
| Pancreatic | 1 (3.5) | 5 (18.5) | 6 (10.7) | 0 | 0 | 0 |
| DM/DKA | 5 (17.2) | 0 | 5 (8.9) | 0 | 0 | 0 |
| Musculoskeletal | 1 (3.5) | 2 (7.1) | 3 (5.4) | 0 | 0 | 0 |
| Cardiovascular | 0 | 2 (7.4) | 2 (3.6) | 0 | 1 (10) | 1 (5) |
| Reproductive | 0 | 0 | 0 | 0 | 0 | 0 |
| Sepsis | 0 | 0 | 0 | 1 (10) | 1 (10) | 2 (10) |
DM, diabetes mellitus; DKA, diabetic ketoacidosis.
Moderate hypernatremia, 11–15 mmol/L higher than the highest reference concentration; severe hypernatremia, ≥16 mmol/L higher than the highest reference concentration.
Pathophysiologic factors potentially contributing to the development of hypernatremia are shown in Table 3. In dogs, gastrointestinal (GI) fluid loss caused by vomiting and diarrhea was the most common identified factor (22/56, 39.3%), followed by central diabetes insipidus (CDI) (13/56, 23.2%), and fever or hyperthermia (13/56, 23.2%). In cats, the most common pathophysiologic factors potentially contributing to the development of hypernatremia were chronic kidney disease (6/20, 30%) and nonoliguric acute kidney injury (5/20, 25%), followed by GI fluid loss (5/20, 25%). Many animals had ≥1 pathophysiologic factor potentially leading to hypernatremia.
Table 3.
Potential pathophysiologic factors that might have contributed to development of hypernatremia in dogs and cats identified with moderate or severe hypernatremia. Note that individual animals may have >1 condition
| Pathophysiologic Factors | Dogs with Hypernatremia N (%) | Cats with Hypernatremia N (%) | ||||
|---|---|---|---|---|---|---|
| Moderate | Severe | Total | Moderate | Severe | Total | |
| Total | 29 | 27 | 56 | 10 | 10 | 20 |
| GI loss | 12 (41.4) | 10 (37) | 22 (39.3) | 2 (20) | 3 (30) | 5 (25) |
| CDI | 2 (6.9) | 11 (40.7) | 13 (23.2) | 0 | 0 | 0 |
| Fever/Hyperthermia | 5 (17.2) | 8 (29.6) | 13 (23.2) | 0 | 1 (10) | 1 (5) |
| NDI | 5 (17.2) | 1 (3.7) | 6 (10.7) | 0 | 0 | 0 |
| DM/DKA | 4 (13.8) | 1 (3.7) | 5 (8.9) | 0 | 0 | 0 |
| Hyperadrenocorticism | 1 (3.4) | 3 (11.1) | 4 (7.1) | 0 | 0 | 0 |
| Mannitol infusion | 2 (6.9) | 2 (7.4) | 4 (7.1) | 0 | 0 | 0 |
| Nonoliguric acute renal failure | 2 (6.9) | 1 (3.7) | 3 (5.4) | 3 (30) | 2 (20) | 5 (25) |
| Hypertonic fluid administration | 2 (6.9) | 1 (3.7) | 3 (5.4) | 1 (10) | 0 | 0 |
| Third space loss | 2 (6.9) | 1 (3.7) | 3 (5.4) | 0 | 0 | 0 |
| Primary hypodipsia | 0 | 2 (7.4) | 2 (3.6) | 0 | 0 | 0 |
| Chronic renal failure | 0 | 1 (3.7) | 1 (1.8) | 3 (30) | 3 (30) | 6 (30) |
| Diuretic administration | 0 | 1 (3.7) | 1 (1.8) | 0 | 0 | 0 |
| Postobstructvive diuresis | 0 | 0 | 0 | 0 | 1 (10) | 1 (5) |
| Inadequate access to water | 0 | 0 | 0 | 1 (10) | 0 | 1 (5) |
| Cutaneous loss | 0 | 0 | 0 | 0 | 0 | 0 |
| Salt poisoning | 0 | 0 | 0 | 0 | 0 | 0 |
| Hyperaldosteronism | 0 | 0 | 0 | 0 | 0 | 0 |
| Unknown | 6 (20.7) | 4 (14.8) | 10 (17.9) | 4 (40) | 2 (20) | 6 (30) |
CDI, central diabetes insipidus; DM, diabetes mellitus; DKA, diabetic ketoacidosis GI, gastrointestinal; NDI, neprhogenic diabetes insipidus.
