Abstract
Background
Immune‐mediated polyarthopathy (IMPA) is common in dogs, and is monitored by serial arthrocenteses.
Hypothesis/Objectives
Plasma C‐reactive protein (CRP), interleukin‐6 (IL‐6), and CXCL8 (interleukin‐8) would serve as noninvasive markers of joint inflammation in IMPA.
Animals
Nine client‐owned dogs with idiopathic IMPA; 6 healthy controls.
Methods
Prospective study. Plasma CRP, IL‐6, and CXCL8 were measured by ELISA at baseline, 2, and 4 weeks during treatment with prednisone at 50 mg/m2/day. Arthrocenteses, the canine brief pain inventory (CBPI), and accelerometry collars were used to assess joint inflammation, lameness, and mobility at all 3 time points.
Results
C‐reactive protein concentrations were higher in IMPA dogs (median 91.1 μg/mL, range 76.7–195.0) compared with controls (median <6.3 μg/mL, <6.3–13.7; P = .0035), and were significantly lower at week 2 (10.6 μg/mL, <6.3–48.8) and week 4 (<6.3 μg/mL, <6.3–24.4; P < .001).
C‐reactive protein was correlated with median CBPI scores (r = 0.68; P = .0004), joint cellularity (r = 0.49, P = .011), and mobility by accelerometry (r = −0.42, P = .048). Plasma IL‐6 concentrations were also higher in IMPA dogs (median 45.9 pg/mL), compared with controls (median <15.7 pg/mL; P = .0008). IL‐6 was lower in IMPA dogs by week 4 (<15.7 pg/mL; P = .0099), and was modestly correlated with CBPI scores (r = 0.47, P = .023). CXCL8 did not differ significantly between IMPA and healthy dogs.
Conclusions
Plasma CRP and IL‐6 might be useful surrogate markers of synovial inflammation and disease activity in dogs with IMPA.
Keywords: C‐reactive protein, Cytokines, IL‐6, Polyarthritis
Abbreviations
- CBPI
canine brief pain inventory
- CRP
C‐reactive protein
- CXCL8
CXC chemokine‐ligand‐8; a.k.a. interleukin‐8
- IL‐6
interleukin 6
- IMPA
immune‐mediated polyarthropathy
- RA
rheumatoid arthritis
Immune‐mediated polyarthropathy (IMPA) is the most common cause of chronic relapsing fevers in dogs.1 It is typically seen in young adult dogs, and is associated with significant pain, shifting leg lameness, reluctance to rise, and anorexia. Any breed of dog may develop IMPA; however, Rottweilers, Labrador Retrievers, Golden Retrievers, Shetland Sheepdogs, Irish Setters, Cocker Spaniels, and American Eskimo dogs are overrepresented.2, 3, 4, 5, 6 The diagnosis of IMPA is made by arthrocentesis and synovial fluid analysis that demonstrates a neutrophilic inflammation in multiple joints, typically the carpi and tarsi, without serologic or microbiologic evidence of underlying infection. Serial arthrocenteses and synovial fluid analyses are recommended to monitor response to treatment;7 however, repeated joint taps are invasive, require sedation, and can be expensive. Because of this, treatment adjustments are often made without any objective measures of disease remission.
Several proinflammatory molecules show promise as potential noninvasive markers of joint inflammation in dogs with IMPA, to include C‐reactive protein (CRP), interleukin‐6 (IL‐6), and interleukin‐8 (IL‐8; CXCL8).8, 9 Serum CRP concentrations were elevated in dogs with IMPA, but objective markers of clinical response were not included.9, 10 IL‐6 is increased in the synovial fluid of dogs with rheumatoid arthritis (RA),11 but has not been evaluated in IMPA. CXCL8 concentrations were increased in the synovial fluid of dogs with IMPA,8 but plasma concentrations and their relationship with clinical response have not been evaluated.
The purpose of this study, therefore, was to determine whether plasma concentrations of the inflammatory mediators CRP, IL‐6, and CXCL8 correlate with clinical and cytologic response to treatment in idiopathic immune‐mediated polyarthropathy in dogs, as measured by a validated pain inventory questionnaire, increased mobility as measured by accelerometry, and serial synovial fluid analyses.
