Abstract
Background
Long‐duration beta‐lactam antibiotics are used for empirical treatment in female dogs with uncomplicated bacterial cystitis. However, women with bacterial cystitis are treated with short‐duration potentiated sulfonamides because longer courses of beta‐lactams result in lower cure and higher recurrence rates.
Hypothesis/Objectives
Short‐duration potentiated sulfonamide treatment is more efficacious than long‐duration beta‐lactam treatment in achieving clinical and microbiological cures in female dogs with uncomplicated bacterial cystitis.
Animals
Thirty‐eight client‐owned female dogs.
Methods
Randomized, double‐blinded, placebo‐controlled clinical trial. Dogs were treated with TMP‐SMX (15 mg/kg PO q12h for 3 days followed by a placebo capsule PO q12h for 7 days; Group SDS; n = 20) or cephalexin (20 mg/kg PO q12h for 10 days; Group LDBL; n = 18). Dogs were monitored for clinical and microbiological cure during treatment and at short‐ and long‐term follow‐up.
Results
No statistically significant differences were found between treatment groups in clinical cure rates after 3 days of treatment (89% SDS, 94% LDBL; P = 1.00) and 4 days (85% SDS, 72% LDBL; P = .44) or >30 days (50% SDS, 65% LDBL; P = .50) after conclusion of treatment or in microbiological cure rates 4 days (59% SDS, 36% LDBL; P = .44) or >30 days (44% SDS, 20% LDBL; P = .40) after conclusion of treatment.
Conclusions and Clinical Importance
We did not identify a difference in cure rates between short‐duration sulfonamide and long‐duration beta‐lactam treatments in female dogs with uncomplicated cystitis. Long‐term cure rates in both treatment groups were low. In some female dogs, “uncomplicated” bacterial cystitis may be more complicated than previously recognized.
Keywords: Beta‐lactam, Cephalexin, Lower urinary tract infection, Sulfonamide
Abbreviations
- ABU
asymptomatic bacteriuria
- anti‐SMX
anti‐sulfamethoxazole
- LDBL
long‐duration beta‐lactam treatment
- PBMC
peripheral blood mononuclear cells
- SDS
short‐duration sulfonamide treatment
- SI
stimulation indices
- SMX‐NO
sulfamethoxazole‐nitroso
In female dogs, as in women, a clinical diagnosis of uncomplicated bacterial cystitis is relatively common and is based on consistent clinical signs of acute dysuria in an otherwise healthy patient.1 Empirical antimicrobial selection is the cornerstone of treatment and is based on the predictability of common uropathogens. Resolution of clinical signs typically is used as a marker for successful treatment in both dogs and women. Documentation of a positive urine culture at diagnosis and confirmation of resolution of bacteriuria after treatment are not routinely performed for first time infections in dogs or women.
For decades, short‐duration antibiotic treatment has been the standard of care for uncomplicated bacterial cystitis in healthy women. This approach arose from evidence that antibiotic administration for a week or more was not necessary to cure superficial infections of the bladder.2, 3, 4, 5 In women, 3‐day regimes of potentiated sulfonamides6, 7 or fluoroquinolones7, 8 show equal clinical efficacy when compared with longer courses.9, 10 Advantages of short‐duration treatment include lower cost, better compliance, fewer adverse effects, and decreased antimicrobial resistance.2, 4, 11 To avoid unnecessary fluoroquinolone use, a 3‐day course of trimethoprim‐sulfamethoxazole (TMP‐SMX) is the recommended empirical treatment for bacterial cystitis in women with no known history of previous TMP‐SMX sensitization and when the local prevalence of Escherichia coli resistance to sulfonamides is <20%.12, 13 Beta‐lactam antibiotics are not recommended for empirical treatment in women because they result in lower bacterial eradication rates and higher recurrence rates, even with extended durations of treatment.4, 9, 12, 14
In contrast to the treatments recommended in women, empirical long‐duration (7–14 days) beta‐lactams are a common first‐line treatment in female dogs with uncomplicated cystitis.15, 16 The routine clinical use of potentiated sulfonamide antibiotics in dogs is limited by the potential for hypersensitivity reactions that include blood dyscrasias, hepatotoxicity, polyarthropathy, and cutaneous skin eruptions.17, 18 However, sulfonamide hypersensitivity reactions occur ≥5 days after beginning drug treatment in both humans and dogs,17, 18, 19 and clinically relevant reactions have not been reported in women in association with widespread use of short‐duration TMP‐SMX regimens.12