Moderate hypernatremia, 11–15 mmol/L higher than the highest reference concentration; severe hypernatremia, ≥16 mmol/L higher than the highest reference concentration.
Signs of dehydration were noted in the medical records in 44.6% (25/56) of dogs and 90% (18/20) of cats at the time moderate or severe hypernatremia was identified. No overhydrated dogs and cats were identified in this population. Of dogs with moderate or severe hypernatremia, 26.8% (15/56) showed clinical signs of hypovolemia, 71.4% (40/56) were considered euvolemic, and 1.8% (1/56) had signs of hypervolemia. In cats, 55% (11/20) showed signs of hypovolemia, while 45% (9/20) were considered euvolemic when hypernatremia was first identified. No hypervolemic cats were identified.
The most frequent clinical signs of dogs when moderate or severe hypernatremia were identified included obtundation (27/56, 48.2%), vomiting (22/56, 39.3%), and lethargy (7/56, 25%). Cats with moderate or severe hypernatremia showed lethargy (13/23, 56.5%), obtundation (13/23, 56.5%), and vomiting (8/23, 34.8%) at the time of diagnosis (Table 4).
Table 4.
Clinical signs present at the time of identification of moderate or severe hypernatremia in dogs and cats. Note that individual animals may have >1 clinical sign
| Clinical Signs | Dogs with Hypernatremia N (%) | Cats with Hypernatremia N (%) | ||||
|---|---|---|---|---|---|---|
| Moderate | Severe | Total | Moderate | Severe | Total | |
| Total | 29 | 27 | 56 | 10 | 10 | 20 |
| Obtundation | 15 (51.7) | 12 (44.4) | 27 (48.2) | 5 (50) | 7 (70) | 12 (60) |
| Vomiting | 13 (44.8) | 9 (33.3) | 22 (39.3) | 2 (20) | 3 (30) | 5 (25) |
| Lethargy | 7 (24.1) | 7 (25.9) | 14 (25) | 7 (20) | 5 (50) | 12 (60) |
| Ataxia | 4 (13.8) | 6 (22.2) | 10 (17.9) | 0 | 2 (20) | 2 (10) |
| Weakness | 5 (17.2) | 5 (18.5) | 10 (17.9) | 0 | 3 (30) | 3 (15) |
| Stuporous | 4 (13.8) | 5 (18.5) | 9 (16.1) | 2 (20) | 2 (20) | 4 (20) |
| Seizure | 3 (10.3) | 5 (18.5) | 8 (14.3) | 0 | 1 (10) | 1 (5) |
| Tremor | 3 (10.3) | 2 (7.4) | 5 (8.9) | 0 | 0 | 0 |
| Comatose | 2 (6.9) | 0 | 2 (3.6) | 0 | 0 | 0 |
| None | 3 (10.3) | 5 (18.5) | 8 (14.3) | 1 (10) | 0 | 1 (5) |
Moderate hypernatremia, 11–15 mmol/L higher than the highest reference concentration; severe hypernatremia ≥16 mmol/L higher than the highest reference concentration.
Discussion
In this study, the overall case fatality rates of dogs and cats with hypernatremia was significantly higher than those animals with a normal sodium concentration. In addition, case fatality rate linearly increased with increasing sodium concentration. An increased risk of death in animals with hypernatremia in this study was present even with borderline abnormalities (a sodium concentration ≥5 mmol/L higher than the highest reference concentration). There are similar relationships in human patients at hospital admission, in patients hospitalized in the general ward as well as in ICU patients.2, 10, 11, 13, 14 All of these human studies have found an independent effect of ICU‐acquired hypernatremia on death after adjusting for other risk factors. However, it is unknown if hypernatremia has a direct effect on death or if it is purely a marker of disease severity. Previous human studies demonstrated that concomitant sepsis or pneumonia and increasing rates of fluid replacement were important prognostic indicators of death with hypernatremia.4, 15, 16, 17 On the other hand, the hyperosmolar state associated with hypernatremia alters a variety of cellular functions, which might contribute to metabolic, neurological, and cardiovascular complications.2, 18, 19, 20 Therefore, the association of hypernatremia with death is likely to be a combination of the effects of underlying organ dysfunction and harmful consequences of the hypernatremia itself. Interestingly, a recent study found that correction of hypernatremia was associated with improved survival in critically ill human patients.21 This finding also might imply that hypernatremia directly affects human patient death.