Materials and Methods
Dog Screening
Dogs of any breed with a suspected diagnosis of polyarthropathy were screened for the study. To be eligible, dogs could not have received immunosuppressive drugs (eg, glucocorticoids, azathioprine, or cyclosporine) within 1 month before sampling for the study. Following informed consent from owners, a standardized diagnostic work‐up was performed for each patient, to include a CBC, biochemical panel, urinalysis, and determination of serum antibodies against B. burgdorferi and A. phagocytophilum. Dogs with positive serologies to B. burgdorferi or A. phagocytophilum, while not definitive for active infection, were not eligible for inclusion. Thoracic radiographs and abdominal ultrasonography were performed to help rule out the presence of secondary causes of IMPA. Dogs with historical, physical exam, biochemical, or imaging evidence of underlying disease, to include systemic infection, primary gastrointestinal or hepatic disease, or systemic neoplasia, which could lead to secondary immune‐mediated polyarthropathy (Types II‐IV),12 were not eligible for enrollment. The study was approved by the Institutional Animal Care and Use Committee at the University of Wisconsin, and all owners provided written informed consent.
Measures of Lameness
Owners of all dogs completed a standardized pain inventory questionnaire (Canine Brief Pain Inventory [CBPI])13 at each study visit. In addition, each enrolled dog was fitted at baseline with a collar containing an omnidirectional accelerometer (Actical1),14 which was worn continuously throughout the 4‐week study period.
Direct Measures of Joint Inflammation
Dogs were sedated with butorphanol (0.2–0.4 mg/kg IV) and dexmedetomidine (2–5 μg/kg IV) for joint radiographs, and for collection of synovial fluid by arthrocentesis of the stifle, tarsal, and carpal joints. Synovial fluid was submitted for cytology and aerobic bacterial culture. If <0.25 mL of synovial fluid was obtained from a joint, direct smears of the fluid were made, and cell counts were extrapolated from cytology.15 A diagnosis of IMPA in otherwise eligible dogs was defined as suppurative inflammation in 2 or more joints (synovial fluid total nucleated cell count >3,000/μL and neutrophils >12% of the nucleated cells),2, 4, 5 with a negative bacterial culture and without radiographic evidence of erosion.
Surrogate Plasma Markers of Inflammation
Six milliliters of whole blood in EDTA was obtained at baseline from all dogs, and plasma was harvested and stored at −80°C for measurement of canine CRP (Canine C‐Reactive Protein ELISA Kit2),16, 17 IL‐6 (Quantikine® Canine IL‐6 immunoassay3),18, 19 and CXCL8 (Quantikine® Canine CXCL8/IL‐83).18, 20All assays were run with concurrent standard curves using canine kit standards, and all time points for individual dogs were measured within the same assay run. In our hands, the CRP assay showed interassay CV's of 1.0–7.9% over the range of relevant concentrations, intra‐assay CV's of 2.3–8.9%, and a limit of detection of 6.3 μg/mL. The IL‐6 assay had interassay CV's of 3.5–20.2%, intra‐assay CV's of 1.7–8.6%, and a limit of detection of 15.7 pg/mL, and the CXCL8 assay had interassay CV's of 9.5–11.0%, intra‐assay CV's of 2.8–9.6%, and a limit of detection of 15.6 pg/mL. For the purposes of statistical analyses, samples with CRP, IL‐6, or CXCL8 that were detected below the limits of detection were encoded as 6.2 μg/mL, 15.6 pg/mL, and 15.5 pg/mL, respectively.
Standardized Treatment
After baseline evaluation, dogs were initially treated with a standard protocol of tramadol (2–5 mg/kg PO q 6–8 h) to control pain, and any previously administered NSAIDs were discontinued. Once joint cultures were returned negative and dogs had been off of NSAIDs (if given) for at least 7 days, all study dogs were treated with prednisone (50 mg/m2/day PO) for 4 weeks. Dogs that did not show a resolution of fever and a substantial reduction in clinical lameness by week 2, as determined by physical exam and owner assessment, were eligible to receive a rescue protocol of oral azathioprine (50 mg/m2/day) as an adjunct to prednisone.