The purpose of our study was to compare the efficacy of 3 days of treatment using a potentiated sulfonamide antibiotic (TMP‐SMX) with 10 days of treatment using a first‐generation beta‐lactam (cephalexin) in female dogs with uncomplicated bacterial cystitis. We hypothesized that short‐duration sulfonamide (SDS), the standard of care for uncomplicated cystitis in women, would be more efficacious than long‐duration beta‐lactam (LDBL), the historical first‐line treatment for female dogs, in achieving clinical and microbiological cures. Our primary outcomes were clinical and microbiological cure rates, both short term (4 days after antibiotic discontinuation) and long term (>30 days after antibiotic discontinuation). A secondary objective was to determine whether there was any evidence of immunologic sensitization to SMX in dogs treated for 3 days with TMP‐SMX.
Materials and Methods
Selection of Dogs
Enrollment was considered for client‐owned female dogs that presented to the University of Wisconsin Veterinary Medical Teaching Hospital from October 2010 to December 2012 with clinical signs consistent with bacterial cystitis. Each dog was evaluated for the presence of ≥1 of the following primary complaints: dysuria, pollakiuria, stranguria, hematuria, or malodorous urine. Eligible dogs needed to have a complete medical history, including any drug treatment during the previous 3 months. In addition, each owner was asked to commit to returning with his or her dog for 3 additional assessments on study days 7, 14, and 42 after the start of antibiotic treatment.
A complete physical examination, including neurological and digital rectal examination, was performed on each dog to rule out any underlying causes that might predispose the dog to bacterial cystitis. Dogs were not eligible if they were systemically ill, had a history of bacterial cystitis or lower urinary tract signs within the previous 6 months, had received antibiotic, anti‐inflammatory, or immunosuppressive treatment within the previous 3 months, were found to have an underlying predisposing condition for cystitis, including a history of urinary incontinence, urolithiasis, azotemia (serum creatinine concentration >1.4 mg/dL and urine specific gravity <1.030), hyperadrenocorticism, diabetes mellitus, or myelopathy, or had a prior history of hypersensitivity to potentiated sulfonamides or keratoconjunctivitis sicca. Doberman Pinscher dogs also were excluded because of reported predisposition to sulfonamide hypersensitivity.20
Eligible dogs were further screened to rule out the presence of radiopaque uroliths using a right lateral abdominal radiograph. A complete blood count (CBC), serum biochemistry profile, and urinalysis (UA) were performed to further exclude possible predisposing conditions for cystitis and to serve as baseline data to monitor for adverse effects of treatment. A Schirmer tear test (STT) was performed before enrollment to document normal tear production. All owners provided informed consent at the time of enrollment, and all study protocols were reviewed, approved, and conducted in accordance to the University of Wisconsin Animal Care and Use Committee.
Study Design
This study was a prospective, randomized, investigator‐ and owner‐blinded, placebo‐controlled clinical trial. A computer‐generated randomization table was used to allocate dogs into 1 of 2 treatment groups. Dogs assigned to the SDS group were treated with 15 mg/kg TMP‐SMX PO q12h for 3 days, followed by a placebo capsule PO q12h for 7 days. Dogs assigned to the LDBL group were treated with 20 mg/kg cephalexin PO q12h for 10 days. A pharmacist dispensed study medications directly to owners to ensure that investigators remained blinded to treatment group assignment. After the initial screening process and before starting treatment, a urine sample was submitted for bacterial culture and antimicrobial susceptibility, and serum and peripheral blood mononuclear cells (PBMC) were banked as baseline controls for assessment of sulfonamide sensitization.
