Abstract
Background
Chronic kidney disease (CKD) in cats is associated with gastrointestinal signs commonly attributed to uremic gastropathy. Consequently, patients often are treated with antacids and gastrointestinal protectants. This therapeutic regimen is based on documented gastric lesions in uremic humans and dogs, but the nature and incidence of uremic gastropathy in cats are unknown.
Hypothesis/Objectives
Evaluate uremic gastropathy in CKD cats to facilitate refinement of medical management for gastrointestinal signs.
Animals
Thirty‐seven CKD cats; 12 nonazotemic cats
Methods
Stomachs were evaluated for the presence of classic uremic gastropathy lesions. Histopathologic lesions were compared with serum creatinine concentrations, calcium‐phosphorus product (CPP), and serum gastrin concentrations.
Results
Gastric ulceration, edema, and vascular fibrinoid change were not observed. The most important gastric lesions in CKD cats were fibrosis and mineralization. Sixteen CKD cats (43%) had evidence of gastric fibrosis of varying severity and 14 CKD cats (38%) had gastric mineralization. CKD cats were more likely to have gastric fibrosis and mineralization than nonazotemic controls (P = .005 and P = .021, respectively). Only cats with moderate and severe azotemia had gastric mineralization. CPP was correlated with disease severity; severely azotemic CKD cats had significantly higher CPP when compared with nonazotemic controls, and to mildly and moderately azotemic cats (P < .05). Gastrin concentrations were significantly higher in CKD cats when compared with nonazotemic controls (P = .003), but increased concentrations were not associated with gastric ulceration.
Conclusions and Clinical Importance
Uremic gastropathy in CKD cats differs from that described in other species and this difference should be considered when devising medical management.
Keywords: Chronic renal failure, Feline, Gastritis, Stomach
Abbreviations
- CKD
chronic kidney disease
- CPP
calcium‐phosphorus product
- CSU‐VTH
Colorado State University Veterinary Teaching Hospital
- SCr
serum creatinine concentration
- USG
urine specific gravity
Chronic kidney disease (CKD) is a common clinical problem that affects a substantial proportion of older cats.1 Progression of CKD typically results in uremia, a syndrome that manifests clinically as a variety of signs, including weight loss, vomiting, and inappetence.1, 2, 3, 4 These clinical signs frequently are ascribed to uremic gastritis, a condition attributed to the presence of uremic toxins and gastric hyperacidity secondary to hypergastrinemia.5 However, there are substantial gaps in our understanding of the pathogenesis and prevalence of uremic gastrointestinal disease in cats.
Uremic gastritis was first described in 1934 from autopsies in 135 uremic human patients; lesions varied from mild edema to hemorrhage, ulceration, and necrosis.6 Although complications of uremia such as gastritis, ulceration, and hemorrhage are common in humans,7 they appear to be less common and less severe in dogs.8 In 1979, Cheville described vascular injury, calcification, mucosal edema, and necrosis in the stomachs of a small group of uremic dogs with renal amyloidosis and pyelonephritis.9 In a more recent study, mucosal necrosis was found to occur less frequently, whereas mineralization, edema, and vascular injury frequently were present in dogs with renal disease.8 The prevalence of uremic gastritis has not been systematically evaluated in cats. A report of 70 cats with acute renal toxicity caused by contamination of food with melamine and cyanuric acid indicated that a small percentage of animals had gastric mineralization, but ulceration was not observed.10 In addition, an experimental study of Easter lily poisoning in cats reported an absence of gastric lesions in 10 cats that were examined by necropsy.11 Finally, in a case series and literature review of gastric ulceration in cats, CKD was not found to be an underlying cause.12
Cats with CKD have been shown to have high concentrations of gastrin that increase with the severity of renal failure,5 but the relationship among gastrin, gastric acid secretion, and gastric pathology has not been investigated. Gastrin is secreted by G cells in the gastric antrum and stimulates the secretion of gastric acid by the parietal cells. The presence of gastric acid results in negative feedback to decrease the secretion of gastrin. In humans and dogs, gastrin is excreted by the kidneys, and it is hypothesized that as renal function declines, hypergastrinemia develops, resulting in gastric hyperacidity.5 Cats that have gastrin‐secreting tumors with hypergastrinemia similar in severity to that found in cats with CKD have considerable gastric pathology, but no study has shown this to be the case in cats with CKD.12 Even in humans, the development of hyperacidity in association with CKD appears to be inconsistent, and may be related to the presence of Helicobacter spp. infection.13 Thus, there is very little available evidence on which to base recommendations for the use of acid‐decreasing medications such as H2 blockers, proton pump inhibitors, or sucralfate in cats with uremia.1, 2, 3, 4 The aims of this study were to evaluate the type and prevalence of histopathologic lesions in the stomach of cats with CKD, and to determine whether degree of azotemia, calcium‐phosphorus product (CPP), and serum gastrin concentration are correlated with gastric pathology. A better understanding of gastric pathology in CKD cats will facilitate refinement of medical management strategies for gastrointestinal signs.