Although hypernatremia in this group of dogs and cats was much less common than hyponatremia in the same population, it was associated with a higher case fatality rate.12 Hypernatremia was uncommon in noncritically ill human patients with a prevalence of 0.2% upon admission and 1.0% for patients developing it during their hospital stays.3 In contrast, hypernatremia is a relatively common finding in critically ill human patients treated in ICU.2, 6, 7, 13, 14 The overall incidence of hypernatremia in this study was similar to that reported in human studies. However, as this study included a wide variety of animal types, it was difficult to directly compare these results with human studies.
Hypernatremia is more commonly acquired during hospitalization than it is to develop before admission.3, 5, 7, 11 In human patients, elderly persons with infirmity or febrile illness and infants with diarrhea have the highest risk of developing community‐acquired hypernatremia.4, 17, 19 The majority of human patients who develop hypernatremia during hospitalization have pure water or hypotonic fluid loss in urine because of urine concentration defects, primarily as a results of the use of diuretics or solute diuresis.3, 7, 8, 9, 22 In this study, hospital acquired moderate or severe hypernatremia was as common as community‐acquired hypernatremia in dogs. While in cats with moderate or severe hypernatremia, hospital‐acquired was more common than community‐acquired hypernatremia. Although the proportion of hospital‐acquired compared to community‐acquired hypernatremia in human patients is higher than those in dogs and cats, the trend is similar. Hospital‐acquired hypernatremia might be associated with the medical and fluid therapy provided by clinicians.3, 8, 23
There were a large variety of primary disease processes identified in this group of dogs and cats with hypernatremia, although it is not possible in this study to determine the relationship between underlying disease and development of hypernatremia. Of the primary disease processes, neurological and urological diseases have well‐identified mechanisms by which they can cause hypernatremia. Neurological disease in this study included animals with intracranial, spinal and peripheral neurological abnormalities. Abnormal mentation or decreased mobility might limit an animal's access to water, which can promote the development of hypernatremia. In addition, intracranial diseases can result in CDI, if they involve the hypothalamus, caudal pituitary gland or both.1, 2, 24 Neurological disease is also well recognized in human patients as a risk factor for development of hypernatremia.25 It is important to note that hypernatremia can cause neurological abnormalities and it was not possible in this study to differentiate neurological signs caused by the primary disease versus those that might have occurred secondary to hypernatremia.
In cats with moderate or severe hypernatremia, urological diseases were the most common primary disease process (55%). Several factors can contribute to the development of hypernatremia in urological diseases, including osmotic diuresis resulting from increased solute excretion in the remaining functioning nephrons, decreased tubular responsiveness to ADH (partial nephrogenic diabetes insipidus [NDI]), and interference with the countercurrent mechanism in disorders affecting the renal medulla. The result might be excess hypotonic fluid loss into the urine.1, 2, 24 Urological disease was not as common in hypernatremic dogs (12.5%) compared to cats, and this might reflect a lower incidence of renal disease in dogs compared to cats in our hospital population, or it might mean that the nature of renal disease in the dog differs from the cat.
In this study, 39% of dogs and 25% of cats with moderate or severe hypernatremia had episodes of GI signs such as vomiting and diarrhea. In a human study, 35% of hypernatremic patients in hospital had increased gastrointestinal fluid loss.3 Fluid deficits were evident in many animals with hypernatremia in this study. Twenty‐seven percent of dogs and 55% of cats were considered hypovolemic and 45% of dogs and 87% of cats with moderate or severe hypernatremia had signs of dehydration on physical examination when hypernatremia was identified. As body fluid loss can be hypertonic, isotonic or hypotonic, dehydration and hypovolemia can occur in association with a normal, increased or decreased sodium concentration.1 Sodium concentration might be further altered by the quantity of water intake. As a result, serum sodium concentration is not a sensitive marker of dehydration or hypovolemia. Gastrointestinal signs are common in many different primary disease processes and might explain how hypernatremia occurs in diseases such as neoplasia, where a direct cause of hypernatremia might not be obvious.