Treatment was initiated based on a clinical diagnosis of uncomplicated bacterial cystitis, defined as compatible clinical signs of acute dysuria in an otherwise healthy dog. Three days after starting antimicrobial treatment, each owner was contacted by phone or email regarding his or her dog's clinical response and status of presenting clinical signs. Clinical and microbiological outcomes were evaluated 4 and >30 days after discontinuation of antibiotics. To retain investigator and owner blinding, all dogs were re‐evaluated at the hospital on study days 7, 14, and 42 after the start of antimicrobial treatment. Study day 7 served as the short‐term assessment point (4 days after antibiotics were completed) for SDS dogs, and study day 14 served as the comparable short‐term assessment point (4 days after antibiotics were completed) for LDBL dogs. Study day 42 served as the long‐term assessment point (>30 days after antibiotics were completed) for dogs in both groups. At each re‐evaluation, complete history, physical examination, urine culture, and STT were performed. In addition, on study day 7, CBC, serum biochemistry profile, and UA were performed to evaluate for hematologic abnormalities or evidence of organ dysfunction. On study day 42, serum and PBMC were obtained for assessment of immunologic sensitization to SMX. Dogs were monitored throughout the study for occurrence of inappetence, vomiting, diarrhea, lethargy, fever, skin eruption, joint swelling or pain, petechiae, ecchymoses, jaundice, pallor, ocular discharge, and decreased tear production (STT <8 mm/min).
Primary outcomes were determined 4 days and >30 days after discontinuation of antibiotics and defined as follows: (1) dogs with clinical failure had persistent clinical signs independent of culture results; (2) dogs with clinical cure had resolution of clinical signs independent of culture results; and (3) dogs with microbiological cure had resolution of bacteriuria independent of clinical signs. Dogs with persistent clinical signs on study day 3 were considered clinical failures and were removed from the study protocol; these dogs were eligible for rescue antibiotic treatment as indicated by culture and susceptibility results or were considered for additional diagnostic tests. Dogs that had a positive clinical response to antibiotic treatment within the first 3 days but had a negative urine culture at the time of enrollment were included in the study on an intention‐to‐treat basis, given that approximately 10% of women with uncomplicated bacterial cystitis have negative urine cultures at the time of diagnosis, but still respond clinically to antibiotic treatment.21 Dogs that had recurrence of clinical signs during the course of the study also were considered treatment failures and underwent an unscheduled urine culture and susceptibility to guide further treatment outside of the study protocol. Dogs that had recurrence of bacteriuria without clinical signs were diagnosed with asymptomatic bacteriuria (ABU), which in women is defined as a positive urine culture in a patient without clinical signs of fever, urgency, increased frequency, dysuria, or some combination of these. In healthy women, treatment of ABU is not indicated because it does not decrease the frequency of symptomatic infection nor does it prevent further episodes of ABU.22 These dogs were monitored at the scheduled re‐evaluation time points, but were not treated unless clinical signs developed.