Materials and Methods
Animals
Feline CKD patients necropsied at the Colorado State University Veterinary Teaching Hospital (CSU‐VTH) between years 2009 and 2012 were prospectively included in the study. Inclusion criteria included historical and clinicopathologic findings consistent with CKD, complete necropsy with evaluation of all major organs, and serum biochemistry profile and urinalysis performed within 2 weeks of euthanasia or death. All owners signed the CSU‐VTH consent form for euthanasia (when applicable) and educational necropsy; no cats were euthanized for the purpose of this study. Exclusion criteria included concurrent primary gastrointestinal disease, such as neoplasia, administration of nonsteroidal anti‐inflammatory drugs or glucocorticoids within 2 weeks before euthanasia, and ureteral obstruction identified as a postrenal cause of azotemia. CKD cats were defined as those with serum creatinine concentration (SCr) >1.6 mg/dL, urine specific gravity (USG) <1.035, and evidence of histologic changes consistent with CKD on renal histopathology. Cats were grouped based on severity of azotemia as follows: mild (SCr: 1.6–2.8 mg/dL), moderate (SCr: 2.9–5.0 mg/dL), and severe (SCr: >5.0 mg/dL). Although this grouping is in accordance with International Renal Interest Society CKD staging system,2 staging could not be performed because 2 SCr during a clinically stable period were not available for all cats. Clinicopathologic data obtained when marked dehydration or clinical decompensation was noted in the medical record were not included in the analysis. Information regarding administration of antacid medications and phosphate binders was recorded. Nonazotemic control cats were young, apparently healthy cats in good body condition and free of reported gastrointestinal disease that were euthanized at a local humane society, according to Humane Society guidelines and protocols. Study samples were obtained from these cats after euthanasia, and no cats were euthanized for the purpose of this study. Age was estimated by Humane Society staff based on surrender history, dental assessment, or both. Nonazotemic status was defined as cats with USG >1.035, SCr <1.6 mg/dL, and no evidence of CKD on renal histopathology.
Clinicopathologic Data
For the CKD cats, SCr, serum total calcium concentration, serum phosphorus concentration, and USG values measured within 2 weeks of euthanasia were obtained from the medical record. CPP was calculated as serum total calcium concentration multiplied by serum phosphorus concentration and expressed in mg2/dL2. Information regarding gastrointestinal signs of vomiting and inappetence was obtained from the medical record. A history of vomiting and inappetence was defined as documentation of the clinical signs prior to the last 24–48 hours before euthanasia. For the nonazotemic control cats, SCr, total calcium and phosphorus concentrations, serum gastrin concentration, and USG were obtained on samples obtained immediately postmortem by cardiac puncture or by cystocentesis, respectively.
Gastrin Assay
Serum gastrin concentrations were measured at Michigan State University with a commercially available radioimmunoassay kit, with assay procedures performed according to the manufacturer's protocol.1 Synthetic human gastrin17‐I standards were used to make the displacement curve, with the highest standard being 1000 ng/L. The manufacturer reported the following percent cross‐reactivity with related compounds: gastrin 17‐I (100%), gastrin 17‐II (77%), gastrin 34‐I (42%), gastrin 5–17 (54%), cholecystokinin‐PZ (<0.1%), and cholecystokinin‐8 (10.9%). The manufacturer‐reported sensitivity of detection was 3 ng/L. For intra‐assay repeatability (10 replicates), the coefficient of variation was 8.6% for a feline sample with a gastrin concentration of 45 ng/L. For interassay repeatability (10 replicates), the coefficient of variation was 9.2% for a feline sample with a concentration of 54 ng/L. Three feline samples with higher concentrations of gastrin (461, 145, 124 ng/L) were diluted with 0 standard at rates of 1 : 2, 1 : 4, and 1 : 8. The average recovery (observed/expected) at these dilutions was 121, 130, and 138%, respectively.
Gross and Histopathologic Evaluation
Complete necropsies were performed on all animals with evaluation of all major organs. Specifically, the stomach was incised along the lesser curvature from cardia to pylorus and grossly evaluated for the presence of edema, hemorrhage, ulceration, or other visible abnormalities. Kidneys were cut longitudinally along the long axis (cranial to caudal pole).
Gastric Histopathology
Three full thickness cross‐sections from 5 anatomic locations of the stomach (cardia, fundus, body, antrum, and pylorus) were sampled in a routine manner. Tissues were preserved in 10% neutral buffered formalin, paraffin embedded, and sectioned at 5 μm. All sections were stained with standard hematoxylin and eosin stain and evaluated independently by 2 pathologists (SM and CD), blinded to clinical data. For final lesion score, discrepancies between the pathologists were resolved by joint review of tissues. Lesion description and scoring rationale are outlined in Table 1. All sections were systematically reviewed for the presence or absence of histologic changes consistent with uremic gastropathy as observed in dogs (ie, edema, vascular fibrinoid change, fibrosis, and mineralization).8, 9 An overall qualitative score was given for mineralization in all gastric sections highlighted by Von Kossa stain. Evaluation of fibrosis utilizing Masson's trichrome was restricted to sections from the gastric body, and a semiquantitative scoring scheme for fibrosis was adapted from WSAVA gastrointestinal evaluation guidelines.14, 15 Finally, sections from the gastric body were evaluated for the presence and character of superficial mucosal inflammation, ulceration or erosion, and presence of lamina proprial lymphoid nodules. Inflammatory infiltrates in the body, if present, were graded using a modification of previously published guidelines and visual scoring scale.14, 16
Table 1.
Scoring descriptions for stomach and kidneys.
| Tissue | |
|---|---|
| Lesion Description | Score |
| Stomach | |
| Amyloid | |
| Absent | 0 |
| Rare | 1 |
| <50% of the section | 2 |
| >50% of the section | 3 |
| Edema | |
| Absent | 0 |
| Rare | 1 |
| <50% of the section affected | 2 |
| >50% of the section affected | 3 |
| Vascular fibrinoid changea | |
| Absent | 0 |
| Rare, focal | 1 |
| Segmental to regionally extensive | 2 |
| Diffusely affecting a majority of vessels | 3 |
| Fibrosis/mucosal atrophyb | |
| Glands closely packed, separated by scant connective tissue | 0 |
| Mild increase in proprial connective tissue | 1 |
| Separation of glands and moderate thickening of lamina propria | 2 |
| Marked lamina propria connective tissue with loss of glands | 3 |
| Mineralization | |
| Absent | 0 |
| Scattered, patchy, or both | 1 |
| Multifocal to coalescing | 2 |
| Diffuse/serpentiguous | 3 |
| Superficial mucosal inflammationc | |
| No inflammation | 0 |
| <30% of lamina propria populated by leukocytes | 1 |
| 30–60% of lamina propria affected | 2 |
| >60% of lamina propria infiltrated by leukocytes that aggregate and crowd glands | 3 |
| Mucosal erosions or ulceration | |
| Yes | 1 |
| No | 2 |
| Lymphoid nodules | |
| Yes | 1 |
| No | 2 |
| Kidney | |
| Amyloid | |
| Absent | 0 |
| Rare | 1 |
| <50% of glomeruli affected | 2 |
| >50% affected, diffuse, or both | 3 |
| Interstitial fibrosisd | |
| Absent | 0 |
| <25% of the section | 1 |
| 25–50% of the section | 2 |
| >50% of the section | 3 |
Renal Histopathology
Renal sections from CKD and control cats were examined independently by 2 pathologists blinded to clinical and gastric findings for confirmation of CKD and lesion scoring. Descriptions of lesion scoring are outlined in Table 1 . For final lesion score, discrepancies between pathologists were resolved by joint review of tissues. Semiquantitative analyses for the presence of amyloid (Congo red) and fibrosis (Masson's trichrome) in the kidney were given scores from 0 to 3 by each pathologist.