Insensible fluid loss from the respiratory tract is generally hypotonic to plasma. In people, 55% of hypernatremia patients in one study had signs of increased insensible hypotonic fluid losses because of fever, hyperthermia, and respiratory diseases.3 Fever and hyperthermia were also one of the common potential pathophysiologic factors seen in dogs with moderate or severe hypernatremia in this study (23.2%). The reference range of insensible water loss in dogs is between 20.5–26.2 ml/kg/d depending on the physiologic condition and diet.1 Fever or hyperthermia results in increased rate of water evaporation and minute ventilation, and thereby increase loss via the skin or lungs.24 It is also possible that human patients with fever or hyperthermia might have decreased water intake because of illness or altered mentation, further contributing to the development of hypernatremia.
In our study, 23% of dogs with moderate or severe hypernatremia were diagnosed with CDI and 11% were diagnosed with NDI. Central diabetes insipidus is caused by partial or complete lack of ADH production and release from the neurohypophysis. It might result from head trauma, pituitary malformation, cysts, encephalitis, or neoplasia or might be idiopathic in dogs and cats.26, 27, 28, 29 Congenital CDI is rare, but has been reported in case reports.30, 31 Nephrogenic diabetes insipidus is a congenital or acquired disorder in which hypothalamic function and ADH release are normal, but the ability to concentrate the urine is reduced because of diminished or absent renal responsiveness to ADH. Congenital NDI is a rare disorder in small animals.32 There are various causes of acquired NDI reported in human patients and small animals.1, 24, 33 It is important to note that the majority of these animals maintain water balance with a near normal sodium concentration if their thirst mechanism is intact and an adequate amount of water is provided. Hypernatremia develops when the thirst mechanism or access to water are compromised. Central and nephrogenic diabetes insipidus were not reported in cats in this study population. As the diagnosis of diabetes insipidus requires specific tests, it is likely that it would be under recognized in this retrospective study.
The clinical signs associated with hypernatremia are primarily neurological as a consequence of water movement out of brain cells. Studies in experimental animals and in humans have revealed that this decrease in brain volume causes rupture of the cerebral veins, resulting in focal intracerebral and subarachnoid hemorrhages.19, 34 The severity of clinical signs are related more to the rapidity of onset of hypernatremia than to the magnitude of hypernatremia. If hypernatremia develops slowly, the brain has time to adapt to the hypertonic state by production of intracellular solutes and thus human patients with chronic hypernatremia are relatively asymptomatic.35 Clinical signs of acute hypernatremia include anorexia, lethargy, vomiting, muscular weakness, disorientation, ataxia, seizure, coma, and death.19, 36, 37 In this study, the common clinical signs evident in animals with moderate or severe hypernatremia were obtundation (48.2% in dogs, 60% in cats), vomiting (39.3% in dogs, 25% in cats), lethargy (25% in dogs, 60% in cats), weakness (17.9% in dogs, 15% in cats), and ataxia (17.9% in dogs, 10% in cats). Some animals also showed more severe clinical signs such as stupor, coma, tremors, and seizures. However, as previously mentioned, clinical signs because of hypernatremia could not be differentiated from clinical signs of the underlying disease in this study.
Our study has limitations inherent to a retrospective study. The direct and indirect effects of medical interventions on serum sodium concentration could not be determined. Similarly, possible causes and clinical signs of hypernatremia were identified in this study, but there was no way to confirm any relationship between these findings and the occurrence of hypernatremia. The true incidence of hypernatremia in dogs and cats cannot be determined by this study, because only animals that had a blood or serum sodium concentration analyzed were included. A prospective study in which all animals presenting to a veterinary facility would have blood or serum sodium concentration measured on admission and followed during the hospital stay would be ideal.
In conclusion, hypernatremia was an infrequent abnormality in both dogs and cats at a veterinary medical teaching hospital. Hospital‐acquired hypernatremia was as common as community‐acquired hypernatremia in dogs, and it was more common than community‐acquired hypernatremia in cats. The overall case fatality rate of animals with hypernatremia was significantly higher than that of animals with a normal sodium concentration or those with hyponatremia. A significant linear association withwards higher case fatality rate with greater hypernatremia in both dogs and cats was found, with even borderline hypernatremia associated with an increased case fatality rate. Future studies to identify the causes of hypernatremia in animals and the potential benefit of prevention or treatment of this abnormality are warranted.