Sample Collection and Analysis
Cystocentesis was the preferred method of urine collection, but if cystocentesis was not possible because of patient compliance, a midstream voided sample was obtained. Urine was collected by the same method for each individual dog throughout the study, whenever possible. For voided samples, the perineum of dogs was not routinely cleaned before urine collection. Significant bacteriuria (>100,000 colony forming units [CFU]/mL) was not observed in urine samples collected from normal dogs by means of this midstream collection technique.23
Urine was submitted directly to the laboratory or stored at 4°C if submission was delayed. Urine cultures were plated for a quantitative culture within 24 hours of collection. Urine cultures were evaluated for growth at 24 and 48 hours. Growth was quantitated as CFU/mL of urine. Bacterial growth cut‐offs were defined for each urine collection method used and based on both human and veterinary recommendations.24, 25, 26, 27 Significant growth from voided samples was defined as having 1 to 3 pathogens present at >10,000 CFU/mL. Because low bacteria counts can be obtained in samples collected during voiding, presumably as a result of bacterial contamination of the urethra and skin, the presence of >3 different microorganisms or <10,000 CFU/mL indicated probable urethral contamination and these cultures were considered insignificant or negative.23 Any microbial growth from urine collected by cystocentesis was considered significant, unless the growth was suggestive of gastrointestinal contamination during collection.27
A representative colony of each isolated pathogen was identified by standard microbiological methods, and minimum inhibitory concentrations (MICs) were determined for each pathogen isolated (see below). Breakpoints were assigned using the Clinical Laboratory Standards Institute (CLSI) interpretive standards.28, 29, 30
Antimicrobial Susceptibility Testing
Antimicrobial MICs were determined for all isolates by the Sensititre antimicrobial susceptibility system1 according to the manufacturer's instructions. Cephalothin, amoxicillin/clavulanic acid, and trimethoprim/sulfamethoxazole MICs were determined for all isolates by the Etest susceptibility method2 according to the manufacturer's instructions with Mueller Hinton agar.3 Quality control was performed for both assays according to CLSI recommendations.28, 29, 30 MIC results were interpreted according to CLSI standards criteria.28, 29, 30 Duplicate isolates were removed from MIC 50 and MIC 90 analysis and were defined as isolates from the same patient of the same bacterial species with <1 well difference in MIC for the antibiotic tested.
Anti‐SMX Antibody Enzyme‐Linked Immunosorbent Assay (ELISA)
Anti‐sulfamethoxazole (anti‐SMX) antibody and drug‐specific T‐cell proliferation assays were performed in a subset of dogs on study day 42 to monitor for immunologic sensitization to SMX. Serum was separated within 30 minutes of phlebotomy, harvested, and stored at −80°C until analysis for anti‐SMX antibodies by ELISA, as previously described.31 , 32 Serum from a confirmed TMP‐SMX hypersensitive dog that previously tested positive for anti‐SMX antibodies served as a positive control,31 and dog sera from 10 SMX‐naïve dogs were used as negative controls. A result was considered positive for anti‐SMX antibodies if the difference in absorbance between SMX‐containing and control wells was >2 SD above the difference in absorbance for the 10 sulfonamide‐naïve control dogs.31, 33
Drug‐Specific T‐Cell Proliferation Assay
Peripheral blood mononuclear cells were isolated from 2 to 4 mL of fresh heparinized blood from treated dogs, using density gradient centrifugation in Histopaque 1077 media.4 Cells were slow frozen in cryopreservation media (10% DMSO in fetal bovine serum) and were stored at −80°C until analysis. PBMC were thawed quickly at 37°C, washed, counted, and checked for viability with a hemacytometer and Trypan blue dye exclusion. Cells were then subjected to the lymphocyte transformation assay as previously described34 with the following modifications: cells were incubated for 48 hours in the presence of 1 mM SMX4 or 100 μM SMX‐nitroso5 (the immunogenic oxidative metabolite of SMX35), with phytohemagglutinin6 and drug vehicle alone (0.25% DMSO) used as positive and negative controls, respectively. During the final 18 hours, PBMC were pulsed with [3H]‐thymidine (1 μCi/well) to assess cell proliferation. Proliferative responses were expressed as stimulation indices (SI): mean counts per minute (cpm) in drug‐treated cultures/mean cpm in vehicle cultures alone. Drug‐stimulated cultures with an SI >2 were considered positive for drug‐specific T cells.35
Statistical Analysis
A target enrollment of 30 dogs in each treatment group (60 dogs in total) was estimated based on published clinical cure rates in women to provide 80% power to detect a 20% difference in clinical efficacy between groups.9, 36 However, an interim analysis performed after 2 years of recruitment failed to show superior efficacy of TMP‐SMX at 4 or >30 days after discontinuation of treatment, and the study was stopped before meeting this target population number.
An intention‐to‐treat analysis was used for all outcomes. Baseline characteristics of the 2 treatment groups, including age, body weight, and sex, were compared by a Mann‐Whitney U‐test. Clinical and microbiological cure rates at 4 and >30 days after completing treatment were compared by a Fisher's exact test. Calculations were performed by a commercial software package7 with P < .05 considered significant.
