Abstract
Intrauterine growth restriction (IUGR) has been defined in several ways, but in general describes a condition in which the fetus exhibits poor growth in utero. This complication of pregnancy poses a significant public health burden as well as increased morbidity and mortality for the offspring. In human IUGR, alteration in fetal glucose and insulin homeostasis occurs in an effort to conserve energy and survive at the expense of fetal growth in an environment of inadequate nutrient provision. Several animal models of IUGR have been utilized to study the effects of IUGR on fetal glucose handling, as well as the postnatal reprogramming of energy metabolite handling, which may be unmasked in adulthood as a maladaptive propensity for cardiometabolic disease. This developmental programming may be mediated in part by epigenetic modification of essential regulators of glucose homeostasis. Several pharmacological therapies and nonpharmacological lifestyle modifications have shown early promise in mitigating the risk for or severity of adult metabolic phenotypes but still require further study of unanticipated and/or untoward side effects.
Intrauterine growth restriction (IUGR) is a poorly understood complication of pregnancy, affecting up to 10% of live-born infants. It is characterized as a rate of fetal growth less than normal for the gestational-age appropriate growth potential (84). Quantitatively, this poor fetal growth has been defined by the American College of Obstetricians and Gynecologists as estimated fetal weight of <10th percentile for gestational age, often times with ultrasound evidence of growth deceleration late in gestation or abnormal Doppler indexes in the umbilical artery or middle cerebral artery. The mechanisms underlying IUGR are poorly understood, since there is much heterogeneity of disease etiology. In general, IUGR has been associated with 1) fetal etiologies, such as genetic abnormalities (syndromes, chromosomal abnormalities), 2) maternal factors (vascular disease, persistent hypoxia or undernutrition, and toxins), and 3) placental etiologies (10). It is thought that 40% of birth weight is ascribable to genetic factors and that the remaining 60% is due to fetal environmental exposures. Therefore, it is clear that poor macronutrient provision modified by micronutrient supply to the fetus is a critical component in the pathogenesis of IUGR. Nutrient provision is largely dependent on availability of nutrients to the fetus via maternal circulation, and as facilitated by placental transport (9).
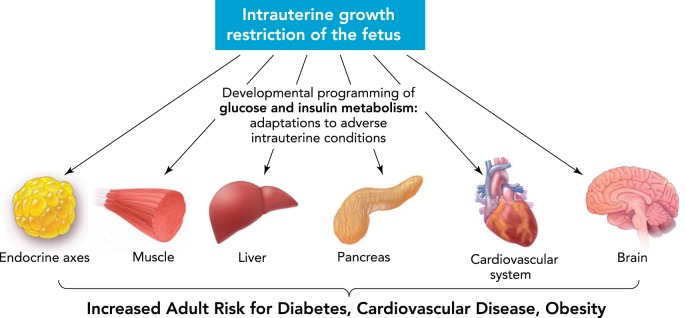
Adequate fetal growth is essential for later health and development, and therefore IUGR carries significant short-term and long-term morbidity and mortality. In the immediate postnatal period, newborns affected by IUGR are at significant risk for metabolic, hematological, immunological, and thermoregulatory abnormalities. In addition, there is an associated increased risk of respiratory distress (RDS), necrotizing enterocolitis (NEC), and cholestasis, conditions that require prolonged postnatal hospitalization and medical intervention. It is even more alarming to note that poor fetal growth also has been associated with adverse adult (and childhood) outcomes, including increased risk for Type 2 diabetes mellitus, cardiovascular disease, obesity, dyslipidemia, and metabolic syndrome (FIGURE 1). Other adverse sequelae include short stature, premature adrenarche, and impaired neurodevelopmental outcomes (84).
FIGURE 1.
The postnatal and adult effects of intrauterine growth restriction
Figure was modified from Ref. 116a with permission from Nature Reviews Endocrinology.
Inadequate nutrient provision may alter fetal physiological processes through epigenetic mechanisms, leading not only to altered growth phenotype in the short term but also to dysregulation of metabolic and cardiovascular processes in adulthood when there is a mismatch between resources and programmed adaptations. It is clear from this growing body of evidence that the study of the pathophysiology underlying IUGR is essential to develop early screening of IUGR, therapeutic interventions for IUGR, and treatment strategies to mitigate risk for long-term consequences of IUGR. The purpose of this review is to 1) describe fetal glucose metabolism and insulin regulation in normal pregnancy and in pregnancies associated with IUGR, 2) describe the organ-specific postnatal effects of IUGR on glucose and insulin handling, 3) review existing evidence as to epigenetic mechanisms underlying developmental programming of adult disease in IUGR offspring, and, finally, 4) present potential therapeutic interventions to alleviate development of adult metabolic maladaptations.
Human and Animal Models of IUGR
Before review of the many studies conducted on glucose homeostasis in IUGR, it is important to note the key differences between the human condition and in vitro and in vivo models of fetal growth restriction. Human placental villous explants have been used to study the maternal-fetal interface to extrapolate selected aspects of pregnancy, such as trophoblast development and placental transport (97). Primary cell culture, largely trophoblasts, has also be used to study cell function and placental modeling in vitro (64). While these studies have the advantage of being species-specific, they are limited by their translatability to actual human disease, given that these employ isolated cells and structures maintained in artificial conditions to ensure cellular viability. The in vivo use of animal models allows for improved fidelity to the human condition, specifically as they better mimic the complexity of pregnancy and placental function (116). There are several animal models of IUGR, and none of these models in isolation can faithfully recapitulate the multifactorial etiology thought to underlie human IUGR. As noted above, the primary cause of idiopathic IUGR in Western countries (not attributable to genetic or structural defects of the fetus) is placental insufficiency. Broadly, adequate provision of nutrients and oxygen to the fetus is dependent on multiple factors, including maternal and fetal blood flow, as well as structural and functional integrity of the placenta. The two most commonly employed broad categories of IUGR models in animals include 1) uterine artery ligation, which reflects an acute insult, and 2) nutrient restriction reflective of a chronic effect.
Uterine artery ligation has been shown in different species to result in fetal growth restriction (12, 32, 74, 115, 128), and variations on this maternal intervention include arterial embolization (96) or radial artery diathermy in guinea pigs (123). This model disrupts uteroplacental circulation, limiting their use in testing maternal therapies that target uterine blood flow or the placental barrier directly (116). In addition, there is a more abrupt nature to this model of IUGR, which differs from the chronicity of the placental insufficiency seen in the human condition. This may limit the ability of these models to encompass the gestational time-dependent nature of human IUGR. The other category of global intervention is maternal nutrient restriction. Based on studies from the Dutch famine of World War II, in humans, a major reduction (equivalent to 75% food restriction) in caloric intake is needed to influence fetal weight (∼300-g reduction in weight compared with siblings not born during the Dutch Hunger Winter) (85). Generally, milder reductions in nutrient restriction have been shown to result in growth restriction in several animal models, including mice, rat, rabbits, and sheep (31, 55, 83, 91, 103). Depending on the study design, nutrient restriction can refer to a global reduction in total caloric intake or to a restriction of specific nutrients, such as low protein or low sodium intake (4, 7). Other models have used manipulation of environmental conditions during pregnancy, such as hypoxia and hyperthermia, to produce fetal growth restriction (38, 71). The advantage of these models is the more chronic nature of insult, but effects on the fetus can vary widely, depending on species (hemochorial vs. epitheliochorial placentation) and gestational timing of the insult. Last, genetic models of fetal growth restriction have also been created, largely in the mouse, such as the IUGR seen resulting from knockout of insulin-like growth factor 2 (IGF2) (19) and more recently knockout of glucose transporter isoforms (42). While not the focus of this review, postnatal growth restriction has also been accomplished by maternal nutritional restriction during lactation (118) or creation and maintenance of a larger litter size compared with the normal counterpart (106). Most often, postnatal growth restriction has been superimposed on intra-uterine growth restriction to mimic the real human condition where often fetal growth restriction is followed by postnatal growth restriction in some cases (poorly resourced regions and critically ill IUGR infants in the Western world), whereas others are exposed to unlimited availability of calorie-rich nutrients following IUGR (Western, well resourced regions, causing a mismatch between intra-uterine and postnatal environments).
Throughout our review, important consideration should be given to which model is used and whether its use appropriately frames the scientific question being asked and how the specific model affects applicability of findings to human disease. Animal models should be chosen with careful consideration of advantages and disadvantages, such as size, cost, length of gestation, litter size, and similarity to human placental and/or fetal physiology (116).
Fetal Glucose Metabolism and Insulin Secretion
Glucose Handling and Insulin Secretion in Normal Pregnancy
Glucose serves as the main energy substrate for the developing fetus. Since the fetus does not endogenously produce glucose under normal conditions (28), maternal glucose must cross the placenta to become available as the exclusive fuel for fetal energy metabolism. At the endocrine level, maternal and fetal glucose availability are regulated largely by the hormone insulin and to some extent by counterregulatory hormones as well. During pregnancy, the mother develops insulin resistance with advancing gestation, which allows for increased glucose availability to the rapidly growing fetus, thereby ensuring the maintenance of a glucose gradient between the mother and the fetus. The higher level of circulating glucose in the mother is then passively transported to the fetus by facilitative diffusion mediated by various isoforms of a family of membrane-spanning proteins known as the facilitative glucose transporters (GLUTs) (67, 75). The predominant isoforms expressed in the mammalian placenta are GLUT1 and GLUT3, present in polarized trophoblast cells (109). Other isoforms (GLUT4 and GLUT12), while expressed, are mainly present in first trimester syncytiotrophoblasts (34, 35, 57). GLUT8, GLUT9, and GLUT10 also have been shown to be expressed in human placenta to a much lesser extent (8, 24, 29). In humans, GLUT1 is expressed in high concentrations throughout pregnancy and is preferentially present in the maternal facing membrane of syncytiotrophoblasts to help maintain the glucose gradient (11, 22). GLUT3 is most abundant in the first and second trimesters, found in both cytotrophoblasts and syncytiotrophoblasts, and has a higher affinity and greater transport capacity for glucose than GLUT1 (fivefold), although it is less ubiquitous than GLUT1 (21, 68). In murine studies, absence of either isoform results in early embryonic demise (GLUT1 = e13, GLUT3 = e6.5), and a 50% reduction in the protein expression of either of these isoforms results in mild fetal growth restriction or reduced adult body weight (42, 127).