Statistical Analysis
SCr, USG, CPP, serum gastrin concentration, and gastric lesion scores were compared between nonazotemic control cats and CKD cats with mild, moderate, or severe azotemia using ANOVA and Bonferroni's multiple comparison posthoc test with Prism software.2 Scoring consensus between the 2 pathologists for each category was evaluated by Cohen's kappa coefficient with SAS software.3 Comparison between individual groups (presence of gastric lesion scores in normal versus CKD cats, CPP of CKD cats with gastric mineralization versus those without gastric mineralization, cats receiving phosphate binders or antacids versus those that were not, gastrin concentrations in cats with mineralization and in those without mineralization, gastrin concentrations in cats with and without fibrosis, and gastrin concentrations in cats with gastrointestinal signs of vomiting and inappetence) was performed using a Mann‐Whitney test with Prism software. Relationship between presence of gastrointestinal signs and presence of gastric mineralization and fibrosis was assessed using a Fisher's exact test with Prism software. Statistical significance for all analyses was set at P ≤ .05.
Results
Fifty‐nine cats with CKD were necropsied during this time period; 37 met the inclusion criteria for the study. Twenty‐two were excluded for the following reasons: routine gastric samples not obtained (7), no laboratory testing near the time of euthanasia or marked dehydration or decompensation at the time clinicopathologic data were collected (7), intestinal neoplasia (6), autolysis (1), and FIV positive status (1). In total, 9 mildly azotemic cats, 9 moderately azotemic cats, 19 severely azotemic cats, and 12 nonazotemic controls were included in the study.
The mean age of the CKD cats was 13.8 years (range: 5–21). There were 24 spayed females and 13 castrated male cats. All but 2 CKD cats were euthanized because of poor or declining quality of life. The remaining 2 cats died at home, and were kept cold and submitted for necropsy within 12 hours of death. Five of the CKD cats included in this study were receiving famotidine antacid treatment at the time of death (mild azotemia, 2 cats; moderate azotemia, 1 cat; severe azotemia, 2 cats), whereas 9 were receiving aluminum hydroxide phosphate binder treatment PO (mild azotemia, 1 cat; moderate azotemia, 1 cat; severe azotemia, 7 cats). Average estimated age of control cats was 2.7 years (range: 0.8–4). There were 2 male intact cats and 1 female intact cat; 3 neutered male cats and 6 spayed female control cats. Control cats had no known history of renal disease, gastrointestinal disease, or medication administration.
Clinicopathologic Data
USG, CPP, and gastrin results for all groups are summarized in Table 2. USG in nonazotemic control cats was significantly greater than USG in each CKD group (P < .05) when analyzed using one‐way ANOVA. CPP was correlated with disease severity; when analyzed using one‐way ANOVA, severely azotemic CKD cats had significantly higher CPP when compared with nonazotemic controls, mildly azotemic cats, and moderately azotemic cats (P < .05) (Fig 1). No change in statistical significance was noted when cats that had received phosphate binders were removed from analysis. The CPP of severely azotemic cats receiving phosphate binders was not significantly different from severely azotemic cats not receiving phosphate binders when compared with a Mann‐Whitney test. Although cats with gastric mineralization had higher CPP relative to cats without mineralization (114.6 mg2/dL2 and 82.5 mg2/dL2, respectively), this difference was not statistically significant (P = .058).
Table 2.
Clinicopathologic values for cats separated by severity of renal disease (creatinine).
| Parameter | Azotemia | |||
|---|---|---|---|---|
| Control (n = 12) | Mild (n = 9) | Moderate (n = 9) | Severe (n = 19) | |
| (SCr: 0.8–1.5 mg/dL) | (SCr: 1.6–2.8 mg/dL) | (SCr: 2.9–5.0 mg/dL) | (SCr >5.0 mg/dL) | |
| Creatinine (mg/dL) | ||||
| Mean | 1.2 | 2.2 | 3.9 | 9.1 |
| Range | 0.8–1.5 | 1.7–2.7 | 3.0–4.6 |
5.5–15.5 5.5–15.5 |
| Urine specific gravity | ||||
| Mean | 1.066 | 1.016 | 1.015 | 1.012 |
| Range | 1.048–1.084 | 1.009–1.036 | 1.012–1.020 | 1.007–1.017 |
| Ca (mg/dL) × Phos (mg/dL) | ||||
| Mean | 43.3 | 53.7 | 70.4 | 122.1 |
| Range | 34.0–58.8 | 32.3–92.9 | 29.4–119.6 | 33.0–208.2 |
| Gastrin (pg/mL) | ||||
| n | 9 | 3 | 5 | 15 |
| Mean | 46 | 143 | 74 | 149 |
| Range | 17–94 | 24–234 | 42–191 | 39–288 |
SCr = serum creatinine concentration.