Immune‐mediated polyarthropathy dogs were rechecked at 2 and 4 weeks after starting prednisone, and were reevaluated with a complete history and physical exam, CBC, blood chemistry, and urinalysis. Lameness was evaluated by the owner at each recheck, using the CBPI questionnaire. Plasma was again obtained at 2 and 4 weeks for measurement of CRP, IL‐6, and CXCL8. Plasma was also obtained from 6 healthy, staff‐owned adult dogs at 3 time points (each 2 weeks apart) and was run in parallel with patient samples as normal controls. For IMPA dogs, daily activity counts were retrieved from the accelerometer at the end of the study. For statistical analyses, activities during the 48 hours before initiation of treatment were compared to activities during the 48 hours immediately before the 2‐ and 4‐week rechecks.21
Sample Size and Statistical Analyses
Based on a narrow reference range for CRP in dogs (0.5–10 μg/mL) and previously reported CRP responses in dogs with steroid‐responsive meningitis,22 a pilot sample size of 10 dogs was planned to provide >90% power to show significant changes in CRP in dogs with IMPA during active disease and clinical remission. Friedman ANOVA by ranks, followed by Dunn's multiple comparison testing, was used to compare median scores from the CBPI questionnaire, daily activity by accelerometry, synovial fluid total nucleated cell counts, and ELISA results for inflammatory mediators over time during treatment. A Spearman rank correlation was used to associate inflammatory mediators and measures of clinical remission, to include joint cellularity, median CBPI scores, and accelerometry data. P < .05 was considered statistically significant.
Results
Dog Characteristics at Enrollment
Of 15 dogs that were screened, 4 dogs did not meet the criteria for diagnosis of idiopathic IMPA, and 11 were enrolled. Of these, 9 dogs completed the 4‐week study and were included in data analyses. Included dogs had a median age of 4.0 years (range 1.5–12.0) and a median body weight of 29.6 kg (range 5.4–83.4), and comprised 6 males (5 neutered, 1 intact) and 3 spayed females, of various breeds (4 mixed breeds, 2 Great Danes, and one each of Cockapoo, Golden Retriever, and Border Collie).
All dogs had arthrocentesis of 6 joints, to include both carpi, both tarsi, and both stifles, with a nucleated cell count >3,000/μL and >12% neutrophils in a median of 5 joints (range 2–6) for each dog (Table 1). The number of joints in each dog at baseline with moderate‐to‐severe inflammation, ranged from 0 to 5 (median 2). CBC, biochemical panel, and urinalyses were within reference intervals, except for subclinical thrombocytopenia in 2 dogs.
Table 1.
Changes in joint cytology in 9 dogs with immune‐mediated polyarthropathy (IMPA) treated with prednisone (50 mg/m2) for 4 weeks. Data shown are the median with ranges for the number of affected joints per dog (of 6 sampled) at each visit.
| Synovial fluid cytology | Baseline | Week 2 | Week 4 |
|---|---|---|---|
| Joints per dog with >3,000 nucleated cells/μL | 5 (2–6)a | 1 (0–6)a | 1 (0–6) |
| Joints per dog with>12% neutrophils | 5 (2–6) | 1 (0–6) | 3 (0–6) |
| Joints per dog with moderate‐to‐severe inflammation | 2 (0–5) | 0 (0–3) | 0 (0–3) |
Significantly different (P = .008)
Treatment Response
Prednisone dosages (calculated as 50 mg/m2/day) ranged from 0.96 to 2.27 mg/kg/day as administered, with the lowest per kg dosages given to the 2 Great Danes. All dogs improved clinically over the 4‐week course of the study, and no dogs were given azathioprine rescue. Eight of 9 dogs had increased serum alkaline phosphatase (ALP) activities by week 2. Thrombocytopenia resolved in both affected dogs by weeks 2–4.
Median CBPI scores for each of 11 questions improved significantly in all dogs at weeks 2 and 4 compared with baseline (P = .0017; Fig 1A). Mobility as measured by accelerometry was significantly greater at week 4 compared with baseline (P = .0024; Fig 1B). One dog (SB) did not wear the collar consistently, and had a cruciate ligament rupture newly diagnosed at week 4 of prednisone treatment. This dog's accelerometry and 4‐week CBPI scores were not included in data analyses.
Figure 1.