While many hormones and growth factors act in concert to maintain euglycemia in the fetus, insulin serves as a major fetal growth potentiator. However, its mediator effect on placental glucose transport is controversial. Early in the first trimester of pregnancy, insulin stimulates glucose uptake in villous fragments (34). However, this effect disappears with gestational age, and with further study no significant effect is seen after 8 wk of gestation (34). While the use of isolated villous extracts was reasonable for the question being posed (the effect of local hormonal concentration on glucose uptake across the trophoblast layer), the effector concentrations used in the study were higher than physiological maternal and fetal plasma concentrations. This may or may not have been reasonable, since the differences between plasma and local placental hormonal concentrations are not precisely known. There is controversy over the effects of insulin on placental glucose uptake at term, with some studies demonstrating increased uptake and others demonstrating no effect (2, 15). This is despite the presence of GLUT4, the insulin responsive glucose transporter isoform noted in placenta (70), which is restricted mainly to non-trophoblastic cells. It has been proposed that the placenta's nutrient-sensing properties and adaptive abilities are mediated through the mammalian target of rapamycin (mTOR) signaling pathway. Insulin is one of the key positive regulators of mTOR, an atypical serine/threonine protein kinase located in trophoblast cells and integrating signals from both the maternal nutrient supply and the fetus's energy demands (9). mTOR then mediates several downstream effects, the best characterized being protein transport via system A and system L amino acid transporters (104, 105). More recently, mTOR has also been shown to regulate human placental GLUT3 concentrations (130). Animal and human studies have shown that placental mTOR is decreased in human IUGR and in animal nutrient restriction models (41). These studies suggest that the placenta responds to nutrient availability by regulating its major nutrient sensor. However, other studies demonstrate that the placenta stimulates pathways besides mTOR, thereby compensating for suboptimal nutrient provision (86). In conclusion, the fetal nutrient provision as mediated by the placenta is a complex system that acts to optimize in utero energy supply by balancing mechanisms of glucose, protein, and fatty acid transport (61, 75, 78).
Within the fetus, insulin regulates glucose utilization and is produced in increasing amounts as gestation progresses. Fetal insulin secretion responds to glucose concentration changes contingent on absolute amount, duration, magnitude, and pattern of change (62). Glucose utilization in insulin-sensitive tissues (skeletal muscle, liver, heart, and adipose tissue) increases, especially during late gestation, resulting in exponential organ growth and thereby fetal growth. Insulin secretion is downregulated by constant hyperglycemia related to glucotoxicity of the fetal beta islet cells (13) but enhanced by pulsatile hyperglycemia that preserves the fetal islets (14). Hypoglycemia leads to both decreased basal and decreased glucose-induced insulin secretion (80) due to the lack of the glucose stimulus. Amino acid concentrations also regulate insulin secretion, but significant changes in concentration are required to elicit clinically relevant changes in fetal insulin concentration (56).
Alterations in Glucose Transport and Metabolism in IUGR
While fetal hypoglycemia has been associated with IUGR, there appears to be no change in GLUT1 transporter protein expression but rather a compensatory increase of GLUT3 in term human placentas associated with IUGR (70). This is hypothesized to reflect increased placental glucose consumption at the expense of glucose availability to the fetus (68, 69). However, other groups have shown no change in term human placental GLUT3 with IUGR pregnancies (72). This variability in human studies suggests an association between the severity of IUGR encountered vs. the observed change in placental GLUT3 concentrations (increasing when mild and decreasing when severe, with no change reflecting an in-between situation). A similar situation with GLUT3 was described previously in chronic maternal hyperinsulinemic hypoglycemia imposed only during late gestation in sheep placenta (21), where mild IUGR was seen. Our own experiments in mice with 50% reduction in maternal diet spanning mid- to late gestation caused a 50% reduction in placental GLUT3 protein expression and glucose transporting function, with no such change in GLUT1 (41). These animal studies provide credence to the variability observed in human studies, supporting the role of gestational timing and severity of the condition on placental GLUT3 expression and function.
In a murine model of maternal nutrient restriction (80% of control diet), placental mRNA expression of GLUT1 at day 16 (term of ∼21 days) was reduced by 17%, although at day 19 GLUT1 mRNA expression was significantly higher than in controls. Total glucose transfer as measured by unidirectional materno-fetal clearance of tracer glucose was not different at either time point (17). Another group demonstrated that, in multiparous ewes, 50% nutrient restriction during the first half of pregnancy resulted in increased placental GLUT1 mRNA and protein expression (86). Recent work in a primate model, which demonstrates fidelity to the human in terms of reproductive biology and placental structure, demonstrated decreased GLUT1 protein expression in the maternal villous membrane at term after 70% maternal control diet conditions (73). Glut4 protein expression appears similar in term placentas taken from normal and IUGR-associated human pregnancies (70), although it was only detected in very small amounts (72). These studies collectively demonstrate that there is placental plasticity in response to altered maternal nutrient restriction but that, in these conditions, adaptations are sufficient for fetal survival but insufficient to prevent IUGR and that these changes are likely gestational period and severity related. This compensation to increase placental glucose availability serves to ensure fetal survival by ensuring placental maintenance but does not increase the overall glucose availability to the fetus, as evidenced by persistent poor fetal growth. Evolutionarily, the self-maintenance of the placenta is essential to protect downstream fetal survival, but at the expense of growth. While discrepancies in findings may be related to model-specific conditions (116), the nutrient restriction during early vs. late gestation demonstrates enhanced compensation toward preservation of the feto-placental unit overall by increasing placental GLUT1 and GLUT3, and increasing severity of restriction even during late gestation, resulting in a reduction in placental GLUT1 and GLUT3 concentrations.
In human IUGR from placental insufficiency, diminished fetal insulin production in response to reduced fetal glucose concentrations is secondary to decreased pancreatic β-cell mass and impaired islet cell insulin secretion in response to exogenous stimuli (81, 82, 89, 92). Fetal metabolic rate as measured by net fetal oxygen uptake or fetal glucose utilization rates, remains relatively constant despite significant decreases or increases in glucose supply (62). While this observation suggests a role for other substrates, glucose utilization on an individual organ or tissue basis may vary significantly in its responsiveness to glucose and insulin, as opposed to that of the whole fetus. Therefore, relative protection of energy substrate provision to one organ (the brain) at the expense of other organs may result in no detectable net change in metabolic rate and would be consistent with the asymmetric IUGR often seen in placental insufficiency. In addition, the mechanisms underlying altered glucose availability may be gestation time-point specific. While there is evidence that placental nutrient transporters can be altered in early stages of gestation in response to maternal nutrient restriction (86), and early gestation deficiencies of nutrient transporters (in knockout models) can be associated with congenital malformations (88), it may be that fetal tissue glucose uptake rates and glucose transporters are affected more in late rather than mid-pregnancy, during the period of rapid fetal growth, which may help explain IUGR being characterized by late-gestation fetal growth deceleration. Therefore, we now review glucose transport, uptake, and metabolism on an organ-specific basis.
The In Utero Organ-Specific Effects of Abnormal Glucose/Insulin Metabolism in IUGR
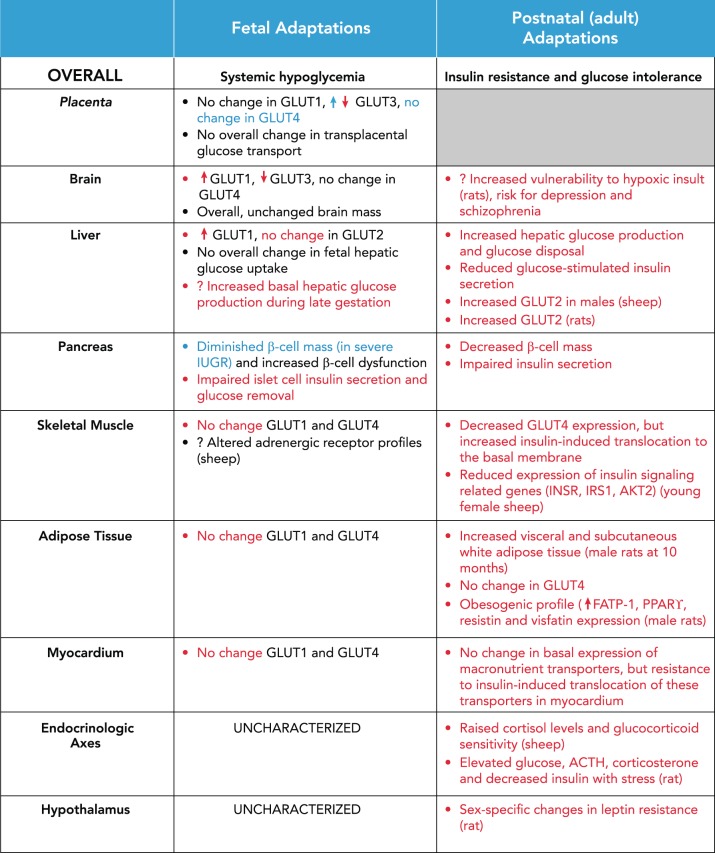
Human IUGR has been shown to be associated with chronic fetal hypoglycemia. In the face of chronic hypoglycemia, fetal tissues' capacity for glucose uptake and utilization increases in compensation (summarized in FIGURE 2). In animal models mimicking chronic hypoglycemia secondary to maternal insulin infusion, brain GLUT3 protein expression decreases, brain GLUT1 protein increases, and liver GLUT1 protein levels increase. There was no reported change in myocardial, skeletal muscle, or adipose tissue GLUT1 or insulin-responsive GLUT4 protein expression (23, 107, 108).
FIGURE 2.
Summary of overall and organ-specific glucose and insulin metabolism adaptations in IUGR, the fetus, and the adult
Red coloring indicates finding in animal models of IUGR, and blue indicates findings in human IUGR studies. Discrepancies exist between human and animal studies, and among animal studies depending on experimental conditions.
This tissue-specific effect may represent the relative fetal preservation of energy fuel to the brain across the blood-brain barrier over glucose utilization by somatic tissues such as the liver and other insulin-responsive tissues in the face of poor glucose provision. For example, acute hypoglycemia induced by insulin infusion in IUGR rats (due to bilateral uterine artery ligation) results in neuronal injury of the brain cortex but to a lesser degree than the injury encountered in response to insulin-induced hypoglycemia in normally grown neonatal rats. No difference in brain GLUT1 or GLUT3 mRNA expression was evident between groups (87). Therefore, the IUGR rat pups are relatively protected against acute hypoglycemic neuronal injury. The limitation of this study stems from the lack of measurements related to substrate utilization by the brain.
As a major player in metabolic regulation, the study of the fetal liver in IUGR has also yielded evidence that organ-specific glucose uptake and production is regulated in a complex manner. Although fetal GLUT1 protein expression in the whole liver appears to increase in IUGR, this observation may actually reflect a parallel expansion in hematopoietic cell mass seen in IUGR (108). The evaluation of GLUT transporter expression in whole fetal liver samples is limited by the differences in the function and location of the specific transporters, GLUT1 as the predominant fetal hematopoietic cellular isoform, whereas GLUT2 is the isoform found in fetal and postnatal hepatocyte cell membranes, responsible for glucose transport across hepatocytes (108). GLUT2 protein does not change in IUGR fetal livers compared with controls, whereas GLUT1 increases as observed in the rat model (108). Overall, there is no evidence that fetal hepatic glucose uptake is changed in IUGR.