Acknowledgment
Grant Support: This study was not supported by a grant.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
ABL 705, Radiometer Medical A/S, Copenhagen, Denmark
Chemistry analyzer, Hitachi 917, Roche Diagnostics, Indianapolis, IN
Chemistry analyzer, Hitachi c501, Roche Diagnostics, Indianapolis, IN
GraphPad Prism 6.0, Graph Pad Software, La Jolla, CA
References
- 1. DiBartola SP. Disorders of sodium and water: Hypernatremia and hyponatremia In: DiBartola SP, ed. Fluid, Electrolyte, and Acid‐Base Disorders in Small Animal Practice, 4th ed St Louis, MO: Saunders; 2012:47–79. [Google Scholar]
- 2. Lindner G, Funk GC. Hypernatremia in critically ill patients. J Crit Care 2013;28:216 e211–216 e220. [DOI] [PubMed] [Google Scholar]
- 3. Palevsky PM, Bhagrath R, Greenberg A. Hypernatremia in hospitalized patients. Ann Intern Med 1996;124:197–203. [DOI] [PubMed] [Google Scholar]
- 4. Long C, Marin P, Bayer A. Hypernatremia in an adult in patient population. Postgrad Med J 1991;67:643–645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Liamis G, Tsimihodimos V, Doumas M, et al. Clinical and laboratory characteristics of hypernatraemia in an internal medicine clinic. Nephrol Dial Transplant 2008;23:136–143. [DOI] [PubMed] [Google Scholar]
- 6. Stelfox HT, Ahmed SB, Khandwala F, et al. The epidemiology of intensive care unit‐acquired hyponatraemia and hypernatraemia in medical‐surgical intensive care units. Crit Care 2008;12:R162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lindner G, Funk GC, Schwarz C, et al. Hypernatremia in the critically ill is an independent risk factor for mortality. Am J Kidney Dis 2007;50:952–957. [DOI] [PubMed] [Google Scholar]
- 8. Polderman KH, Schreuder WO, Strack van Schijndel RJ, et al. Hypernatremia in the intensive care unit: An indicator of quality of care? Crit Care Med 1999;27:1105–1108. [DOI] [PubMed] [Google Scholar]
- 9. Hoorn EJ, Betjes MG, Weigel J, et al. Hypernatraemia in critically ill patients: Too little water and too much salt. Nephrol Dial Transplant 2008;23:1562–1568. [DOI] [PubMed] [Google Scholar]
- 10. Darmon M, Timsit JF, Francais A, et al. Association between hypernatraemia acquired in the ICU and mortality: A cohort study. Nephrol Dial Transplant 2010;25:2510–2515. [DOI] [PubMed] [Google Scholar]
- 11. Funk GC, Lindner G, Druml W, et al. Incidence and prognosis of dysnatremias present on ICU admission. Intensive Care Med 2010;36:304–311. [DOI] [PubMed] [Google Scholar]
- 12. Ueda Y, Hopper K, Epstein SE. Incidence, severity and prognosis associated with hyponatremia in dogs and cats. J Vet Intern Med 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Stelfox HT, Ahmed SB, Zygun D, et al. Characterization of intensive care unit acquired hyponatremia and hypernatremia following cardiac surgery. Can J Anaesth 2010;57:650–658. [DOI] [PubMed] [Google Scholar]
- 14. Lindner G, Funk GC, Lassnigg A, et al. Intensive care‐acquired hypernatremia after major cardiothoracic surgery is associated with increased mortality. Intensive Care Med 2010;36:1718–1723. [DOI] [PubMed] [Google Scholar]
- 15. Himmelstein DU, Jones AA, Woolhandler S. Hypernatremic dehydration in nursing home patients: An indicator of neglect. J Am Geriatr Soc 1983;31:466–471. [DOI] [PubMed] [Google Scholar]
- 16. Arieff AI, Carroll HJ. Cerebral edema and depression of sensorium in nonketotic hyperosmolar coma. Diabetes 1974;23:525–531. [DOI] [PubMed] [Google Scholar]
- 17. Synder C, Feigal D, Arieff A. Hypernatremia in elderly patients: A heterogeneous, morbid, and iatrogenic entity. Ann Intern Med 1987;107:309–319. [DOI] [PubMed] [Google Scholar]