Results
Study Population
Forty‐six dogs were screened for enrollment (Fig 1). Of the 46 dogs screened, 8 dogs were ineligible for the following reasons: observed signs consistent with behavioral marking (2), isolated episode of hematuria after intense exercise that resolved before evaluation (1), primary polydipsia (1), clinical evidence of vaginitis (1), owner unwilling to return for re‐evaluations (1), urinary incontinence and vaginitis (1), and paraparesis (1). Thirty‐eight female dogs were enrolled and randomized to either SDS (n = 20) or LDBL (n = 18) treatment groups.
Figure 1.
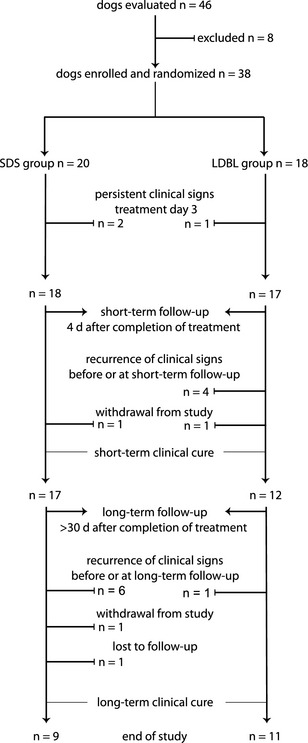
Allocation of treatment groups for female dogs treated for bacterial cystitis with SDS treatment versus long‐duration beta‐lactam treatment. Lines with arrows indicate progression of dogs that entered the study, clinically responded to treatment, and did not have a recurrence of clinical signs. Lines with terminal ends indicate when dogs were excluded or withdrawn from the study. SDS, short‐duration sulfonamide treatment; LDBL, long‐duration beta‐lactam treatment; n, number of dogs in group.
The baseline descriptive characteristics of the SDS versus the LDBL groups are summarized in Table 1. No statistically significant differences were found between the treatment groups with regard to sex (spayed versus intact), breed (pure versus mixed), age, or weight.
Table 1.
Study population characteristics by treatment group in female dogs treated for bacterial cystitis with short‐duration sulfonamide treatment versus long‐duration beta‐lactam treatment.
| SDS (n = 20) | LDBL (n = 18) | P value | |
|---|---|---|---|
| Age (years)a | 4 (2–8.3) | 8 (5–10.8) | .07 |
| Weight (kg)a | 12.9 (10–20.9) | 20.9 (6.7–27.3) | .37 |
| Spayed (versus intact)b | 80% (16 of 20) | 89% (16 of 18) | .66 |
| Purebred (versus mixed)b | 75% (15 of 20) | 78% (14 of 18) | 1.00 |
| Breeds (top 2)c , d |
Miniature Dachshund (4) Boxer (2) |
Labrador Retriever (3) Yorkshire Terrier (2) |
N/A |
SDS, short‐duration sulfonamide treatment; LDBL, long‐duration beta‐lactam treatment; n, number of dogs in group; UC, urine culture.
Continuous data are reported as median and range.
Categorical data are reported as percent (n of total).
Common breeds are reported as breed (n).
The remaining dogs included 1 each of other purebreds.
Culture Results
Eighty percent (16 of 20) of the SDS group and 61% (11 of 18) of the LDBL group had positive urine cultures initially, which was not statistically different between groups (P = 0.29). Bacterial species isolated from the urine of the dogs included E. coli (8), Proteus spp. (4), Staphylococcus spp. (3) and Klebsiella pneumoniae with E. coli (1) in the SDS‐treated group and E. coli (7), Proteus spp. (3), and E. coli with Streptococcus canis (1) in the LDBL group. All organisms isolated were identified as susceptible to both TMP‐SMX and cephalexin. For the E. coli isolates cultured during the study period (n = 21), the MIC50 (or median MIC) and the MIC90 (or MIC required to inhibit the growth of 90% of organisms) were calculated for trimethoprim/sulfamethoxazole (MIC50 <0.5 μg/mL/MIC90 <0.5 μg/mL), cephalothin (MIC50 12 μg/mL/MIC90 24 μg/mL), and amoxicillin/clavulanic acid (MIC50 4 μg/mL/MIC90 12 μg/mL).