However, it may be that hepatic glucose production is changed in the IUGR fetus. Throughout most of gestation, maternal glucose supply is sufficient for feto-placental utilization under normal conditions. In periods of stress, meaning several days of decreased materno-placental-fetal glucose supply, the fetus experiences sustained hypoglycemia and hypoinsulinemia, and may demonstrate endogenous glucose production (28). In most species, functional activity of the hepatic gluconeogenic enzymes begins to mature over gestation, supportive of the fetal liver being capable of producing glucose, should the situation demonstrate a need (36, 54). A recent study in fetal sheep demonstrated that IUGR fetuses have increased basal hepatic glucose production during late gestation, as measured by fetal hyperinsulinemic-euglycemic clamp study. Isolated IUGR fetal hepatocytes grown in culture maintained increased glucose production. Based on increased mRNA and protein expression of hepatic gluconeogenic gene expression (specifically PEPCK and glucose-6-phosphatase), a novel mechanism of persistent hepatic transcriptional activation in IUGR was proposed to be mediated by counterregulatory hormones (catecholamines and cortisol) but not suppressed by insulin (119).
The effects of IUGR on insulin secretion in utero have also been characterized. The fetal pancreas begins to produce measurable insulin levels by mid-gestation, and there is a gradual increase of basal insulin concentration as gestation progresses to term (62). In human IUGR, glucose-stimulated insulin secretion is impaired, leading to delayed return to basal glucose levels (92). Animal models of IUGR also consistently demonstrate impaired insulin secretion. In fetal sheep at 120 and 140 days gestational age exposed to placental restriction (induced by removal of endometrial caruncles from the uterus of non-pregnant ewes before mating), glucose- and arginine-stimulated insulin secretion were impaired, although insulin sensitivity was unchanged (98). The potential mechanisms underlying impaired insulin secretion include decreased β-cell mass and function (51). Severely IUGR human fetuses (<1.5 kg) demonstrate reduction in β-cell mass (124). However, human studies utilizing less stringent criteria for IUGR in humans (<10% for birth weight) did not demonstrate differences in β-cell mass compared with AGA infants (6). This discrepancy in human findings likely results from differences in cohorts studied. Animal models of IUGR demonstrating impaired insulin secretion have found associated defects in pancreatic β-cell expression of Pdx1, a transcription factor important for both early development of the pancreas as well as later differentiation of β-cells (30, 59, 114). Interestingly, an associated change in epigenetic modification at the proximal promoter region of Pdx1 has been identified in this animal model (112), and similar epigenetic modifications have been observed at Pdx1 in islets from humans with Type 2 diabetes mellitus (131). These findings may, in part, explain the decrease seen in β-cell mass. β-Cell dysfunction may result from decreased islet vascularity (60, 93), which may be associated with mitochondrial dysfunction (113). The potential effect of IUGR on fetal pancreatic GLUT2 expression and function in the fetal pancreas requires further study, since GLUT2 is a bidirectional glucose transporter, essential in allowing free flow of glucose to the intracellular glucose-sensing mechanisms within β-cells. Further evaluation of how insulin secretion by the fetal pancreas is affected by GLUT2 as a mediator of glucose transfer to glucose-sensing cells in the pancreas is also warranted.
Postnatal Effects of IUGR and Potential Therapeutic Interventions
The fetal adaptations to suboptimal intrauterine nutrient provision have long-lasting implications to fetal health and persist postnatally into adult life. Overall, early postnatal life is characterized by enhanced insulin sensitivity, which then progresses to insulin resistance and altered glucose homeostasis in adults (98, 117, 120). Glucose tolerance testing on IUGR rat pups at 2 days showed that they were glucose intolerant, had increased hepatic glucose production, and had increased glucose disposal compared with controls (48). IUGR juvenile sheep at 1 mo of age demonstrated impaired insulin sensitivity and mRNA expression of insulin signaling and glucose transporter genes in skeletal muscle but not in the liver (25). Insulin disposition indexes for glucose and free fatty acids were also increased in the young lamb and can predict both catch-up growth and early onset visceral obesity (26). At 15 mo, the IUGR male rat offspring with prenatal nutrient restriction demonstrated greater weight gain and hyperinsulinemia compared with age-matched counterparts exposed to both pre- and postnatal nutrient restriction. Additionally, visceral and subcutaneous adiposity accumulation was evident in the former group (48). Age-matched IUGR male rat offspring with both pre- and postnatal nutrient restriction were smaller and hypoinsulinemic, with no change in glucose tolerance, hepatic glucose production, or clearance (48). Further study into the potential therapeutic effect of postnatal calorie restriction in IUGR rat offspring showed that postnatal caloric restriction protects against ad libitum feeding and high-fat-diet-induced adult obesity, and normalizes whole-body insulin sensitivity and energy expenditure with increasing physical activity levels at 10 (45) and 17 mo of age (20). Thus postnatal catch-up growth superimposed on IUGR propagates detriment in the adult stages of development, setting the stage for chronic diseases.
Of note, in both human and animal studies, growth-restricted males seem to be at a relative disadvantage for adverse outcomes compared with females, in terms of both neonatal complications (111) as well as long-term outcomes. It is a recurring theme that females are protected from metabolic programming adaptations seen after adverse in utero exposures. This pattern holds true across risk for metabolic disease (122), hypothalamic energy balance (3), renal disease, and adult hypertension/cardiovascular disease (39) in fetuses exposed to adverse in utero environments, including placental insufficiency resulting in IUGR. The reasons for this sex-specific effect is unknown but has been proposed to result from sex-specific transcriptional and epigenetic regulation in the developing fetus, which may affect sex-determined response to adverse environmental conditions (76). Anthropologically speaking, this female-sparing effect may serve to protect the postnatal survival of the sex that disproportionately carries the burden of reproductive survival. In addition, this protection may be hormonally influenced, and epidemiological data suggests that females born to mothers with preeclampsia are at risk for preeclampsia during their own pregnancies (129). In addition, animal studies of female IUGR progeny are at increased risk of developing gestational diabetes. Furthermore, it is known that women with preeclampsia (hypertension and proteinuria) during pregnancy go on to be two to four times more likely to develop high blood pressure, heart disease, or stroke (5). It may be that hormonal changes during pregnancy create an imbalance that unmasks programming of cardiometabolic dysfunction and that, with time and aging, this propensity toward adult cardiovascular disease is even more evident. Future translational studies aimed at understanding the sex-specific effects of the interaction between early environmental exposures and programming of adult disease are essential.
Hepatic Glucose and Insulin Signaling
At the hepatic level, young sheep (at 30 days) did not have altered mRNA expression of insulin signaling and related genes but increased GLUT2 mRNA expression in males (25). Fifteen-month-old adult male rats who were IUGR were also found to be glucose intolerant, have reduced glucose-stimulated insulin secretion, have decreased total glucose clearance, but have unsuppressed hepatic glucose production. They also demonstrate increased liver GLUT1 protein expression compared with controls, suggestive of increased hepatic glucose uptake. Postnatal nutrient restriction in these IUGR rat offspring rescues glucose tolerance and hepatic glucose production, but with increased glucose-stimulated insulin secretion, glucose clearance rate, and hepatic GLUT1 protein expression (43). Using Affymetrix microarray-based expression profiling techniques, IUGR rats also subjected to postnatal caloric restriction demonstrated only seven genes that were differentially expressed in the liver at 15 mo of age, suggesting that the metabolic adult sequelae of IUGR that were also calorie restricted during postnatal life are not evident, being protective and thereby not transcriptionally mediated (37). These studies make it clear that hepatic glucose and insulin metabolism is affected via several pathways by both prenatal and postnatal nutrition, demonstrating the plasticity of the fetus and neonate toward adaptation in overcoming the dire environmental conditions. Thus an in utero to postnatal match in nutrition proves to be protective against cardiometabolic disorders. In contrast, a mismatch as seen with availability of normal nutrition (perceived as increased nutrition), which causes catch-up growth in the IUGR fetus, sets up an unhealthy trajectory.
Pancreatic Alterations in IUGR
Pancreatic β-cell mass has been reported as decreased in the rat IUGR male and female offspring (43). In IUGR offspring, pancreatic β-cell mass was decreased by 45% compared with offspring exposed to postnatal nutrient restriction, who demonstrated a 30% increase in pancreatic mass. Rats subjected to both pre- and postnatal caloric restriction had an 80% decrease in pancreatic β-cell mass (89). In another rat model of IUGR with adult-onset diabetes, it appears that placental insufficiency leads to dysfunction of the electron transport chain in β-cells, leading to production of reactive oxygen species and loss of β-cell proliferation and impaired function (113). In a sheep model of IUGR, pancreata collected from sheep as a lamb (42 days) and as a young adult sheep (age 556 days) showed that β-cell insulin secretory capacity is impaired in male sheep with aging, despite increases in β-cell mass (50).
Skeletal Muscle Insulin-Responsive Glucose Handling in IUGR
Since skeletal muscle is a key player in insulin-responsive glucose uptake, its role in glucose homeostasis is critical. Female IUGR rats have decreased skeletal muscle mRNA and protein expression and increased insulin-induced translocation of GLUT4 to the basal membrane in adulthood (118). Perturbations in kinase and/or phosphatase arms of post-receptor insulin-signaling pathways in skeletal muscle were seen in the female IUGR adult offspring, and were distinct from those in postnatally restricted IUGR offspring (95). Skeletal muscle insulin-induced glucose uptake is enhanced in postnatal caloric restriction of IUGR offspring (20). In young sheep exposed to placental insufficiency, skeletal muscle mRNA expression of insulin signaling and related genes, such as INSR, IRS1, AKT2, and GLUT4, were reduced in females (25) and correlated with decreased whole-body insulin sensitivity. Other endocrine and metabolic pathways regulated at a skeletal muscle level may also be affected in IUGR as an adaptation to altered glucose handling. Yates et al. demonstrated adaptations in skeletal muscle adrenergic receptor expression profiles in IUGR sheep fetuses, which may act to suppress insulin signaling, muscle fiber hypertrophy, and therefore glucose oxidation (132). Overall, these studies suggest poor insulin-responsive glucose uptake by skeletal muscle in IUGR.
Adaptations in Adipose Tissue in IUGR
GLUT4 is the main glucose transporter found in adipose tissues and is generally regulated by insulin and is responsible for insulin-regulated glucose storage. The mRNA and protein expression of white adipose tissue GLUT4 in adult female IUGR rats is unchanged from controls (118), implying that, despite alterations in the in utero metabolic milieu, adipose tissue preserves insulin responsiveness and absorbs postprandial glucose not efficiently utilized by skeletal muscle. In male 10-mo-old adult rats exposed to prenatal caloric restriction, visceral and subcutaneous white adipose tissue mass was increased, as measured by abdominal computed tomography (45). At 15 mo of age, fatty acid transport protein-1 (FATP-1), peroxisomal proliferator-activated receptor-γ (PPARγ), resistin, and visfatin mRNA expression were increased in IUGR (43). Taken together, this profile of gene expression reflects an obesogenic phenotype, as FATP1 and PPARγ mediate fatty acid synthesis in adipose tissue, and resistin and visfatin are adipokines that mediate the development of obesity and insulin resistance (43). However, further study assessing the functional change in white adipose tissue glucose storage in IUGR offspring would strengthen these findings further.