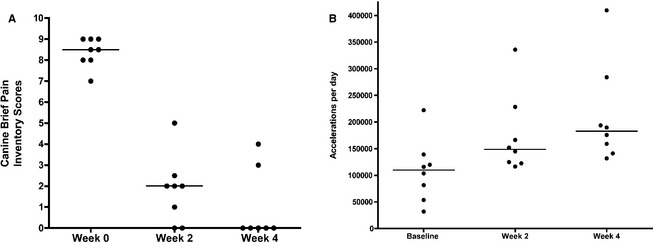
(A) Canine brief pain inventory scores (CBPI) in dogs with immune‐mediated polyarthropathy (IMPA) treated with prednisone (50 mg/m2) over 4 weeks. Scores were reported by owners at each visit; data shown are the median individual scores of responses to 11 questions for each dog. Pain scores at weeks 2 and 4 are significantly lower than at baseline (P = .0017). (B) Accelerometry data as a measure of daily activity in dogs with IMPA treated with prednisone (50 mg/m2) over 4 weeks. Data shown are accelerations per day for each dog, as measured over the first 48 hours before the start of treatment, compared to the 48 hours before the 2‐ and 4‐week rechecks. Median accelerations were significantly higher at week 4 compared with baseline (P = .0024).
Cytologic Response
The number of joints per dog with total nucleated cell counts >3000/μL decreased significantly between baseline and week 2 (P = .008; Table 1). The number of joints with >12% neutrophils or with moderate‐to‐severe inflammation varied among dogs, and was not significantly different over time (Table 1).
ELISA Results
Baseline plasma concentrations of CRP were significantly higher in dogs with IMPA (median 91.1 μg/mL, range 76.7–195.0) compared with healthy controls (<6.3 μg/mL, <6.3–13.7; P < .001), with no overlap between groups (Fig 2). CRP concentrations were stable in healthy dogs over 4 weeks (range <6.3–16.8 μg/mL). During IMPA treatment, CRP concentrations were significantly lower at both week 2 (10.6 μg/mL, <6.3–48.8) and week 4 (<6.3 μg/mL, <6.3–24.4; P < .001) compared with baseline, and by week 4, were not statistically different from healthy dogs (P = .61). CRP was significantly and positively correlated with median CBPI scores across all IMPA dogs and time points (r = 0.68; P < .001), and showed a modest positive correlation with joint cellularity (number of joints with >3,000 nucleated cells/μL; r = 0.49, P = .011) and a modest inverse correlation with mobility as measured by accelerometry (r = −0.42, P = .048).
Figure 2.
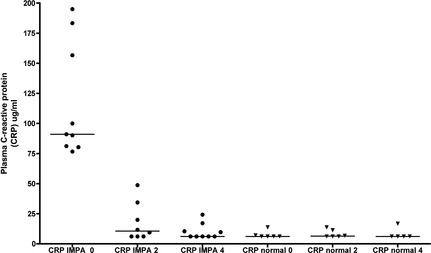
Plasma C‐reactive protein (CRP) concentrations over time in 6 untreated normal control dogs, and in 9 dogs with IMPA during treatment with prednisone. CRP concentrations in IMPA dogs were significantly higher at baseline compared with healthy control dogs (P < .001), and also decreased significantly during treatment at weeks 2 and 4 (P < .001).
Plasma IL‐6 concentrations were also significantly increased in IMPA dogs before treatment (median 45.9 pg/mL, range 28.6–871.5), compared with healthy controls (median <15.7 pg/mL, <15.7–31.0; P < .001; Fig 3). By week 4 of treatment, IL‐6 concentrations were significantly decreased in IMPA dogs (<15.7 pg/mL, <15.7–157; P = .0099) compared with baseline, with median concentration no higher than healthy dogs (P = .39). IL‐6 was significantly but modestly correlated with CBPI scores (r = 0.47, P = .023) across all time points in IMPA dogs.
Figure 3.
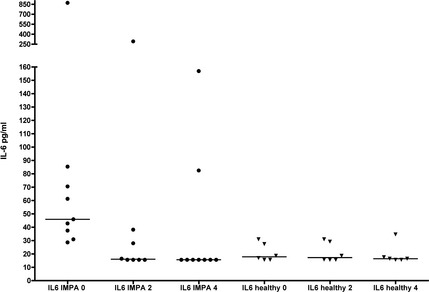
Plasma interleukin‐6 (IL‐6) concentrations over time in 6 untreated healthy control dogs, and in 9 dogs with IMPA during treatment with prednisone. IL‐6 concentrations in IMPA dogs were significantly higher at baseline compared with healthy control dogs (P < .001), and were also decreased significantly at week 4 of treatment (P = .0099).