Clinical and Microbiological Outcomes
Improvement or resolution of clinical signs on treatment day 3 was not significantly different between the SDS‐ and LDBL‐treated dogs (Table 2). In addition, there were no differences between treatment groups in clinical or microbiological cure rates at either short‐term follow‐up (4 days after completion of treatment) or long‐term follow‐up (>30 days after completion of treatment; Table 2).
Table 2.
Clinical and microbiological cure rates in female dogs treated for bacterial cystitis with short‐duration sulfonamide treatment versus long‐duration beta‐lactam treatment.
| Clinical Cure | Microbiological Cure | |||||
|---|---|---|---|---|---|---|
| SDS | LDBL | P‐value | SDS | LDBL | P‐value | |
| Day 3 of tx. | 89% (17 of 19) | 94% (17 of 18) | 1.00 | N/A | N/A | N/A |
| Day 4 after tx. | 85% (17 of 20) | 72% (13 of 18) | .44 | 59% (10 of 17) | 36% (4 of 11) | .44 |
| >30 days after tx. | 50% (9 of 18) | 65% (11 of 17) | .50 | 44% (7 of 16) | 20% (2 of 10) | .40 |
All data are reported as percent (n of total). SDS, short‐duration sulfonamide treatment; LDBL, long‐duration beta‐lactam treatment; tx., treatment; N/A, not applicable for assessment.
Dogs with persistent or recurrent clinical signs are presented in Figures 1 and 2. Persistent clinical signs were present on treatment day 3 in 2 SDS group dogs and 1 LDBL group dog; these 3 dogs were considered treatment failures. At the short‐term follow‐up (4 days after completion of treatment), 1 dog in the SDS group had been withdrawn from the study because of an acute onset of gastrointestinal illness (see Adverse Events), and 1 dog in the LDBL group was withdrawn because the owners wanted to treat their dog's ABU. At long‐term follow‐up (>30 days after completion of treatment), 1 dog in the SDS group had been withdrawn because of an acute onset of tick‐associated illness (see Adverse Events) and another dog in the SDS group was lost to follow‐up.
Figure 2.
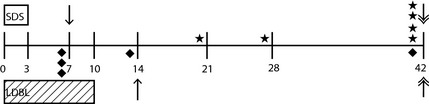
Timeline of study events for female dogs treated for bacterial cystitis with SDS treatment versus long‐duration beta‐lactam treatment. Numbers correspond to study day; day 0 = enrollment and start of treatment, day 3 = completion of SDS treatment, day 10 = completion of LDBL treatment. Boxes denote duration of each treatment; open box = duration of SDS treatment, diagonal hatched box = duration of LDBL treatment. Symbols above the line correspond to SDS group events. Symbols below the line correspond to LDBL group events. Single‐headed arrows represent short‐term follow‐up time point for each treatment. Double‐headed arrows represent long‐term follow‐up time point for each treatment. Each star marks an individual dog's time point of recurrence of clinical signs in the SDS group; diamonds mark the same for dogs in the LDBL group. SDS, short‐duration sulfonamide treatment; LDBL, long‐duration beta‐lactam treatment.
Eleven dogs had recurrence of clinical signs during the study observation period (6 in the SDS group and 5 in the LDBL group). Nine of these dogs cultured positive when clinical signs recurred. Eight were rescued with a 2‐week fluoroquinolone course (enrofloxacin 10 mg/kg PO q24h or ciprofloxacin 20 mg/kg PO q24h), and 1 was rescued with a 2‐week course of cephalexin prescribed by a veterinarian not involved with this study. Two dogs had negative urine cultures and were not treated with antibiotics.