Cardiovascular Sequelae of IUGR
As IUGR offspring are also at risk for cardiovascular disease, it is interesting to note the effects of IUGR on cardiac structure and glucose handling. Induction of diabetes in IUGR rat offspring at 24 wk of age resulted in cardiac hypertrophy 8 wk later, characterized by posterior wall thickening and ventricular interstitial fibrosis. While this effect was also seen in non-IUGR rats, the effect was greatest in the IUGR diabetes-induced group (79). As measured by protein expression of glucose and fatty acid transporters, female IUGR offspring demonstrate resistance to insulin-induced translocation of several macronutrient transporters in the heart but overall increased expression of these proteins in the basal state (1). We have also previously demonstrated enhanced atherosclerotic patches in IUGR adult male mouse offspring associated with higher circulating cholesterol concentrations (7).
These studies highlight the importance of further work to understand the effects of metabolic reprogramming in IUGR offspring as related to cardiovascular disease such as myocardial infarction and its associated complications. In addition, evaluation of myocardial glucose and energy substrate handling in response to stress, aging, and worsening metabolic disease in the IUGR population may demonstrate dysfunction not seen at basal, normal healthy conditions but surface after certain unhealthy life style choices. Thus lifestyle interventions can prevent the development of cardiometabolic disease.
Endocrinological Axes Affecting Glucose and Insulin Homeostasis in IUGR
Pre- and postnatal growth restriction may affect other endocrine axes. In a twin-sheep model of 40% maternal caloric restriction during late gestation (day 110 until term, ∼147 days), IUGR offspring raised in a low-activity obesogenic environment had enhanced postnatal growth as well as insulin and leptin resistance. In addition, this group demonstrated raised cortisol levels and increased gene (mRNA) expression for glucocorticoid sensitivity in the hypothalamus. These findings were attenuated in the IUGR offspring raised in a standard post-weaning environment (27). (We review the hypothalamus and leptin pathway specifically in further detail below.) In IUGR rat offspring exposed to a high-fat diet after weaning, they demonstrated normal serum glucose levels, and lower serum levels of adrenocorticotrophic hormone and corticosterone. However, after unpredictable chronic stress, they had significant elevations of glucose, ACTH, and corticosterone and decreases in insulin levels (133). In addition, in IUGR rats, adipose deposition is dysregulated in males and may be accompanied by proinflammatory signals such as TNF-α, which is known to impair insulin signaling. A recent study has implicated TNF-α in the activation of the unfolded protein response and the impairment of glucose tolerance in IUGR rats (102). We have also previously observed perturbed TNF-α activation in the liver of IUGR rat offspring compared with controls (33). Again, these studies demonstrate that various stressors may uncover developmental programming of metabolic derangements and may also help to explain the propensity for inflammatory diseases seen in the IUGR offspring.
Hypothalamus and Energy Balance in IUGR
IUGR specifically affects the hypothalamus and centrally mediated energy balance. Energy balance is regulated by circulating leptin concentrations in combination with the hypothalamic leptin receptor-signaling pathway. Leptin signaling overall enhances anorexigenic neuropeptides and suppresses orexigenic neuropeptides. Both male and female rat IUGR offspring are hypoleptinemic on postnatal day 2, but females demonstrate evidence of leptin resistance, whereas males demonstrate leptin sensitivity. At postnatal day 21, this sex-specific effect in leptin resistance and sensitivity is persistent, although males are now euleptinemic and females are hyperleptinemic. Postnatal growth restriction abolishes this sex-specificity, overall favoring hyperphagia and diminished energy expenditure post-weaning (110). However, leptin replenishment during this early postnatal period overall restores leptin sensitivity by reversing the hypothalamic orexigenic-to-anorexigenic neuropeptide ratio, as mediated by the STAT3 signaling arm of the hypothalamic leptin receptor pathway (53). Again, the differential sex-specific effects on leptin regulation and adiposity likely result from the thematic pattern in developmental programming that emphasizes the timing of in utero insult during different critical windows of development and the differential effects on males and females (65). Overall, these studies highlight the important effects IUGR has on the hypothalamus and energy balance, which has implications for adult energy expenditure, satiety, and risk for obesity.
Brain and Neurodevelopmental Changes in IUGR
In a rat model of IUGR induced by uterine artery ligation, fetal body weight was decreased by 25% but was not accompanied by a significant change in fetal brain weight. GLUT1 protein levels in the brain were increased ∼45% in fetal brain, and this change was persistent through postnatal day 21 (107). This compensation in GLUT1 levels may represent a brain protective effect that persists postnatally. However, while the brain weight is preserved in IUGR, its vulnerability to insult may be altered. Compared with control rat pups, IUGR rat pups exposed to perinatal hypoxia by placing maternal rats in 14% FiO2 3 h before delivery demonstrated increased cerebral apoptosis (77a). These findings are important to note, since human IUGR fetuses are also at particular risk for preterm delivery and exposure to perinatal stress, and hypoxia is not well tolerated (90).
While several of the aforementioned studies demonstrated the protective effect of postnatal nutrient restriction against metabolic derangements, there are concerns on the effect of postnatal nutrient restriction on brain development. Epidemiological studies have associated early nutrient restriction to depression and schizophrenia (58). Rat pups exposed to late gestation 50% in utero nutrient restriction and then fostered by 50% food-restricted lactating mothers demonstrated decreased CSF glucose and lactate levels, and increased ketone levels at postnatal day 21 in suckling and postnatal day 50 post-weaned stages. Postnatally restricted offspring also exhibited anxiety but had no indicators of social or cognitive impairments. Interestingly, female offspring with postnatal caloric restriction demonstrated increased brain GLUT1 and GLUT3, increased brain-derived neurotrophic factor, and reduced GLUT4 protein expression. Aging female offspring expressed reduced brain protein levels of IRS-2, pAkt, and pGSK-3β, and higher glial fibrillary acidic protein, spinophilin, and amyloid-β42 (121). These findings are concerning since they demonstrate the effect of postnatal nutritional restriction on neurobehavior and possible predilection for adult neurological disease, and again highlight the importance of monitoring sex-specific effects. Thus postnatal growth restriction, while proving protective of cardiometabolic disorders, is detrimental toward neurodevelopment.
Other Proposed Therapeutic Interventions for IUGR Metabolic Derangements
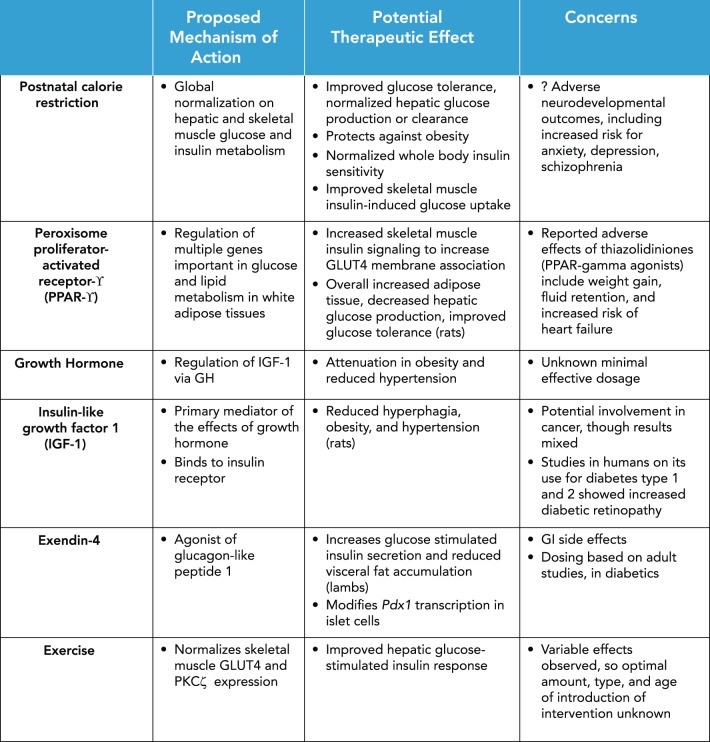
Given the neurological concerns with postnatal caloric restriction, the pursuit of other avenues for mitigating risk for metabolic derangements in IUGR is necessary (summarized in FIGURE 3). Peroxisome proliferator-activated receptor-γ (PPARγ) is predominantly expressed in white adipose tissues, and its activation regulates the expression of multiple genes important in glucose and lipid metabolism. The thiazolidinediones, a class of antidiabetic drugs, are thought to activate PPARγ. Therefore, the study of the therapeutic effect of PPARγ in IUGR offspring has shown that early introduction of an PPARγ agonist improves skeletal muscle insulin signaling to increase GLUT4 membrane association in muscles (94). Two-month-old female rats subjected to prenatal IUGR and treated with a PPARγ agonist had increased subcutaneous white and brown adipose tissue, decreased hepatic glucose production, and improved glucose tolerance with lower glucose-stimulated insulin release compared with placebo IUGR rats (47).
FIGURE 3.
Therapeutic interventions for the adult risk of cardiometabolic disease seen in IUGR
Other therapeutics with metabolic effect, such as growth hormone and IGF-1, have also been studied. Adult growth hormone treatment in IUGR rats resulted in attenuation in obesity and reduced hypertension (125). The proposed mechanism for this effect is via the regulation of IGF-1 by growth hormone. The same group demonstrated that IGF-1 treatment also reduces hyperphagia, obesity, and hypertension in a rat model of IUGR-induced adult metabolic disease (126).
Administration of exendin-4, an agonist of glucagon-like peptide-1, to growth-restricted lambs in the neonatal period increased glucose-stimulated insulin secretion and reduced visceral fat accumulation (52). In rat models of IUGR, exendin-4 treatment during the neonatal period also appears to successfully prevent the development of diabetes by preventing hepatic insulin resistance (100, 114). It appears that exendin-4 treatment in these rats may modify histone acetylation activity via regulation of the Pdx1 proximal promoter, leading to overall increased Pdx1 transcription in islets (40a). Therefore, targeting mediators of glucose handling pathways may attenuate downstream effects of chronic hyperglycemia and insulin resistance, as well as associated morbidities. Even more interestingly, further studies of how these therapeutic interventions may restore expression of glucose handling regulators via epigenetic mechanisms could play a critical role in mitigating the development of adult disease. However, a cautionary note in translating these findings to human infants exists. Most drugs have major side effects that may last a lifetime and are concerning when they are administered during the early neonatal period.