Figure 1.
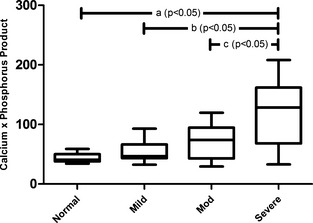
Calcium‐phosphorus product (CPP) is correlated with disease severity. Severely azotemic CKD cats had significantly higher CPP when compared with nonazotemic controls (A), mildly azotemic cats (B), and moderately azotemic cats (C) when analyzed with one‐way ANOVA with Bonferroni's posthoc analysis (P < .05).
Serum gastrin concentrations in cats with CKD were significantly higher than serum gastrin concentrations in control cats (P = .003) when compared using a Mann‐Whitney test. When all groups were compared using one‐way ANOVA, cats with severe azotemia had significantly higher serum gastrin concentrations than the control group (P < .05; Fig 2). No change in statistical significance was noted when cats that had received antacids (3 cats) were removed from analysis. The median serum gastrin concentration of azotemic cats receiving antacids was not significantly different from the median serum gastrin concentration of those azotemic cats not receiving antacids when compared with a Mann‐Whitney test.
Figure 2.
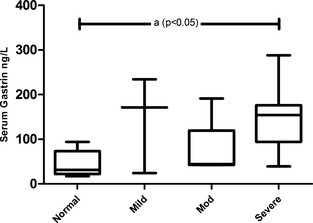
Serum gastrin concentrations are significantly increased in severely azotemic CKD cats. Cats with severe azotemia had significantly higher serum gastrin concentrations when compared with nonazotemic controls (A) when analyzed with one‐way ANOVA with Bonferroni's posthoc analysis (P < .05).
Sufficient information was present in the medical record to determine presence of gastrointestinal signs of vomiting and inappetence in 31/37 cats. A history of inappetence was documented in 84% of CKD cats (26/31) and a history of vomiting was documented in 45% of CKD cats (14/31). No statistically significant relationship between a history of gastrointestinal signs of vomiting and inappetence and gastric lesions of mineralization or fibrosis was observed when analyzed with a Fisher's exact test. However, no cats with mineralization were documented to have a good appetite. There was no statistically significant difference in serum gastrin concentrations between cats with a history of vomiting or inappetence and cats with no history of vomiting or inappetence.
Histopathology
Interobserver variation in histologic scoring was compared between pathologists, yielding an average kappa coefficient of 0.84 (range: 0.78–0.91) for all gastric and renal histologic categories. Of the 37 CKD cats and 12 controls, 3 sections from each anatomic location (cardia, fundus, body, antrum, and pylorus) were evaluated with the exception of 2 CKD cats; only 1 section from the body was available for evaluation in these cases. Both kidneys were available for evaluation on all study cats with the exception of 1 CKD cat that only had the right kidney available for histologic evaluation.
Gastric lesion scores in CKD and control cats are summarized in Table 3. No gross or histologic evidence of gastric mucosal ulcerations, hemorrhage, or edema was observed in either CKD or control gastric tissues. No histologic evidence of edema or vascular fibrinoid change was observed in CKD or control gastric tissues. Gastric fibrosis occurred more frequently in CKD cats when compared with controls using a Mann‐Whitney test (P = .005). When all groups were compared using one‐way ANOVA, there was a statistically significant difference in the degree of fibrosis in severely azotemic CKD cats when compared with the nonazotemic control group (P < .05; Fig 3). When serum gastrin concentrations from CKD cats with and without gastric fibrosis were compared using a Mann‐Whitney test, no significant difference was seen between groups. Fibrosis was absent in the stomachs of control cats, whereas some degree of fibrosis occurred in 16/37 (43%) CKD cats (Fig 4A–D). Fibrosis typically was superficial, with variable expansion of the lamina propria, and minimal nesting of pit and glandular cells in severe cases. Three of 9 mildly azotemic cats had mild increases in superficial proprial fibrous connective tissue (Fig 4B). A single mildly azotemic cat had moderate increased amounts of fibrous connective tissue with separation of superficial glands (Fig 4 C). Two moderately azotemic CKD cats and 8 severely azotemic cats had mild gastric fibrosis. One severely azotemic cat had moderate lamina proprial fibrous expansion and 1 severely azotemic cat had marked lamina proprial fibrosis.
Table 3.
Summarized gastric lesion scores.
| Azotemia | ||||
|---|---|---|---|---|
| Controls (n = 12) | Mild (n = 9) | Moderate (n = 9) | Severe (n = 19) | |
| Stomach Lesions | (SCr <1.6 mg/dL) | (SCr: 1.6–2.8 mg/dL) | (SCr: 2.9–5.0 mg/dL) | (SCr >5.0 mg/dL) |
| Amyloid | 0 | 0 | 0 | 0 |
| Edema | 0 | 0 | 0 | 0 |
| Vascular fibrinoid change | 0 | 0 | 0 | 0 |
| Fibrosis | ||||
| 0 | 12 | 5 | 7 | 9 |
| 1 | 0 | 3 | 2 | 8 |
| 2 | 0 | 1 | 0 | 1 |
| 3 | 0 | 0 | 0 | 1 |
| Mean score | 0a | 0.56 | 0.22 | 0.68a |
| Mineralization | ||||
| 0 | 12 | 9 | 5 | 9 |
| 1 | 0 | 0 | 0 | 1 |
| 2 | 0 | 0 | 2 | 3 |
| 3 | 0 | 0 | 2 | 6 |
| Mean score | 0b | 0c | 1.11 | 1.32b , c |
| Superficial mucosal inflammation | ||||
| 0 | 4 | 0 | 1 | 3 |
| 1 | 6 | 7 | 7 | 12 |
| 2 | 2 | 2 | 1 | 4 |
| 3 | 0 | 0 | 0 | 0 |
| Mean score | 0.83 | 1.22 | 1 | 1.05 |
| Mucosal erosion/ulceration | 0 | 0 | 0 | 0 |
| Lymphoid nodules | 8 | 7 | 6 | 8 |
SCr = serum creatinine concentration.