Plasma concentrations of CXCL8 were more varied among individual dogs, with consistent outliers (>400 pg/mL) in both IMPA and healthy dogs over time, and no statistical differences between groups (Fig 4). However, the IMPA dog (SB) that developed a newly diagnosed cruciate ligament rupture at week 4 of prednisone treatment showed a sharp increase in plasma CXCL8 at this visit (560.6 pg/mL) compared with baseline and week 2 (20.0 and 32.4 pg/mL, respectively). Interestingly, we found comparably high CXCL8 concentrations in 2 dogs that were screened for the study, but were not enrolled because of a final diagnosis of cruciate ligament rupture (plasma IL‐8 653 and 1487 pg/mL, respectively).
Figure 4.
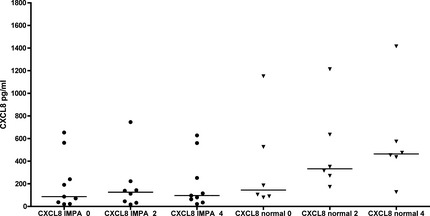
Plasma interleukin‐8 (CXCL8) concentrations over time in 6 untreated normal control dogs, and in 9 dogs with IMPA during treatment with prednisone. CXCL8 concentrations in IMPA dogs were not different at any time point from healthy dogs.
Discussion
The overall goal of this study was to determine whether noninvasive plasma markers of inflammation correlate with several measures of clinical response in dogs with idiopathic IMPA, to include owner's assessment of pain by the CBPI, mobility by accelerometry, and cytologic joint inflammation. The dogs in this small study had an excellent treatment response to prednisone based on body surface area dosing; and no dog required rescue or second‐line immunosuppressive therapy. Synovial fluid neutrophil counts significantly decreased and most were normal by 4 weeks into treatment. Treatment decreased pain, improved function, and increased mobility, as demonstrated by significant changes in CBPI and accelerometry data. Two weeks into treatment, all dogs had detectable clinical improvement and significantly improved CBPI scores; however, canine activity continued to improve and became statistically significant from baseline at the 4‐week recheck.
In all of the dogs with IMPA in this study, plasma CRP concentrations were substantially greater than those observed in our healthy dogs (≤16.8 μg/mL at all time points) and those previously reported in healthy dogs (observed upper limits of normal <8–23 μg/mL)10, 23, 24, 25, 26; in fact, all IMPA dogs had a baseline CRP >75 μg/mL. This is consistent with a previous retrospective study using a different CRP assay (laser nephelometric immunoassay), which demonstrated increased plasma CRP concentrations (~ 25 to >200 μg/mL) in 38 dogs at the time of diagnosis of IMPA.9 In that study, however, CRP concentrations were not followed beyond 2 weeks of treatment, and were not correlated with joint cytology or other objective markers of response over time.9 Plasma CRP levels using a human turbidometric assay were also elevated in a dog with type II IMPA secondary to Anaplasma phagocytophilum; however, this dog had concurrent IMHA.10
In the dogs with IMPA in this study, CRP concentrations fell dramatically with prednisone monotherapy, and were normal in 7 of 9 dogs by week 4. In addition, plasma CRP was significantly correlated with several markers of clinical response, to include CBPI scores, mobility by accelerometry, and synovial fluid total nucleated cell counts. This suggests that plasma CRP may be a useful surrogate marker of both synovial inflammation and clinical improvement at home in dogs with IMPA. Two previous studies further suggest that changes in CRP during treatment may have prognostic significance. In the previous retrospective study of IMPA dogs, those with plasma CRP concentrations >10 μg/mL at approximately 2 weeks of prednisone treatment were more likely to require continued immunosuppressive treatment at 6 months after diagnosis.9 Furthermore, in the dog with IMPA secondary to Anaplasmosis, episodes of clinical relapse were accompanied by increases in CRP, although joint cytologies were not reported.10
CRP has also been evaluated in 34 dogs with stifle osteoarthritis secondary to cranial cruciate rupture (serum CRP <6 μg/mL in all dogs at the time of diagnosis),27 and in 29 dogs with elbow, stifle, or shoulder osteoarthropathies (serum CRP ≤16.1 μg/mL in all dogs).28 In our study, we also found normal CRP concentrations (<6.2 μg/mL) in 2 dogs with a final diagnosis of cruciate ligament rupture, without IMPA, that were screened for eligibility (data not shown). These observations suggest that CRP might be useful to help distinguish between joint swelling attributable to localized osteoarthritis versus systemic inflammation and polyarthritis, although physical exam is usually adequate to address this.