Lifestyle interventions such as exercise also have potential for improving metabolic consequences of IUGR, as has been shown in a few human cohorts (49). The studies evaluating changes of skeletal muscle expression of enzymes important in glucose metabolism in IUGR animal models have yielded mixed results. Exercise does appear to normalize PKCζ and, consequently, GLUT4 protein expression (66). Early exercise regimens that were moderate in nature introduced in pregestational female offspring exposed to IUGR improved hepatic glucose-stimulated plasma insulin response and hepatic glucose production. However, the exercise regimen improved insulin responsiveness of skeletal muscle GLUT4 translocation only in exercised rats that were IUGR but had a detrimental effect when the IUGR offspring was also exposed to postnatal caloric restriction (46), collectively suggesting that the protective effect of exercise may actually act through hepatic adaptation, as opposed to improved skeletal muscle glucose handling. In another rat model of exercise from 20 to 24 wk of age in IUGR offspring resulting from bilateral uterine artery ligation, β-cell mass was restored to normal, and first-phase insulin secretion was improved (77). In conclusion, although exercise does appear to have protective effects against the metabolic consequences of IUGR, there is significant variability in the effects and mechanistic pathways identified. This variability may be dependent on several factors, including the amount, type, and age at which exercise regimens are instituted (49). More importantly, while useful in the IUGR infant, exercise can have a detrimental effect in IUGR infants who are also postnatally growth restricted. Thus this distinction must be made in the clinical arena by pediatricians and internists.
Evidence of Epigenetic Mechanisms Underlying Developmental Programming of Metabolic Disease in IUGR Offspring
It has been observed in large epidemiological studies that not only do IUGR offspring suffer increased risk of adult metabolic disease, but they also pass these phenotypes of aberrant glucose/insulin homeostasis on to their own offspring. In fact, while the male IUGR offspring develop glucose intolerance and obesity, the female IUGR offspring demonstrate gestational diabetes in the form of glucose intolerance (117), propagating the effects of an altered in utero metabolic environment on the next generation. In a rat study using embryo transfer to control for gestational environment, female F2 rats procreated by F1 pre- and postnatally nutrient-restricted mothers but gestating in control mothers demonstrated features of metabolic imbalance. Although the F2 IUGR had the same birth weight and postnatal growth patterns as control F2 offspring, they had increased hepatic weight, fasting hyperglycemia, and hyperinsulinemia, and unsuppressed glucose production. In addition, they had increased skeletal muscle total GLUT4 and pAkt concentrations but decreased membrane-associated GLUT4 and pPKCζ enzyme activity (117). This study elegantly demonstrates that the intergenerational inheritance of abnormal glucose and insulin metabolism and skeletal muscle insulin signaling is independent of immediate intrauterine environment. It is important to note that, in contrast, embryo transfer of F2 embryos from F1 mothers simply exposed to postnatal calorie restriction did not show similar findings (44), supporting the role for the intrauterine environment in modifying the heritability of metabolic phenotypes.
These intergenerational effects may result from epigenetic modifications induced by nutritional (environmental) stressors. In humans, study of individuals prenatally exposed to famine during the Dutch Hunger Winter in 1944-1945 had less DNA methylation of the imprinted IGF2 gene compared with their unexposed, sex-matched siblings (63). This study was among the first to empirically support the hypothesis that early life environmental conditions cause persistent epigenetic changes. However, there are several important limitations to note in these studies, including the samples used and the statistical analysis of methylation changes. Changes seen in DNA methylation were small, and it is unknown what the functional implications are of a 5% change in DNA methylation. Additionally, the samples analyzed were whole blood, which includes a mixed population of cells, which raises the question of how applicable the findings are to human disease. However, the subsequent use of animal studies do provide further evidence for the in utero effects on epigenetic modification. The decline in skeletal muscle GLUT4 mRNA and protein concentrations seen in rat and human IUGR may be transcriptionally regulated through histone modifications. Deacetylation and dimethylation of specific amino acid residues in the NH2-tail of histone 3 of skeletal muscle GLUT4 promote corepressor complex formation to collectively decrease Glut4 transcription (101). In the pancreas, PDX1 (pancreatic and duodenal homeobox 1) is a transcription factor critical for β-cell function and development. In IUGR rats, Pdx1 transcription is reduced, and there are associated epigenetic changes at the promoter (histone modification in late fetal and early postnatal life, and then DNA methylation changes in adulthood) (99).
In addition, IUGR mice demonstrate decreased global placental methylation and sex-specific changes in methylation that are more pronounced in the male offspring. Specific genes are also differentially methylated in IUGR, including micro-RNAs targeting genes important in metabolic, cardiovascular, neurological disease, as well as genes responsible for transplacental nutrient transfer (16). Since placental GLUT3 mRNA expression is decreased by 50% when associated with IUGR murine fetuses, DNA methylation of a CpG island ∼1,000 base pairs upstream of the transcription start site of the Glut3 gene was noted, and by chromatin immunoprecipitation assays, enhanced MeCP2 binding at this site was noted to enhance histone deacetylase 2 recruitment, which interfered with Sp1 transcription factor binding. This change in DNA-nuclear protein association is perhaps responsible for decreased placental GLUT3 mRNA expression (40) and function (41). Taken together, these studies arm healthcare providers with evidence that the effects of IUGR are intergenerational and that the perinatal period is especially vulnerable to the effects of environmental input on later health and disease.
Future Directions
While the mechanisms behind the development of IUGR are being uncovered, the ability to project the development of IUGR is lacking. The only therapy available in the human is delivery of the IUGR infant based on achieved gestational maturity. This crucial intervention prevents intra-uterine demise and stillbirth. However, at times, delivery of the fetus is not plausible if fetal immaturity precludes extra-uterine survival. It therefore becomes essential to develop biomarkers that can accurately predict the development of IUGR and future chronic diseases, so that interventions can be developed, once recognized. Non-invasive modalities, which range from imaging techniques to “-omics,” are essential in determining the signatures predictive of IUGR. More importantly, development of pure cellular isolation methodology is essential if “-omics” is to be useful in clinical diagnosis.
While human studies are necessary both prenatally and during infancy (What is the ideal nutrition that prevents catch-up growth but maximizes the neurodevelopmental potential?), studies in vivo should continue to require animal models that will help in technology/methodology development and in establishing cause-and-effect associations. Animal models should continue to define our ability to undertake life-course and inter-generational studies at the present time. In both cases, prevention of premature aging with the earlier appearance of chronic disorders is essential toward preserving the life expectancy and productivity of IUGR infants similar to their normally grown counterparts.
Conclusions
In conclusion, intrauterine growth restriction results in altered in utero handling of glucose and regulation of glucose metabolism via insulin secretion. These adaptations result in generalized hypoglycemia in the fetus but also affect multiple pathways perturbing glucose homeostasis. Multiple organs, including the brain, liver, skeletal muscle, and pancreas, adapt in different ways to survive in the face of altered nutritional supply, resulting in a compromise to achieve optimal fetal growth. The reprogramming of these pathways may prove to be maladaptive in later life, when resources are more abundant, resulting in a glucose-intolerant phenotype. In animal models and human studies, there is evidence that this metabolic phenotype is intergenerational across generations, and programming of adult disease may result from epigenetic modifications. Several interventions, both lifestyle and drug-related, have been proposed to act on these affected pathways to protect the IUGR offspring from adverse metabolic consequences. More studies are needed to optimize these interventions and to carefully uncover and weigh unintended consequences of these treatments. Most importantly, a balance must be struck while preventing long-term metabolic consequences of IUGR, so that postnatal or other nutrient supply to the developing brain is not compromised. This balance may be achieved by encouraging postnatal catch-up growth in moderation, not to exceed the limits of convention by encroaching into the pre-obesogenic state.
Acknowledgments
We are grateful to the many present and past contributors to this body of work while in the Devaskar laboratory and research group.
Footnotes
S. U. Devaskar is supported by National Institute of Child Health and Human Development Grants HD-041230, HD-081206, and HD-087221-01, and A. Chu is supported by National Institute of Child Health and Human Development Grant 5K12 HD-034610 and American Heart Association Western States Affiliate Beginning Grant-in-Aid 15BGIA25710060.
No conflicts of interest, financial or otherwise, are declared by the author(s).
Author contributions: S.U.D. and A.C. edited and revised manuscript; S.U.D. approved final version of manuscript; A.C. prepared figures; S.U.D. and A.C. drafted manuscript.