Mean fibrosis scores for severely azotemic group significantly higher than for control group (P < .05).
Mean mineralization scores for severely azotemic group significantly higher than for control group (P < .05).
Mean mineralization scores for severely azotemic group significantly higher than for mildly azotemic group (P < .05).
Figure 3.
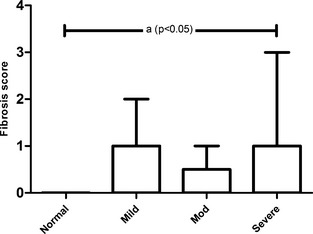
Gastric glandular atrophy and fibrosis occurred more frequently in CKD cats. Fibrosis scores in severely azotemic CKD cats were significantly increased when compared with the nonazotemic controls (A) using one‐way ANOVA with Bonferroni's posthoc analysis (P < .05).
Figure 4.
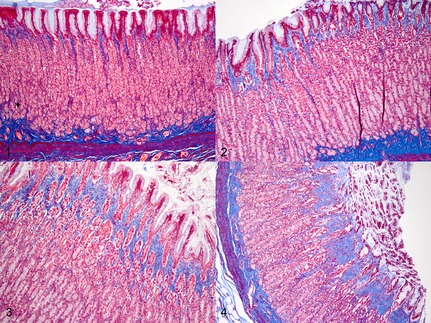
Representative examples of degrees of gastric fibrosis detected in cats with CKD compared with nonazotemic controls (Masson's trichrome stain). (1) Nonazotemic control cat stomach, body. Glands are tightly packed with minimal lamina proprial connective tissue. (2) Cat with CKD, body. Mild expansion of fovalae by lamina proprial fibrous connective tissue. (3) Cat with CKD, body. Moderate expansion of fovalae and gastric glands by lamina proprial connective tissue. (4) Cat with CKD, body. Marked separation and individualization of gastric glands by fibrous connective tissue.
Gastric mineralization was more likely to occur in cats with CKD than in nonazotemic cats (P = .0214) when compared using a Mann‐Whitney test. When all groups were compared using one‐way ANOVA, gastric mineralization scores were significantly higher in cats with severe azotemia compared with nonazotemic and mildly azotemic cats (P < .05; Fig 5). When serum gastrin concentrations from CKD cats with and without gastric mineralization were compared using a Mann‐Whitney test, no significant difference was seen between groups. A total of 14 CKD cats (38%), all from the moderate and severe azotemia groups, had gastric mineralization of varying severity within the apical one‐third to one‐half of the mucosal lamina propria (Fig 6A–D). Within the gastric mucosa of 3 cats, karyorrhectic debris was solely associated with the presence of proprial mineralization. Mineralization was not found in sections of stomach from nonazotemic control or mildly azotemic CKD cats. Moderately azotemic cats had multifocal to coalescing (n = 2) and diffuse (n = 2) gastric mineralization. Mineralization was evident in 10/19 cats (52%) of cats in the severe azotemia group. A single severely azotemic cat had mild, scattered gastric mucosal mineralization. Diffuse, marked mucosal mineralization that formed a serpentine band across the mucosa was prominent in 6 cats with severe azotemia. The remaining 3 had multifocal to coalescing patches of mucosal mineralization. In addition, 3 cats with gastric mineralization (moderate azotemia, 1 cat; severe azotemia, 2 cats) also had mineralization of gastric arteries and arterioles. One of these cats had vascular mineralization that was so severe that the tunica media and tunica intima of coronary arteries and the aorta were affected.
Figure 5.
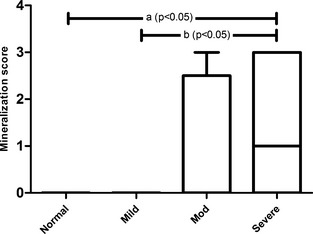
Gastric mineralization was present in moderately and severely azotemic CKD cats. Gastric mineralization scores were significantly greater in cats with severe azotemia when compared with nonazotemic and mildly azotemic cats using one‐way ANOVA with Bonferroni's posthoc analysis (P < .05).
Figure 6.
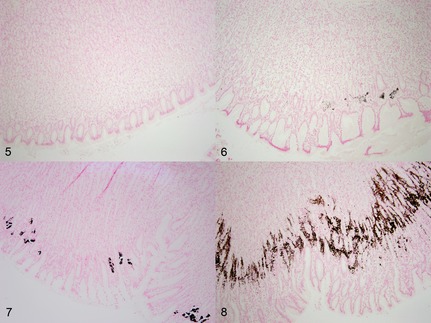
Representative examples of degrees of gastric mineralization present in cats with chronic kidney disease (CKD) in comparison to nonazotemic controls (Von Kossa stain). (5) Nonazotemic control cat stomach, body. Absence of mineral within the mucosa. (6) Cat with CKD, body. Mild, patchy mineralization. (7) Cat with CKD, body. Moderate, multifocal to coalescing mineralization. (8) Cat with CKD, body. Severe, diffuse, serpentine pattern of mineralization in the apical one‐half to one‐third of the mucosa.