IL‐6 is a cytokine with both proinflammatory and anti‐inflammatory effects, which is produced by leukocytes, fibroblasts, synovial cells, chondrocytes, and many other cell types.29 Serum concentrations of IL‐6 are increased in human patients with RA compared with those with osteoarthritis, and serum IL‐6 correlates with clinical disease indices30; furthermore, IL‐6 is targeted in the treatment of RA in humans.31 In this study, dogs with IMPA showed significantly increased plasma IL‐6 concentrations compared with healthy dogs, and by week 4 of treatment, plasma IL‐6 concentrations had fallen to those of healthy dogs in 7 of 9 IMPA dogs. However, unlike CRP, there was some overlap between IL‐6 in affected dogs at the time of diagnosis (28.6–871.5 pg/mL) and in healthy dogs (<15.7–31.0 pg/mL). In addition, plasma IL‐6 was correlated with CBPI scores, but not with synovial fluid cell count or mobility by accelerometry, albeit in a small population. These findings suggest that plasma CRP may be a more useful marker of synovial inflammation than plasma IL‐6 in dogs with IMPA. However, because IL‐6 is an important driver of hepatic CRP expression,29 the role of IL‐6 in the pathogenesis of IMPA in dogs should be explored further.
CXCL8 functions as a chemoattractant of neutrophils to sites of inflammation. However, we found no differences in plasma CXCL8 concentrations between dogs with IMPA and healthy dogs, and no apparent change in plasma CXCL8 with prednisone treatment, despite clinical and cytologic improvement. A similar phenomenon has been demonstrated in children with juvenile rheumatoid arthritis, in which plasma CXCL8 concentrations might be only sporadically elevated in some patients, and do not track with clinical disease activity.32 Together, these data suggest that plasma CXCL8 is not likely to be a sensitive or specific biomarker of IMPA disease activity in dogs.
We observed serendipitously that 3 dogs with cranial cruciate ligament ruptures (2 dogs not enrolled and 1 IMPA dog with a new ligament rupture at week 4) had markedly increased plasma CXCL8 concentrations. Increased CXCL8 protein expression has been shown in the synovia of dogs with cruciate ligament ruptures,33 but plasma CXCL8 concentrations deserve further evaluation in this disease syndrome.
This research was funded as a pilot study, and therefore has the limitation of a small population. However, this is balanced in some respects by the uniform prospective management of all cases. Even given the low numbers of dogs, we were able to show significant associations between plasma CRP and clinically relevant outcome measures for IMPA. However, while CRP might appear to be sensitive for changes in clinical status in dogs with IMPA, CRP is certainly not specific for this disease. For example, CRP is also elevated in dogs with steroid‐responsive meningitis arteritis,22, 34 which is another important differential in young adult dogs with fever, pain, and stiffness. In addition, the dogs in this study had a complete diagnostic work‐up to eliminate other potential causes of systemic inflammation. Without this exclusionary work‐up, an increased plasma CRP would be a very nonspecific finding.
Overall, the results of this study indicate that plasma CRP shows promise as a surrogate marker of synovial inflammation and disease activity in dogs with primary IMPA, both at the time of diagnosis and during prednisone treatment. Plasma CRP might be particularly useful in dogs with questionable clinical response histories or as a surrogate to repeated arthrocenteses. These results support the design of a follow‐up study in a larger number of dogs, to determine whether plasma CRP or IL‐6 concentrations are predictive of a durable response to prednisone alone, or are useful as sensitive markers of clinical relapse during or after treatment discontinuation.