References
- 1.Abbasi A, Thamotharan M, Shin BC, Jordan MC, Roos KP, Stahl A, Devaskar SU. Myocardial macronutrient transporter adaptations in the adult pregestational female intrauterine and postnatal growth-restricted offspring. Am J Physiol Endocrinol Metab 302: E1352–E1362, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Acevedo CG, Marquez JL, Rojas S, Bravo I. Insulin and nitric oxide stimulates glucose transport in human placenta. Life Sci 76: 2643–2653, 2005. [DOI] [PubMed] [Google Scholar]
- 3.Adam CL, Bake T, Findlay PA, Milne JS, Aitken RP, Wallace JM. Impact on birth weight and gender on early postnatal hypothalamic energy balance regulatory gene expression in the young lamb. Int J Dev Neurosci 31: 608–615, 2013. [DOI] [PubMed] [Google Scholar]
- 4.Battista MC, Oligny LL, St-Louis J, Brochu M. Intrauterine growth restriction in rats is associated with hypertension and renal dysfunction in adulthood. Am J Physiol Endocrinol Metab 283: E124–E131, 2002. [DOI] [PubMed] [Google Scholar]
- 5.Bellamy L, Casas JP, Hingorani AD, Williams DJ. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 335: 974, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Beringue F, Blondeau B, Castellotti MC, et al. Endocrine pancreas development in growth-retarded human fetuses. Diabetes 51: 385–391, 2002. [DOI] [PubMed] [Google Scholar]
- 7.Bhasin KK, van Nas A, Martin LJ, Davis RC, Devaskar SU, Lusis AJ. Maternal low-protein diet or hypercholesterolemia reduces circulating essential amino acids and leads to intrauterine growth restriction. Diabetes 58: 559–566, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bibee KP, Illsley NP, Moley KH. Asymmetric syncytial expression of GLUT9 splice variants in human term placenta and alterations in diabetic pregnancies. Reprod Sci 18: 20–27, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brett KE, Ferraro ZM, Yockell-Lelievre J, Gruslin A, Adamo KB. Maternal-fetal nutrient transport in pregnancy pathologies: the role of the placenta. Int J Mol Sci 15: 16153–16185, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brodsky D, Christou H. Current concepts in intrauterine growth restriction. J Int Care Med 19: 307–319, 2003. [DOI] [PubMed] [Google Scholar]
- 11.Carruthers A, DeZutter J, Ganguly A, Devaskar SU. Will the original glucose transporter isoform please stand up! Am J Physiol Endocrinol Metab 297: E836–E848, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Carter AM. Current topic: restriction of placental and fetal growth in the guinea pig. Placenta 14: 125–135, 1993. [DOI] [PubMed] [Google Scholar]
- 13.Carver TD, Anderson SM, Aldoretta PA, Esler AL, Hay WW Jr. Glucose suppression of insulin secretion in chronically hyperglycemic fetal sheep. Pediatr Res 38: 754–762, 1995. [DOI] [PubMed] [Google Scholar]
- 14.Carver TD, Anderson SM, Aldoretta PW, Hay WW Jr. Effect of low-level basal plus marked “pulsatile” hyperglycemia on insulin secretion in fetal sheep. Am J Physiol Endocrinol Metab 271: E865–E871, 1996. [DOI] [PubMed] [Google Scholar]
- 15.Challier JC, Hauguel S, Desmaizieres K. Effect of insulin on glucose uptake and metabolism in the human placenta. J Clin Endocrinol Metab 62: 803–807, 1986. [DOI] [PubMed] [Google Scholar]
- 16.Chen PY, Ganguly A, Rubbi L, et al.. Intrauterine calorie restriction affects placental DNA methylation and gene expression. Physiol Genomics 45: 565–576, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Coan PM, Vaughan OR, Sekita Y, Finn L, Burton GJ, Constancia M, Fowden AL. Adaptations in placental phenotype support fetal growth during undernutrition of pregnant mice. J Physiol 588: 527–538, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Conde-Agudelo A, Papageorghiou AT, Kennedy SH, Villar J. Novel biomarkers for predicting intrauterine growth restriction: a systematic review and meta-analysis. BJOG 120: 681–694, 2013. [DOI] [PubMed] [Google Scholar]
- 19.Constancia M, Hemberger M, Hughes J, Dean W, Ferguson-Smith A, Fundele R, et al. Placental-specific IGF-II is a major modulator of placental and fetal growth. Nature 417: 945–948, 2002. [DOI] [PubMed] [Google Scholar]
- 20.Dai Y, Thamotharan S, Garg M, Shin BC, Devaskar SU. Superimposition of postnatal calorie restriction protects the aging male intrauterine growth-restricted offspring from metabolic maladaptations“. Endocrinology 153: 4216–4226, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Das UG, He J, Ehrhardt RA, Hay WW Jr, Devaskar SU. Time-dependent physiological regulation of ovine placental GLUT3 glucose transporter protein. Am J Physiol Regul Integr Comp Physiol 279: R2252–R2261, 2000. [DOI] [PubMed] [Google Scholar]
- 22.Das UG, Sadiq HF, Soares MJ, Hay WW Jr, Devaskar SU. Time-dependent physiological regulation of rodent and ovine placental glucose transporter (GLUT-1) protein. Am J Physiol Regul Integr Comp Physiol 274: R339–R347, 1998. [DOI] [PubMed] [Google Scholar]
- 23.Das UG, Schroder RE, Hay WW Jr, Devaskar SU. Time-dependent and tissue-specific effects of circulating glucose on fetal ovine glucose transporters. Am J Physiol Regul Integr Comp Physiol 276: R809–R817, 1999. [DOI] [PubMed] [Google Scholar]
- 24.Dawson PA, Mychaleckyj JC, Fossey SC, Mihic SJ, Craddock AL, Bowden DW. Sequence and functional analysis of GLUT10: a glucose transporter in the type 2 diabetes-linked region of chromosome 20q12-13.1. Mol Genet Metab 74: 186–199, 2001. [DOI] [PubMed] [Google Scholar]
- 25.De Blasio MJ, Gatford KL, Harland ML, Robinson JS, Owens JA. Placental restriction reduces insulin sensitivity and expression of insulin signaling and glucose transporter genes in skeletal muscle, but not liver, in young sheep. Endocrinology 153: 2142–2151, 2012. [DOI] [PubMed] [Google Scholar]
- 26.De Blasio MJ, Gatford KL, McMillen IC, Robinson JS, Owens JA. Placental restriction of fetal growth increases insulin action, growth, and adiposity in the young lamb. Endocrinology 148: 1350–1358, 2007. [DOI] [PubMed] [Google Scholar]
- 27.Dellschaft NS, Alexandre-Gouabau MC, Gardner DS, Antignac JP, et al. Effect of pre- and postnatal growth and post-weaning activity on glucose metabolism in the offspring. J Endocrinol 224: 171–182, 2015. [DOI] [PubMed] [Google Scholar]
- 28.DiGiacomo JE, Hay WW Jr. Regulation of placental glucose transfer and consumption by fetal glucose production. Pediatr Res 25: 429–434, 1989. [DOI] [PubMed] [Google Scholar]
- 29.Doege H, Schürmann A, Bahrenberg G, Brauers A, Joost HG. GLUT8, a novel member of the sugar transport facilitator family with glucose transport activity. J Biol Chem 275: 16275–16280, 2000. [DOI] [PubMed] [Google Scholar]
- 30.Dumortier O, Blondeau B, Duvillie B, et al. Different mechanisms operating during different critical time-windows reduce rat fetal beta cell mass due to a maternal low-protein or low-energy diet. Diabetologia 50: 2495–2503, 2007. [DOI] [PubMed] [Google Scholar]
- 31.Eixarch E, Hernandez-Andrade E, Crispi F, Illa M, Torre I, Figueras F, et al. Impact on fetal mortality and cardiovascular Doppler of selective ligature of uteroplacental vessels compared with undernutrition in a rabbit model of intrauterine growth restriction. Placenta 32: 304–309, 2011. [DOI] [PubMed] [Google Scholar]
- 32.Emmanouilides GC, Townsend DE, Bauer RA. Effects of single umbilical artery ligation in the lamb fetus. Pediatrics 42: 919–927, 1968. [PubMed] [Google Scholar]
- 33.Equils O, Singh S, Karaburun S, Lu D, Thamotharan M, Devaskar SU. Intra-uterine growth restriction downregulates the hepatic toll like receptor-4 expression and function. Clin Dev Immunol 12: 59–66, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ericsson A, Hamark B, Jansson N, Johansson BR, Powell TL, Jansson T. Hormonal regulation of glucose and system A amino acid transport in first trimester placental villous fragments. Am J Physiol Regul Integr Comp Physiol 288: R656–R662, 2005. [DOI] [PubMed] [Google Scholar]
- 35.Ericsson A, Hamark B, Powell TL, Jansson T. Glucose transporter isoform 4 is expressed in the syncytiotrophoblast of first trimester human placenta. Hum Reprod 20: 521–530, 2005. [DOI] [PubMed] [Google Scholar]
- 36.Fowden Al Mundy L, Silver M. Developmental regulation of gluconeogenesis in the sheep fetus during late gestation. J Physiol 508: 937–947, 1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Freije WA, Thamotharan S, Lee R, Shin BC, Devaskar SU. The hepatic transcriptome of young suckling and aging intrauterine growth restricted male rats. J Cell Biochem 116: 566–579, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Galan HL, Hussey MJ, Barbera A, Ferrazzi E, Chung M, Hobbins JC, et al. Relationship of fetal growth to duration of heat stress in an ovine model of placental insufficiency. Am J Obstet Gynecol 180: 1278–1282, 1999. [DOI] [PubMed] [Google Scholar]
- 39.Gallo LA, Tran M, Cullen-McEwen LA, Denton KM, Jefferies AH, Moritz KM, Wlodek ME. Transgenerational programming of fetal nephron deficits and sex-specific adult hypertension in rats. Reprod Fertil Dev 26: 1032–1043, 2014. [DOI] [PubMed] [Google Scholar]
- 40.Ganguly A, Chen Y, Shin BC, Devaskar SU. Prenatal caloric restriction enhances DNA methylation and MeCP2 recruitment with reduced murine placental glucose transporter isoform 3 expression. J Nutr Biochem 25: 259–266, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40a.Pinney SE, Jaeckle Santos LJ, Han Y, Stoffers DA, Simmons RA. Exendin-4 increases histone acetylase activity and reverses epigenetic modifications that silence Pdx-1 in the intra-uterine growth retarded rat. Diabetologia 54: 2606–2614, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ganguly A, Collis L, Devaskar SU. Placental glucose and amino acid transport in calorie-restricted wild-type and Glut 3 null heterozygous mice. Endocrinology 153: 3995–4007, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ganguly A, McKnight RA, Raychaudhuri S. Glucose transporter isoform-3 mutations cause early pregnancy loss and fetal growth restriction. Am J Physiol Endocrinol Metab 292: E1241–E1255, 2007. [DOI] [PubMed] [Google Scholar]