There was no statistically significant difference in inflammation scores among any groups. Lymphocytic aggregates forming nodules deep within the mucosal lamina propria with variable expansion were present in 21/37 (57%) CKD cats and 8/12 (67%) control cats. Follicles were not factored into the presence or severity of inflammation in either group. Six control cats had mild inflammation and 2 had moderate inflammation, which was nearly exclusively lymphocytic in character. A single control cat had intraepithelial globular leukocytes present superficially. Severe inflammation was not detected in any control or CKD cat. Seven mildly azotemic cats had mild superficial inflammation that involved no more than one‐third of the section. Two additional cats with mild azotemia had moderate inflammation involving 30–60% of the section. Leukocytic infiltrates primarily were mononuclear (lymphocytes with few plasma cells), whereas 5 cats had intraepithelial globular leukocytes that ranged from 0 to 6 per high‐power field. The majority (7/9) of moderately azotemic cats had mild inflammation with only a single cat (1/9) having moderate inflammation. Mononuclear inflammatory cells (predominantly lymphocytes with occasional macrophages, plasma cells, or both) frequently aggregated around sites of mineralization when present in the moderately azotemic group of cats. Mild inflammation also predominated in cats with severe azotemia (12/19). An additional 4 cats with severe azotemia had moderate inflammation. Within the group of severely azotemic cats, infiltrates in addition to lymphocytes included frequent neutrophils and occasional eosinophils. In a single cat with severe azotemia, eosinophils were aggregated around sites of mineralization.
Renal lesions in the CKD group were consistent with those previously reported including interstitial fibrosis, interstitial nephritis, and tubular atrophy and glomerulosclerosis.17 The frequency and severity of renal fibrosis in study cats are summarized in Table 4. The fibrosis score in CKD kidneys was significantly higher than in controls (P < .001) when compared using a Mann‐Whitney test. When groups were compared using one‐way ANOVA, renal fibrosis scores were significantly higher in all CKD groups in comparison to controls (P < .05). Renal amyloidosis was not observed in either CKD or control groups.
Table 4.
Summarized kidney lesions.
| Azotemia | ||||||||
|---|---|---|---|---|---|---|---|---|
| Control | Mild | Moderate | Severe | |||||
| Kidney Lesions | (SCr <1.6 mg/dL) | (SCr: 1.6–2.8 mg/dL) | (SCr: 2.9–5.0 mg/dL) | (SCr >5.0 mg/dL) | ||||
| Kidney 1 | Kidney 2 | Kidney 1 | Kidney 2 | Kidney 1 | Kidney 2 | Kidney 1 | Kidney 2 | |
| Amyloid | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Interstitial Fibrosis | ||||||||
| 0 | 11 | 12 | 0 | 0 | 0 | 0 | 0 | 0 |
| 1 | 1 | 0 | 3 | 4 | 4 | 4 | 0 | 1 |
| 2 | 0 | 0 | 4 | 5 | 3 | 4 | 17 | 12 |
| 3 | 0 | 0 | 2 | 0 | 2 | 1 | 2 | 5 |
| Mean score | 0.08a | 0a | 1.89a | 1.56a | 1.78a | 1.67a | 2.11a | 2.22a |
SCr = serum creatinine concentration.
Renal fibrosis scores for all CKD groups were significantly greater than for controls (P < .05) when tested by one‐way ANOVA.
Discussion
In this study, CKD cats were more likely to have gastric mineralization, fibrosis, higher CPP, and higher serum gastrin concentrations than nonazotemic controls. Mineralization was only observed in cats with moderate and severe azotemia, and CPP increased significantly with degree of azotemia. Lesions in CKD cats were restricted to mucosal mineralization and fibrosis, whereas classic uremic gastropathy lesions such as gastric ulceration, edema, vascular fibrinoid change, and hemorrhage were not observed in the cats examined. These findings are unique because no studies previously have reported the prospective and systematic evaluation of uremia‐induced gastric lesions in cats with CKD. These findings also have important clinical implications because they suggest that gastrointestinal signs seen in CKD cats may be more likely to be the result of uremic toxins and centrally acting emetogens as opposed to pathology within the stomach.
Gastric mineralization was a common finding in cats with CKD in this study, as also reported in dogs.8 Thirty‐eight percent of stomachs evaluated from cats with CKD contained mineralization within the mucosal lamina propria. Only cats with moderate and severe azotemia had mineralization, suggesting that worsening azotemia and associated derangements in calcium and phosphorus concentrations put these cats at risk for soft tissue mineralization, specifically in the stomach. In this study, CPP was higher in those cats with gastric mineralization (114.6) compared with those without mineralization (82.58), but not significantly so. In general, animals with CPP > 60–70 are at risk for soft tissue mineralization, although increased concentrations probably need to be present for weeks to months.18 Development of gastric mineralization may vary depending on individual patient factors or variation in the time frame of increased plasma mineral concentrations (ie, months versus days). The administration of phosphate binders did not appear to affect the presence of gastric mineralization, but it is difficult to assess the efficacy of phosphate binder treatment from this study because duration of hyperphosphatemia, duration of treatment, and individual trends in serum calcium and phosphorus concentrations during the course of treatment are not known for these patients. In addition, relatively small numbers of cats were receiving phosphate binder treatment (7/18 severely azotemic cats), which is consistent with a recent survey of medication practices in CKD cats in which 78.3% of cats with CKD were not receiving phosphate binders.19
Mucosal mineralization in cases of uremia typically has been attributed to the effects of abnormalities in plasma calcium and phosphorus, local acidosis, and ischemia.9 Our data suggest that calcium‐phosphorus dysregulation may have contributed to gastric mineralization, and although no evidence currently is available to support the involvement of the latter 2 factors, their contribution to gastric mineralization in cats with CKD cannot be ruled out. In this study, serum gastrin concentrations were not significantly higher in CKD cats with mineralization. In addition, it has not yet been documented that hypergastrinemia is associated with gastric hyperacidity in CKD cats, and thus its potential effect on mineralization is unknown. The extent to which gastric mineralization contributes to clinical signs of uremia is unclear and additional studies are needed to fully elucidate the pathophysiology of gastric mineralization in cats with CKD.