Acknowledgments
The authors thank Dr Erica Behling‐Kelly, now an Assistant Professor, Population Medicine and Diagnostic Services, College of Veterinary Medicine, Cornell University, for invaluable assistance with initial selection of the ELISA assays, and Mr Brett Nemke for kind assistance with data analyses. This project was funded by an ACORN grant from the American Kennel Club Canine Health Foundation.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
These data were presented, in part, in abstract form at the 2013 ACVIM Forum in Seattle WA
Footnotes
Mini Mitter, Respironics Inc., Bend, OR
BD Biosciences, San Jose, CA
R&D Systems, Minneapolis, MN
References
- 1. Dunn KJ, Dunn JK. Diagnostic investigations in 101 dogs with pyrexia of unknown origin. J Small Anim Pract 1998;39:574–580. [DOI] [PubMed] [Google Scholar]
- 2. Rondeau MP, Walton RM, Bissett S, et al. Suppurative, nonseptic polyarthropathy in dogs. J Vet Intern Med 2005;19:654–662. [DOI] [PubMed] [Google Scholar]
- 3. Bennett D. Immune‐based non‐erosive inflammatory joint disease of the dog. 3. Canine idiopathic polyarthritis. J Small Anim Pract 1987;28:909–928. [Google Scholar]
- 4. Clements DN, Gear RN, Tattersall J, et al. Type I immune‐mediated polyarthritis in dogs: 39 cases (1997‐2002). J Am Vet Med Assoc 2004;224:1323–1327. [DOI] [PubMed] [Google Scholar]
- 5. Jacques D, Cauzinille L, Bouvy B, et al. A retrospective study of 40 dogs with polyarthritis. Vet Surg 2002;31:428–434. [DOI] [PubMed] [Google Scholar]
- 6. Stull J, Evason M, Carr A, et al. Canine immune‐mediated polyarthritis: Clinical and laboratory findings in 83 cases in western Canada (1991‐2001). Can Vet J 2008;49:1195–1203. [PMC free article] [PubMed] [Google Scholar]
- 7. Bennett D. Immune‐mediated and infective arthritis In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 6th ed St. Louis, MO: Elsevier; 2005:1958–1965. [Google Scholar]
- 8. Hegemann N, Wondimu A, Kohn B, et al. Cytokine profile in canine immune‐mediated polyarthritis and osteoarthritis. Vet Comp Orthop Traumatol 2005;18:67–72. [PubMed] [Google Scholar]
- 9. Ohno K, Yokoyama Y, Nakashima K, et al. C‐reactive protein concentration in canine idiopathic polyarthritis. J Vet Med Sci 2006;68:1275–1279. [DOI] [PubMed] [Google Scholar]
- 10. Kjelgaard‐Hansen M, Jensen AL, Houser GA, et al. Use of serum C‐reactive protein as an early marker of inflammatory activity in canine type II immune‐mediated polyarthritis: Case report. Acta Vet Scand 2006;48:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Carter SD, Barnes A, Gilmore WH. Canine rheumatoid arthritis and inflammatory cytokines. Vet Immunol Immunopathol 1999;69:201–214. [DOI] [PubMed] [Google Scholar]
- 12. Johnson KC, Mackin A. Canine immune‐mediated polyarthritis: Part 1: Pathophysiology. J Am Anim Hosp Assoc 2012;48:12–17. [DOI] [PubMed] [Google Scholar]
- 13. Brown D, Boston R, Coyne J, et al. Ability of the canine brief pain inventory to detect response to treatment in dogs with osteoarthritis. J Am Vet Med Assoc 2008;233:1278–1283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hansen BD, Lascelles BD, Keene BW, et al. Evaluation of an accelerometer for at‐home monitoring of spontaneous activity in dogs. Am J Vet Res 2007;68:468–475. [DOI] [PubMed] [Google Scholar]
- 15. Gibson NR, Carmichael S, Li A, et al. Value of direct smears of synovial fluid in the diagnosis of canine joint disease. Vet Rec 1999;144:463–465. [DOI] [PubMed] [Google Scholar]
- 16. Seo KW, Lee JB, Ahn JO, et al. C‐reactive protein as an indicator of inflammatory responses to experimentally induced cystitis in dogs. J Vet Sci 2012;13:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Carney PC, Ruaux CG, Suchodolski JS, et al. Biological variability of C‐reactive protein and specific canine pancreatic lipase immunoreactivity in apparently healthy dogs. J Vet Intern Med 2011;25:825–830. [DOI] [PubMed] [Google Scholar]