- 43.Garg M, Thamotharan M, Dai Y, Lagishetty V, Matveyenko AV, Lee WNP, Devaskar SU. Glucose intolerance and lipid metabolic adaptations in response to intrauterine and postnatal calorie restriction in male adult rats. Endocrinology 154: 102–113, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garg M, Thamotharan M, Dai Y, Lee PW, Devaskar SU. Embryo-transfer of the F2 postnatal calorie restricted female rat offspring into a control intra-uterine environment normalizes the metabolic phenotype. Metabolism 62: 432–441, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Garg M, Thamotharan M, Dai Y, Thamotharan S, Shin BC, Stout D, Devaskar SU. Early postnatal caloric restriction protects adult male intrauterine growth-restricted offspring from obesity. Diabetes 61: 1391–1398, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Garg M, Thamotharan M, Oak SA, Pan G, MacLaren DC, Lee PWN, Devaskar SU. Early exercise regimen improves insulin sensitivity in the intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab 296: E272–E281, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garg M, Thamotharan M, Pan G, Lee PWN, Devaskar SU. Early exposure of the pregestational intrauterine and postnatal growth-restricted female offspring to a peroxisome proliferator-activated receptor-γ agonist. Am J Physiol Endocrinol Metab 298: E489–E498, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garg M, Thamotharan M, Rogers L, Bassillian S, Lee WN, Devaskar SU. Glucose metabolic adaptations in the intrauterine growth-restriction adult female rat offspring. Am J Physiol Endocrinol Metab 290: E1218–E1226, 2006. [DOI] [PubMed] [Google Scholar]
- 49.Gatford KL, Kaur G, Falcao-Tebas F, Wadley GD, Wlodek ME, Laker RC, Ebeling PR, McConell GK. Exercise as an intervention to improve metabolic outcomes after intrauterine growth restriction. Am J Physiol Endocrinol Metab 306: E999–E1012, 2014. [DOI] [PubMed] [Google Scholar]
- 50.Gatford KL, Mohammad SN, Harland ML, De Blasio MJ, Fowden AL, Robinson JS, Owens JA. Impaired beta-cell function and inadequate compensatory increases in beta-cell mass after intrauterine growth restriction in sheep. Endocrinology 149: 5118–5127, 2008. [DOI] [PubMed] [Google Scholar]
- 51.Gatford KL, Simmons RA. Prenatal programming of insulin secretion in intrauterine growth restriction. Clin Obstet Gynecol 56: 520–528, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gatford KL, Sulaiman SA, Mohammed S, De Blasio MJ, Harland ML, Simmons RA, Owens JA. Neonatal exendin-4 reduces growth, fat deposition and glucose tolerance during treatment in the intrauterine growth-restricted lamb. PLos One 8: e56553, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gibson LC, Shin BC, Dai Y, Freije W, Kositamongkol S, Cho J, Devaskar SU. Early leptin intervention reverses perturbed energy balance regulating hypothalamic neuropeptides in the pre- and postnatal calorie-restricted female rat offspring. J Neurosci Res 93: 902–912, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Girard J. Gluconeogenesis in late fetal and early neonatal life. Biol Neonate 50: 237–258, 1986. [DOI] [PubMed] [Google Scholar]
- 55.Girard JR, Ferre P, Gilbert M, Kervran A, Assan R, Marliss EB. Fetal metabolic response to maternal fasting in the rat. Am J Physiol Endocrinol Metab Gastrointest Physiol 232: E456–E463, 1977. [DOI] [PubMed] [Google Scholar]
- 56.Gresores A, Anderson S, Hood D, Zerbe GO, Hay WW Jr. Separate and joint effects of arginine and glucose on ovine fetal insulin secretion. Am J Physiol Endocrinol Metab 272: E68–E73, 1997. [DOI] [PubMed] [Google Scholar]
- 57.Gude NM, Stevenson JL, Rogers S, Best JD, Kalionis B, Huisman MA, Erwich JJ, Timmer A, King RG. GLUT12 expression in human placenta in first trimester and term. Placenta 24: 566–570, 2003. [DOI] [PubMed] [Google Scholar]
- 58.Guimaraes LR, Jacka FN, Gama CS, Berk M, Leitao-Azevedo CL, Belmonte de Abreu MG, Lobato MI, Andreazza AC, Cereser KM, Kapczinski F, Belmonte-de-Abreu P. Serum levels of brain-derived neurotrophic factor in schizophrenia on a hypocaloric diet. Prog Neuropsychopharmacol Biol Psychiatry 32: 1595–1598, 2008. [DOI] [PubMed] [Google Scholar]
- 59.Habener JF, Stoffers DA. A newly discovered role of transcription factors involved in pancreas development and the pathogenesis of diabetes mellitus. Proc Am Assoc Physicians 110: 12–21, 1998. [PubMed] [Google Scholar]
- 60.Ham JN, Crutchloww MF, Desai BM, et al. Exendin-4 normalizes islet vascularity in intrauterine growth restricted rats: potential role of VEGF. Pediatr Res 66: 42–46, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hay WW., Jr Development of the fetus: carbohydrate and lipid metabolism. In: Nutrition in Pediatrics: Basic Science, Clinical Applications, edited by Duggan C, Watkins JB, Walker I. Hamilton, Canada: BC Decker, 2008. [Google Scholar]
- 62.Hay WW., Jr Recent observations on the regulation of fetal metabolism by glucose. J Physiol 572: 17–24, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heijmans BT, Tobi EW, Stein AD, Putter H, Blauw GJ, Susser ES, Slagboom PE, Lumey LH. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc Natl Acad Sci USA 105: 17046–17050, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hills FA, Elder MG, Chard T, Sullivan MH. Regulation of human villous trophoblast by insulin-like growth factors and insulin-like growth factor binding protein-1. J Endocrinol 183: 487–496, 2004. [DOI] [PubMed] [Google Scholar]
- 65.Howie GJ, Sloboda DM, Vickers MH. Maternal undernutrition during critical windows of development results in differential and sex-specific effects on postnatal adiposity and related metabolic profiles in adult rat offspring. Br J Nutr 108: 298–307, 2012. [DOI] [PubMed] [Google Scholar]
- 66.Huber K, Miles JL, Normal AM, Thompson NM, Davidson M, Breier BH. Prenatally induced changes in muscle structure and metabolic function facilitate exercise-induced obesity prevention. Endocrinology 150: 4135–4144, 2009. [DOI] [PubMed] [Google Scholar]
- 67.Illsley NP. Glucose transporters in the human placenta. Placenta 21: 14–22, 2000. [DOI] [PubMed] [Google Scholar]
- 68.Jansson T, Wennergren M, Illslsey NP. Glucose transporter protein expression in human placenta throughout gestation and in intrauterine growth retardation. J Clin Endocrinol Metab 77: 1554–1562, 1993. [DOI] [PubMed] [Google Scholar]
- 69.Jansson T, Ylven K, Wennergren M, Powell TL. Glucose transport and system A activity in syncytiotrophoblast microvillous and basal plasma membranes in intrauterine growth restriction. Placenta 23: 392–399, 2002. [DOI] [PubMed] [Google Scholar]
- 70.Janzen C, Lei MY, Cho J, Sullivan P, Shin BC, Devaskar SU. Placental glucose transporter 3 (GLUT3) is up-regulated in human pregnancies complicated by late-onset intrauterine growth restriction. Placenta 34: 1072–1078, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Julian CG, Vargas E, Browne VA, Wilson MJ, Bigham AW, Rodriguez C, et al. Potential role for elevated maternal enzymatic antioxidant status in Andean protection against altitude-associated SG. J Mater Fetal Neonatal Med 25: 1233–1240, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kainulainen H, Jarvinen T, Heinonen PK. Placental glucose transporters in fetal intrauterine growth retardation and macrosomia. Gynecol Obstet Invest 44: 89–92, 1997. [DOI] [PubMed] [Google Scholar]
- 73.Kavitha JV, Rosario FJ, Nijland MJ, McDonald TJ, Wu G, Kanai Y, Powell TL, Nathanielsz PW, Jansson T. Down-regulation of placental mTOR, insulin/IGF-1 signaling and nutrient transporters in response to maternal nutrient restriction in the baboon. FASEB J 28: 1294–1305, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lafeber HN, Rolph TP, Jones CT. Studies on the growth of the fetal guinea pig. The effects of ligation of the uterine artery on organ growth and development. J Dev Physiol 6: 441–459, 1984. [PubMed] [Google Scholar]
- 75.Lager S, Powell TL. Regulation of nutrient transport across the placenta. J Pregnancy 2012: 179827, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Laguna-Barraza R, Bermejo-Alvarez P, Ramos-Ibeas P, de Frutos C, Lopez-Cardona AP, Calle A, Fernandez-Gonzalez R, Pericuesta E, Ramirez MA, Gutierrez-Adan A. Sex-specific embryonic origin of postnatal phenotypic variability. Reprod Fertil Dev 25: 38–47, 2012. [DOI] [PubMed] [Google Scholar]
- 77.Laker RC, Gallo LA, Wlodek ME, Siebel AL, Wadley GD, McConell GK. Short-term exercise training early in life restores deficits in pancreatic beta-cell mass associated with growth restriction in adult male rats. Am J Physiol Endocrinol Metab 301: E931–E940, 2011. [DOI] [PubMed] [Google Scholar]
- 77a.Lane RH, Ramirez RJ, Tsirka AE, Kloesz JL, McLaughlin MK, Gruetzmacher EM, Devaskar SU. Uteroplacental insufficiency lowers the threshold towards hypoxia-induced cerebral apoptosis in growth retarded fetal rats. Brain Res 23: 186–193, 2001. [DOI] [PubMed] [Google Scholar]
- 78.Liechty EA, Boyle DW. Protein metabolism in the fetal-placental unit. In: Principles of Perinatal-Neonatal Metabolism, edited by Cowett RM. New York: Springer, 1998. [Google Scholar]
- 79.Lim K, Lombardo P, Schneider-Kolsky M, Black MJ. Intrauterine growth restriction coupled with hyperglycemia: effects on cardiac structure in adult rats. Pediatr Res 72: 344–351, 2012. [DOI] [PubMed] [Google Scholar]
- 80.Limesand SW, Hay WW Jr. Adaptation of ovine fetal pancreatic insulin secretion to chronic hypoglycaemia and euglycaemic correction. J Physiol 547: 95–105, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Limesand SW, Jensen J, Hutton JC, Hay WW Jr. Diminished beta-cell replication contributes to reduced beta-cell mass in fetal sheep with intrauterine growth restriction. Am J Physiol Regul Integr Comp Physiol 288: R1297–R1305, 2005. [DOI] [PubMed] [Google Scholar]
- 82.Limesand SW, Rozance PJ, Zerbe GO, Hutton JC, Hay WW Jr. Attenuated insulin release and storage in fetal sheep pancreatic islets with intrauterine growth restriction. Endocrinology 147: 1488–1497, 2006. [DOI] [PubMed] [Google Scholar]
- 83.Lingas R, Dean F, Matthews SG. Maternal nutrient restriction (48 h) modifies brain corticoid receptor expression and endocrine function in the fetal guinea pig. Brain Res 846: 236–242, 1999. [DOI] [PubMed] [Google Scholar]
- 84.Longo S, Bollani L, Decembrino L, Di Comite A, Angelini M, Stronati M. Short-term and long-term sequelae in intrauterine growth retardation (IUGR). J Matern Fetal Neonatal Med 26: 222–225, 2013. [DOI] [PubMed] [Google Scholar]
- 85.Lumey LH, Stein AD. Offspring birth weights after maternal intrauterine undernutrition: a comparison within sibships. Am J Epidemiol 146: 810–819, 1997. [DOI] [PubMed] [Google Scholar]
- 86.Ma Y, Zhu MJ, Uthlaut AB, Nijland MJ, Nathanielsz PW, Hess BW, Ford SP. Upregulation of growth signaling and nutrient transporters in cotyledons of early to mid-gestational nutrient restricted ewes. Placenta 32: 255–263, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Maliszewski-Hall AM, Stein AB, Alexander M, Ennis K, Rao R. Acute hypoglycemia results in reduced cortical neuronal injury in the developing IUGR rat. Pediatr Res 78: 7–13, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Marín-Juez R, Rovira M, Crespo D, van der Vaart M, Spaink HP, Planas JV. GLUT2-mediated glucose uptake and availability are required for embryonic brain development in zebrafish. J Cereb Blood Flow Metab 35: 74–85, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Matveyenko AV, Singh I, Shin BC, Georgia S, Devaskar SU. Differential effects of prenatal and postnatal nutritional environment on β-cell mass development and turnover in male and female rats. Endocrinology 151: 5647–5656, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Murray E, Fernandes M, Fazel M, Kennedy SH, Villar J, Stein A. Differential effects of intrauterine growth restriction on childhood neurodevelopment: a systematic review. BJOG 122: 1062–1072, 2015. [DOI] [PubMed] [Google Scholar]