Fibrosis in the superficial mucosa of the gastric body was a common lesion in cats with CKD. Evaluation of fibrosis and atrophy was restricted to the body because of variability in glandular density and lamina propria connective tissue in other gastric regions.20 Typically, atrophic gastritis is the result of chronic inflammation leading to loss of gastric glands and replacement by fibrosis; however, significant amounts of inflammation were not seen in CKD cats in this study.21, 22, 23 Disorders leading to atrophic gastritis in humans are predominantly Helicobacter pylori infections or autoimmune gastritis.21 Conversely, atrophic gastritis in dogs and cats is not frequently reported and a causative relationship between Helicobacter spp. infection and disease is equivocal.20 In a single study investigating noninflammatory atrophy and fibrosis, 27% of vomiting dogs and 26% of clinically asymptomatic dogs had similar lesions.23 Despite the statistically significant difference between controls and CKD cats, the role of azotemia and uremia in the pathogenesis of gastric fibrosis is unclear. A limitation of this study was that control cats were not aged matched, thus age variation between the 2 groups is a possible explanation for this difference. The amount of fibrous connective tissue normally found in different locations of the cat stomach at different ages has not been established. In addition, without corroborative pathologic evidence of edema or other evidence of mucosal injury preceding the glandular loss and fibrosis, the interpretation that uremia leads to gastric fibrosis should be made with caution. Future studies evaluating cross‐sectional connective tissue density among similarly aged animals are necessary to draw additional conclusions.
Median serum gastrin concentrations were nearly 3 times greater in azotemic cats than in nonazotemic cats. Hypergastrinemia in CKD cats reported here is similar to previously published findings.5 Gastrin signals the release of HCl from parietal cells. Hypergastrinemia may lead to hyperacidity and potential chemical damage to the gastric mucosa. Despite this reasonable theory for the pathogenesis of uremic gastropathy, gastric ulceration was not found in CKD cats in this study, even when serum gastrin concentrations were markedly high, to a degree reported to be associated with gastric ulceration in cats with nonrenal diseases.12 In addition, it has not yet been demonstrated that hypergastrinemia is associated with gastric hyperacidity in CKD cats. In humans, gastric hyperacidity is highly variable in CKD patients and appears to be more heavily influenced by the presence of Helicobacter spp. as opposed to renal function.24 Hypergastrinemia may strictly be a consequence of renal disease. More than one third of plasma gastrin is extracted from circulation in a single pass through the kidney by renal cortical inactivation.25 In 1 study in humans, hypergastrinemia correlated with loss of functional renal mass, but not the degree of uremia,25 and in another study in humans, hypergastrinemia was not alleviated by dialysis.13 Gastrin concentrations did not necessarily correlate with severity of azotemia in the CKD cats in this study, although this has been documented in a previous study.5 It is unlikely that the small number of cats on antacids substantially affected serum gastrin results in this study. Only 3 cats on antacids had serum available for measurement of gastrin and no change in results was found when those cats were removed from analysis. In addition, it had previously been shown in dogs that famotidine results in only a transient increase in serum gastrin and thus serum gastrin concentrations would not be expected to be increased in patients receiving long‐term treatment.26 Given the gastric fibrosis observed in this study, increased concentrations of gastrin may be the result of atrophic gastritis, with decreased gastric acid secretion failing to give appropriate negative feedback. The data presented here showed a significant difference in the presence and severity of fibrosis in cats with CKD in comparison to controls. However, no relationship was seen between serum gastrin concentration and the presence of gastric fibrosis, but only a portion of cats had serum available for gastrin measurement. Thus, it is difficult to determine the role of renal disease in development of gastric fibrosis and its effect on serum gastrin concentrations based on this information. Appropriate morphometric density studies of parietal cells within the gastric mucosa and measurement of gastric acid pH are needed to determine the exact role of hypergastrinemia in gastric pathology in cats with CKD.
Inflammation and lymphoid hyperplasia were similar in the stomachs of CKD and control cats. Therefore, their presence was probably unrelated to renal disease, or perhaps within normal limits of leukocyte constituents of the feline gastric mucosa. Studies characterizing leukocyte subpopulations within the superficial gastric mucosa have been performed in dogs, and standards for infiltrates within a defined unit have been described.14, 15 Similar studies have not been reported in cats.
Gastrointestinal signs were common in CKD cats, with 84% of cats having a history of inappetence and 45% of CKD cats having a history of vomiting. No significant relationship between a clinical history of gastrointestinal signs and gastric lesions or serum gastrin concentrations was documented in this study. Because this was a necropsy study and many cats were euthanized for progression of disease, this information does have inherent bias. To counteract this, information regarding the medical history was collected from the medical record not just at the time of euthanasia, but from the several months preceding euthanasia. However, this should be taken into account when interpreting this information.
In conclusion, cats with CKD appear more likely to have gastric fibrosis and mineralization, rather than uremic gastropathy lesions previously described in dogs and humans. Therefore, the administration of gastric protectants such as sucralfate may not be justified, unless obvious clinical evidence of gastrointestinal hemorrhage such as melena or hematemesis is identified. The notable frequency of gastric mineralization, presumably as a consequence of metastatic mineralization, may highlight the need for more aggressive control of hyperphosphatemia and renal secondary hyperparathyroidism in cats with CKD. The clinical consequences of gastric mineralization are unknown, but an effect on appetite should be considered. Gastrointestinal signs in these animals may not necessarily be the result of gastric lesions such as gastric ulceration and inflammation, but perhaps the consequence of circulating uremic toxins interacting with the chemoreceptor trigger zone in the brain. Medical management of gastrointestinal signs with antiemetic and antinausea drugs may therefore be more appropriate. Lastly, the exact role of hypergastrinemia in contributing to gastric hyperacidity and gastric lesions in cats with CKD still is unclear. Additional studies to determine gastric acidity and parietal cell density in cats are needed to improve our understanding of the etiopathogenesis of hypergastrinemia in uremic cats.