- 18. Bratusch‐Marrain PR, DeFronzo RA. Impairment of insulin‐mediated glucose metabolism by hyperosmolality in man. Diabetes 1983;32:1028–1034. [DOI] [PubMed] [Google Scholar]
- 19. Adrogue HJ, Madias NE. Hypernatremia. N Engl J Med 2000;342:1493–1499. [DOI] [PubMed] [Google Scholar]
- 20. Kozeny GA, Murdock DK, Euler DE, et al. In vivo effects of acute changes in osmolality and sodium concentration on myocardial contractility. Am Heart J 1985;109:290–296. [DOI] [PubMed] [Google Scholar]
- 21. Darmon M, Pichon M, Schwebel C, et al. Influence of early dysnatremia correction on survival of critically ill patients. Shock 2014;41:394–399. [DOI] [PubMed] [Google Scholar]
- 22. Lindner G, Kneidinger N, Holzinger U, et al. Tonicity balance in patients with hypernatremia acquired in the intensive care unit. Am J Kidney Dis 2009;54:674–679. [DOI] [PubMed] [Google Scholar]
- 23. Liamis G, Milionis HJ, Elisaf M. A review of drug‐induced hypocalcemia. J Bone Miner Metab 2009;27:635–642. [DOI] [PubMed] [Google Scholar]
- 24. Rose B, Post T. Hypoosmolal states‐hyponatremia In: Wonsciewics M, McCullough K, Davis K, eds. Clinical Physiology of Acid‐Base and Electrolyte Disorders, 5th ed New York: McGraw‐Hill; 2001:696–733. [Google Scholar]
- 25. Tisdall M, Crocker M, Watkiss J, et al. Disturbances of sodium in critically ill adult neurologic patients: A clinical review. J Neurosurg Anesthesiol 2006;18:57–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Authement JM, Boudrieau RJ, Kaplan PM. Transient, traumatically induced, central diabetes insipidus in a dog. J Am Vet Med Assoc 1989;194:683–685. [PubMed] [Google Scholar]
- 27. Ferguson DC, Biery DN. Diabetes insipidus and hyperadrenocorticism associated with high plasma adrenocorticotropin concentration and a hypothalamic/pituitary mass in a dog. J Am Vet Med Assoc 1988;193:835–839. [PubMed] [Google Scholar]
- 28. Harb MF, Nelson RW, Feldman EC, et al. Central diabetes insipidus in dogs: 20 cases (1986–1995). J Am Vet Med Assoc 1996;209:1884–1888. [PubMed] [Google Scholar]
- 29. Aroch I, Mazaki‐Tovi M, Shemesh O, et al. Central diabetes insipidus in five cats: Clinical presentation, diagnosis and oral desmopressin therapy. J Feline Med Surg 2005;7:333–339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Post K, McNeill JR, Clark EG, et al. Congenital central diabetes insipidus in two sibling Afghan hound pups. J Am Vet Med Assoc 1989;194:1086–1088. [PubMed] [Google Scholar]
- 31. Winterbotham J, Mason K. Congenital diabetes insipidus in a kitten. J Small Anim Pract 1983;24:569–573. [Google Scholar]
- 32. Takemura N. Successful long‐term treatment of congenital nephrogenic diabetes insipidus in a dog. J Small Anim Pract 1998;39:592–594. [DOI] [PubMed] [Google Scholar]
- 33. Breitschwerdt E, Verlander J, Hribernik T. Nephrogenic diabetes insipidus in three dogs. J Am Vet Med Assoc 1981;179:235–238. [PubMed] [Google Scholar]
- 34. AlOrainy IA, O'Gorman AM, Decell MK. Cerebral bleeding, infarcts, and presumed extrapontine myelinolysis in hypernatraemic dehydration. Neuroradiology 1999;41:144–146. [DOI] [PubMed] [Google Scholar]
- 35. Bagley R, deLahunta A, Randolph J. Hypernatremia, adipsia, and diabetes insipidus in a dog with hypothalamic dysplasia. J Am Anim Hosp Assoc 1993;29:267–271. [Google Scholar]
- 36. Barr J, Khan S, McCullough S, et al. Hypernatremia secondary to homemade play dough ingestion in dogs: A review of 14 cases from 1998 to 2001. J Vet Emerg Crit Care 2004;14:196–202. [Google Scholar]
- 37. Puzot C, Descone‐Junot C, Loup J, et al. Successful treatment of severe salt intoxication in a dog. J Vet Emerg Crit Care 2007;17:294–298. [Google Scholar]