- 91.Newnham JP, Kelly RW, Patterson L, James I. The influence of maternal undernutrition in ovine twin pregnancy on fetal growth and Doppler flow-velocity waveforms. J Dev Physiol 16: 277–282, 1991. [PubMed] [Google Scholar]
- 92.Nicolini U, Hubinont C, Santolaya J, Fisk NM, Rodeck CH. Effects of fetal intravenous glucose challenge in normal and growth retarded fetuses. Horm Metab Res 22: 426–430, 1990. [DOI] [PubMed] [Google Scholar]
- 93.Nikolova G, Jabs N, Konstantinova I. The vascular basement membrane: a niche for insulin gene expression and β cell proliferation. Dev Cell 10: 397–405, 2006. [DOI] [PubMed] [Google Scholar]
- 94.Oak S, Tran C, Castillo MO, Thamotharan S, Thamotharan M, Devaskar SU. Peroxisome proliferator-activated receptor-γ agonist improves skeletal muscle insulin signaling in the pregestational intrauterine growth-restricted rat offspring. Am J Physiol Endocrinol Metab 297: E514–E524, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Oak SA, Tran C, Pan G, Thamotharan M, Devaskar SU. Perturbed skeletal muscle insulin signaling in the adult female intrauterine growth-restricted rat. Am J Physiol Endocrinol Metab 290: E1321–E1330, 2006. [DOI] [PubMed] [Google Scholar]
- 96.Ochi H, Matsubara K, Kusanagi Y, Taniguchi H, Ito M. Significance of a diastolic notch in the uterine artery flow velocity waveform induced by uterine embolization in the pregnant ewe. Br J Obstet Gynaecol 105: 1118–1121, 1998. [DOI] [PubMed] [Google Scholar]
- 97.Orendi K, Kivity V, Sammar M, Grimpel Y, Gonen R, Meiri H, et al. Placental and trophoblastic in vitro models to study preventive and therapeutic agents for preeclampsia. Placenta 32, Suppl: S49–S54, 2011. [DOI] [PubMed] [Google Scholar]
- 98.Owens JA, Gatford KL, De Blasio MJ, Edwards LJ, McMillen IC, Fowden AL. Restriction of placental growth in sheep impairs insulin secretion but not sensitivity before birth. J Physiol 584: 935–949, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Park JH, Stoffers DA, Nicholls RD, Simmons RA. Development of Type 2 diabetes following intrauterine growth retardation in rats is associated with progressive epigenetic silencing of Pdx1. J Clin Invest 118: 2316–2324, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Raab EL, Vuguin PM, Stoffers DA, Simmons RA. Neonatal exendin-4 treatment reduces oxidative stress and prevents hepatic insulin resistance in intrauterine growth-retarded rats. Am J Physiol Regul Integr Comp Physiol 297: R1785–R1794, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Raychaudhuri N, Raychaudhuri S, Thamotharan M, Devaskar SU. Histone code modifications repress glucose transporter 4 expression in the intrauterine growth-restricted offspring. J Biol Chem 283: 13611–13626, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Riddle ES, Campbell MS, Lang BY, Bierer R, Wang Y, Bagley HN, Joss-Moore LA. Intrauterine growth restriction increases TNFα and activates the unfolded protein response in male rat pups. J Obes 2014: 829862, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Roberts CT, Sohlstrom A, Kind KL, Earl RA, Khong TY, Robinson JS, et al. Maternal food restriction reduced the exchange surface area and increases the barrier thickness of the placenta in the guinea-pig. Placenta 22: 177–185, 2001. [DOI] [PubMed] [Google Scholar]
- 104.Roos S, Jansson N, Palmberg I, Saljo K, Powell TL, Jansson T. Mammalian target of rapamycin in the human placenta regulated leucine transport and is down-regulated in restricted fetal growth. J Physiol 582: 449–459, 2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Roos S, Kanai Y, Prasad PD, Powell TL, Jansson T. Regulation of placental amino acid transporter activity by mammalian target of rapamycin. Am J Physiol Cell Physiol 296: C142–C150, 2009. [DOI] [PubMed] [Google Scholar]
- 106.Sadagurski M, Landeryou T, Blandino-Rosano M, Cady G, Elghazi L, Meister D, See L, Bartke A, Bernal-Mizrachi E, Miller RA. Long-lived crowded-litter mice exhibit lasting effects on insulin sensitivity and energy homeostasis. Am J Physiol Endocrinol Metab 306: E1315–E1324, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sadiq HF, Das UG, Tracy TF, Devaskar SU. Intrauterine growth restriction differentially regulated perinatal brain and skeletal muscle glucose transporters. Brain Res 823: 96–103, 1999. [DOI] [PubMed] [Google Scholar]
- 108.Sadiq HF, de Mello DE, Devaskar SU. The effect of intrauterine growth restriction upon fetal and postnatal hepatic glucose transporter and glucokinase proteins. Pediatr Res 43: 91–100, 1998. [DOI] [PubMed] [Google Scholar]
- 109.Sakta M, Kurachi H, Imai T. Increase in human placental glucose transpoters-1 during pregnancy. Eur J Endocrinol 132: 206–212, 1995. [DOI] [PubMed] [Google Scholar]
- 110.Shin BC, Dai Y, Thamotharan M, Gibson LC, Devaskar SU. Pre- and postnatal calorie restriction perturbs early hypothalamic neuropeptide and energy balance. J Neurosci Res 90: 1169–1182, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Simchen MJ, Weisz B, Zilberberg E, Morag I, Weissmann-Brenner A, Sivan E, Dulitzki M. Male disadvantage for neonatal complications of term infants, especially in small-for-gestational age neonates. J Matern Fetal Neonatal Med 27: 839–843, 2014. [DOI] [PubMed] [Google Scholar]
- 112.Simmons RA. Developmental origins of diabetes: the role of oxidative stress. Best Pract Res Clin Endocrinol Metab 26: 701–708, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Simmons RA, Suponitsky-Kroyter I, Selak MA. Progressive accumulation of mitochondrial DNA mutations and decline in mitochondrial function lead to β-cell failure. J Biol Chem 280: 28785–28791, 2005. [DOI] [PubMed] [Google Scholar]
- 114.Stoffers DA, Desai BM, DeLeon DD, Simmons RA. Neonatal exendin-4 prevents the development of diabetes in the intrauterine growth retarded rat. Diabetes 52: 734–740, 2003. [DOI] [PubMed] [Google Scholar]
- 115.Supramaniam VG, Jenkin G, Loose J, Wallace EM, Miller SL. Chronic fetal hypoxia increases activin A concentrations in the late-pregnant sheep. BJOG 113: 102–109, 2006. [DOI] [PubMed] [Google Scholar]
- 116.Swanson AM, David AL. Animal models of fetal growth restriction: considerations for translational medicine. Placenta 36: 623–630, 2015. [DOI] [PubMed] [Google Scholar]
- 116a.Symonds M, Sebert S, Hyatt M, Budge H. Nutritional programming of the metabolic syndrome. Nat Rev Endocrinol 5: 604–610, 2009. [DOI] [PubMed] [Google Scholar]
- 117.Thamotharan M, Garg M, Oak S, Rogers LM, Pan G, Fangiorgi S, Lee PWN, Devaskar SU. Transgenerational inheritance of the insulin-resistant phenotype in embryo-transferred intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab 292: E1270–E1279, 2007. [DOI] [PubMed] [Google Scholar]
- 118.Thamotharan M, Shin BC, Suddirikku DT, Thamotharan S, Garg M, Devaskar SU. GLUT4 expression and subcellular locations in the intrauterine growth-restricted adult rat female offspring. Am J Physiol Endocrinol Metab 288: E935–E947, 2005. [DOI] [PubMed] [Google Scholar]
- 119.Thorn SR, Brown LD, Rozance PJ, Hay WW Jr, Friedman JE. Increased hepatic glucose production in fetal sheep with intrauterine growth restriction is not suppressed by insulin. Diabetes 62: 65–73, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Thorn SR, Rozance PJ, Brown LD, Hay WW Jr. The intrauterine growth restriction phenotype: fetal adaptations and potential implications for later life insulin resistance and diabetes. Semin Reprod Med 29: 225–236, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tomi M, Zhao Y, Thamotharan S, Shin BC, Devaskar SU. Early life nutrient restriction impairs blood-brain metabolic profile and neurobehavior predisposing to Alzheimer's disease with aging. Brain Res 1495: 61–75, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Torres-Rovira L, Tarrade A, Astiz S, Mourier E, Perez-Solana M, de la Cruz P, Gomez-Fidalgo E, Sanchez-Sanchez R, Chavatte-Palmer P, Gonzalez-Bulnes A. Sex and breed-dependent organ development and metabolic responses in fetuses from lean and obese/leptin resistant swine. PLos One 8: e66728, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Turner AJ, Trudinger BJ. A modification of the uterine artery restriction technique in the guinea pig fetus produces asymmetrical ultrasound growth. Placenta 30: 236–240, 2009. [DOI] [PubMed] [Google Scholar]
- 124.Van Assche FA, De Prins F, Aerts L, et al. The endocrine pancreas in small-for-dates infants. Br J Obstet Gynaecol 84: 751–753, 1977. [DOI] [PubMed] [Google Scholar]
- 125.Vickers MH, Ikenasio BA, Breier BH. Adult growth hormone treatment reduces hypertension and obesity induced by an adverse prenatal environment. J Endocrinol 175: 615–623, 2002. [DOI] [PubMed] [Google Scholar]
- 126.Vickers MH, Ikenasio BA, Breier BH. IFG-1 treatment reduces hyperphagia, obesity and hypertension in metabolic disorders induced by fetal programming. Endocrinology 142: 3964–3973, 2001. [DOI] [PubMed] [Google Scholar]
- 127.Wang D, Pascual JM, Yang H, Englestad K, Mao X, Cheng J, Yoo J, Noebels JL, De Vivo DC. A mouse model for GLUT1 haploinsufficiency. Hum Mol Genet 15: 1169–1179, 2006. [DOI] [PubMed] [Google Scholar]
- 128.Wigglesworth JS. Fetal growth retardation. Animal model: uterine vessel ligation in the pregnant rat. Am J Pathol 77: 347–350, 1974. [PMC free article] [PubMed] [Google Scholar]
- 129.Williams PJ, Pipkin FB. The genetics of preeclampsia and other hypertensive disorders of pregnancy. Best Pract Res Clin Obstet Gynaecol 25: 405–417, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Xu J, Lu C, Wang J, Zhang R, Qian X, Zhu H. Regulation of human trophoblast GLUT3 glucose transporter by mammalian target of rapamycin signaling. Int J Mol Sci 16: 13815–13828, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Yang BT, Dayeh TA, Volkov PA, et al. Increased DNA methylation and decreased expression of PDX-1 in pancreatic islets from patients with Type 2 diabetes. Mol Endocrinol 26: 1203–1212, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Yates DT, Macko AR, Nearing M, Chen X, Rhoads RP, Limesand SW. Developmental programming in response to intrauterine growth restriction impairs myoblast function and skeletal muscle metabolism. J Pregnancy 2012: 631938, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zhang L, Xu D, Liu Y, Chu F, Guo Y, Gong J, Zheng X, Chen L, Wang H. Prenatal food restriction induces a hypothalamic-pituitary adrenocortical axis-associated neuroendocrine metabolic programmed alteration in adult offspring rats. Arch Med Res 44: 335–345, 2013. [DOI] [PubMed] [Google Scholar]