Acknowledgments
Grant support: This work was supported by a grant from the Colorado State University College of Veterinary Medicine and Biomedical Sciences College Research Council.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Footnotes
Gastrin [125I] Radioimmunoassay Kit, MP Biomedicals, Diagnostics Division, Orangeburg, NY
Prism 5, GraphPad, La Jolla, CA
SAS 9.1.3, SAS Institute Inc, Cary, NC
References
- 1. Bartges JW. Chronic kidney disease in dogs and cats. Vet Clin North Am Small Anim Pract 2012;42:669–692, vi. [DOI] [PubMed] [Google Scholar]
- 2. Polzin DJ. Chronic kidney disease in small animals. Vet Clin North Am Small Anim Pract 2011;41:15–30. [DOI] [PubMed] [Google Scholar]
- 3. Roudebush P, Polzin DJ, Ross SJ, et al. Therapies for feline chronic kidney disease. What is the evidence? J Feline Med Surg 2009;11:195–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Polzin DJ, Osborne C, Ross SJ. Evidence‐based medicine of chronic kidney disease In: Bonagura JD, Twedt DC, eds. Kirk's Current Veterinary Therapy XIV, 14th ed St. Louis, MO: Saunders Elsevier; 2009:872–878. [Google Scholar]
- 5. Goldstein RE, Marks SL, Kass PH, et al. Gastrin concentrations in plasma of cats with chronic renal failure. J Am Vet Med Assoc 1998;213:826–828. [PubMed] [Google Scholar]
- 6. Jaffe R, Laing D. Changes in the digestive tract in uremia. A pathologic anatomic study. Arch Int Med 1934;53:851–864. [Google Scholar]
- 7. Lazarus H. Gastrointestinal abnormalities In: Brenner BM, Rector FC, eds. The Kidney. Philadelphia, PA: WB Saunders; 2000:1634–1659. [Google Scholar]
- 8. Peters RM, Goldstein RE, Erb HN, et al. Histopathologic features of canine uremic gastropathy: A retrospective study. J Vet Intern Med 2005;19:315–320. [DOI] [PubMed] [Google Scholar]
- 9. Cheville NF. Uremic gastropathy in the dog. Vet Pathol 1979;16:292–309. [DOI] [PubMed] [Google Scholar]
- 10. Cianciolo RE, Bischoff K, Ebel JG, et al. Clinicopathologic, histologic, and toxicologic findings in 70 cats inadvertently exposed to pet food contaminated with melamine and cyanuric acid. J Am Vet Med Assoc 2008;233:729–737. [DOI] [PubMed] [Google Scholar]
- 11. Rumbeiha WK, Francis JA, Fitzgerald SD, et al. A comprehensive study of Easter lily poisoning in cats. J Vet Diagn Invest 2004;16:527–541. [DOI] [PubMed] [Google Scholar]
- 12. Liptak JM, Hunt GB, Barrs VR, et al. Gastroduodenal ulceration in cats: Eight cases and a review of the literature. J Feline Med Surg 2002;4:27–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. El Ghonaimy E, Barsoum R, Soliman M, et al. Serum gastrin in chronic renal failure: Morphological and physiological correlations. Nephron 1985;39:86–94. [DOI] [PubMed] [Google Scholar]
- 14. Day MJ, Bilzer T, Mansell J, et al. Histopathological standards for the diagnosis of gastrointestinal inflammation in endoscopic biopsy samples from the dog and cat: A report from the World Small Animal Veterinary Association Gastrointestinal Standardization Group. J Comp Pathol 2008;138(Suppl 1):S1–43. [DOI] [PubMed] [Google Scholar]
- 15. Washabau RJ, Day MJ, Willard MD, et al. Endoscopic, biopsy, and histopathologic guidelines for the evaluation of gastrointestinal inflammation in companion animals. J Vet Intern Med 2010;24:10–26. [DOI] [PubMed] [Google Scholar]
- 16. Price AB. The Sydney System: Histological division. J Gastroenterol Hepatol 1991;6:209–222. [DOI] [PubMed] [Google Scholar]
- 17. Chakrabarti S, Syme HM, Brown CA, et al. Histomorphometry of feline chronic kidney disease and correlation with markers of renal dysfunction. Vet Pathol 2013;50:147–155. [DOI] [PubMed] [Google Scholar]
- 18. Landau D, Krymko H, Shalev H, et al. Transient severe metastatic calcification in acute renal failure. Pediatr Nephrol 2007;22:607–611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Markovich JE, Freeman LM, Labato MA, et al. Survey of the dietary and medication practices of owners of cats with chronic kidney disease. J Vet Intern Med 2013;27:735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Wilcock B. Histopathology In: Washabau R, Day MJ, eds. Canine and Feline Gastroenterology, 1st ed St. Louis, MO: Saunders Elsevier; 2013:333–341. [Google Scholar]
- 21. Rugge M, Pennelli G, Pilozzi E, et al. Gastritis: The histology report. Dig Liver Dis 2011;43(Suppl 4):S373–384. [DOI] [PubMed] [Google Scholar]
- 22. Sullivan M, Yool DA. Gastric disease in the dog and cat. Vet J 1998;156:91–106. [DOI] [PubMed] [Google Scholar]
- 23. van der Gaag I. The histological appearance of peroral gastric biopsies in clinically healthy and vomiting dogs. Can J Vet Res 1988;52:67–74. [PMC free article] [PubMed] [Google Scholar]
- 24. Watanabe H, Hiraishi H, Ishida M, et al. Pathophysiology of gastric acid secretion in patients with chronic renal failure: Influence of Helicobacter pylori infection. J Intern Med 2003;254:439–446. [DOI] [PubMed] [Google Scholar]
- 25. Zelnick EB, Goyal RK. Gastrointestinal manifestations of chronic renal failure. Semin Nephrol 1981;1:124–136. [Google Scholar]
- 26. Mordecai A, Sellon RK, Mealey KL. Normal dogs treated with famotidine for 14 days have only transient increases in serum gastrin concentrations. J Vet Intern Med 2011;25:1248–1252. [DOI] [PubMed] [Google Scholar]