- 18. DeClue AE, Sharp CR, Harmon M. Plasma inflammatory mediator concentrations at ICU admission in dogs with naturally developing sepsis. J Vet Intern Med 2012;26:624–630. [DOI] [PubMed] [Google Scholar]
- 19. Maiolini A, Otten M, Hewicker‐Trautwein M, et al. Interleukin‐6, vascular endothelial growth factor and transforming growth factor beta 1 in canine steroid responsive meningitis‐arteritis. BMC Vet Res 2013;9:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gelaleti GB, Jardim BV, Leonel C, et al. Interleukin‐8 as a prognostic serum marker in canine mammary gland neoplasias. Vet Immunol Immunopathol 2012;146:106–112. [DOI] [PubMed] [Google Scholar]
- 21. Dow C, Michel K, Love M, et al. Evaluation of optimal sampling interval for activity monitoring in companion dogs. Am J Vet Res 2009;70:444–448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lowrie M, Penderis J, McLaughlin M, et al. Steroid responsive meningitis‐arteritis: A prospective study of potential disease markers, prednisolone treatment, and long‐term outcome in 20 dogs (2006‐2008). J Vet Intern Med 2009;23:862–870. [DOI] [PubMed] [Google Scholar]
- 23. Nakamura M, Takahashi M, Ohno K, et al. C‐reactive protein concentration in dogs with various diseases. J Vet Med Sci 2008;70:127–131. [DOI] [PubMed] [Google Scholar]
- 24. Chan DL, Rozanski EA, Freeman LM. Relationship among plasma amino acids, C‐reactive protein, illness severity, and outcome in critically ill dogs. J Vet Intern Med 2009;23:559–563. [DOI] [PubMed] [Google Scholar]
- 25. Griebsch C, Arndt G, Raila J, et al. C‐reactive protein concentration in dogs with primary immune‐mediated hemolytic anemia. Vet Clin Pathol 2009;38:421–425. [DOI] [PubMed] [Google Scholar]
- 26. Israeli I, Steiner J, Segev G, et al. Serum pepsinogen‐A, canine pancreatic lipase immunoreactivity, and C‐reactive protein as prognostic markers in dogs with gastric dilatation‐volvulus. J Vet Intern Med 2012;26:920–928. [DOI] [PubMed] [Google Scholar]
- 27. Bennett D, Eckersall PD, Waterston M, et al. The effect of robenacoxib on the concentration of C‐reactive protein in synovial fluid from dogs with osteoarthritis. BMC Vet Res 2013;9:42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hurter K, Spreng D, Rytz U, et al. Measurements of C‐reactive protein in serum and lactate dehydrogenase in serum and synovial fluid of patients with osteoarthritis. Vet J 2005;169:281–285. [DOI] [PubMed] [Google Scholar]
- 29. Tanaka T, Kishimoto T. Targeting interleukin‐6: All the way to treat autoimmune and inflammatory diseases. Int J Biol Sci 2012;8:1227–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Klimiuk PA, Sierakowski S, Latosiewicz R, et al. Interleukin‐6, soluble interleukin‐2 receptor and soluble interleukin‐6 receptor in the sera of patients with different histological patterns of rheumatoid synovitis. Clin Exp Rheumatol 2003;21:63–69. [PubMed] [Google Scholar]
- 31. Smolen JS, Schoels MM, Nishimoto N, et al. Consensus statement on blocking the effects of interleukin‐6 and in particular by interleukin‐6 receptor inhibition in rheumatoid arthritis and other inflammatory conditions. Ann Rheum Dis 2013;72:482–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Mangge H, Gallistl S, Schauenstein K. Long‐term follow‐up of cytokines and soluble cytokine receptors in peripheral blood of patients with juvenile rheumatoid arthritis. J Interferon Cytokine Res 1999;19:1005–1010. [DOI] [PubMed] [Google Scholar]
- 33. El‐Hadi M, Charavaryamath C, Aebischer A, et al. Expression of interleukin‐8 and intercellular cell adhesion molecule‐1 in the synovial membrane and cranial cruciate ligament of dogs after rupture of the ligament. Can J Vet Res 2012;76:8–15. [PMC free article] [PubMed] [Google Scholar]
- 34. Lowrie M, Penderis J, Eckersall PD, et al. The role of acute phase proteins in diagnosis and management of steroid‐responsive meningitis arteritis in dogs. Vet J 2009;182:125–130. [DOI] [PubMed] [Google Scholar]