The incidence of ABU after treatment of symptomatic bacterial cystitis was 35% (6 of 17) in the SDS group and 43% (6 of 14) in the LDBL group. Of the 6 dogs in the SDS group with ABU, 2 dogs had clinical recurrence (ABU followed by recurrence of clinical signs), 3 dogs had transient ABU (ABU that resolved without additional antibiotic treatment or recurrence of clinical signs), and 1 had persistent ABU (ABU that persisted without recurrence of clinical signs at the end of the study observation period). Of the 6 dogs in the LDBL group with ABU, 1 dog had clinical recurrence and 5 had persistent ABU.
Adverse Events
Two SDS group dogs were withdrawn from the study because of acute onset of illness. The first dog had an acute onset of vomiting and lethargy on study day 5 (2 days after discontinuation of TMP‐SMX), with normal physical examination, CBC, serum biochemistry profile, UA, and negative urine culture. No abnormalities were present that were consistent with sulfonamide hypersensitivity,18 and the dog's clinical signs resolved without treatment within 48 hours. A second dog had an acute occurrence of fever and lethargy and concurrent ectoparasitism (attached ticks) after travel to northern Wisconsin on study day 8 (5 days after discontinuation of treatment). Physical examination, CBC, serum biochemistry profile, UA, and qualitative tick serology8 were performed, and no evidence of sulfonamide hypersensitivity or tick‐borne disease was found. The primary veterinarian initiated treatment with doxycycline, and the clinical signs resolved within 48 hours.
Immunologic Sensitization
Sera from SDS (n = 8) and LDBL (n = 9) group dogs were screened by ELISA for antibodies against SMX. Anti‐SMX antibodies were not detected in sera of dogs treated with a 3‐day course of TMP‐SMX or (as expected) in dogs that received a standard course of cephalexin.
Lymphocyte proliferation in the presence of SMX or its oxidative metabolite, SMX‐NO, was used to determine the presence of drug‐specific T cells. Using conditions that detect SMX‐ and SMX‐NO‐specific T cells in human patients with sulfonamide hypersensitivity,34 no dogs treated for 3 days with TMP‐SMX that had PBMCs available (n = 9) had evidence of drug‐specific T cells against SMX (SI range 0.8–1.2) or SMX‐NO (SI range 0.8–1.4).
Discussion
This study evaluated the clinical and microbiological cure rates of SDS and LDBL antibiotic treatment in female dogs with a clinical diagnosis of uncomplicated bacterial cystitis. Most dogs in both treatment groups had resolution of clinical signs by treatment day 3 (89% in SDS group, 94% in LDBL group). Clinical cure rates 4 days after discontinuation of antibiotics also were comparably high in both groups (85% in SDS group, 72% in LDBL group). However, durable clinical remission rates were lower (only 50% in SDS group and 65% in LDBL group) when evaluated >30 days after completing antibiotics. This demonstrates a relatively high rate of recurrence of clinical infections in this population of female dogs.
Microbiological cure rates were relatively low in both treatment groups. Only 59% of SDS and 36% of LDBL group dogs had microbiological cures 4 days after completion of antibiotics, and only 44% of SDS and 20% of LDBL dogs had microbiological cures >30 days after completing treatment. All 7 dogs that had clinical relapse between short‐term (4 days after treatment was completed) and long‐term (>30 days after treatment was completed) follow‐up also had evidence of recurrent infection. However, some dogs had ABU (positive cultures at follow‐up without clinical signs); specifically, 35% of dogs in the SDS group and 43% in LDBL group, which contributed to the lower microbiological cure rates as compared with clinical cure rates. Most of these ABU dogs (9 of 12) did not develop a recurrence of clinical signs during the study observation period.
The relative importance of clinical versus microbiological cure for bacterial cystitis in dogs is not clear. Clinical cure rather than microbiological cure is the primary outcome for short‐duration antibiotic treatment in women with uncomplicated lower urinary tract infections. Short‐duration antibiotic treatment is aimed at decreasing the bacterial load to a level that allows clinical signs of the infection to be controlled, while the patient's immune system eradicates the remaining bacterial organisms from tissues (ie, bladder, vagina, and rectal reservoirs).10
The clinical cure rates reported in women treated for uncomplicated cystitis are 90% (SDS) and 85% (LDBL) at short‐term follow‐up and 85% (SDS) and 70% (LDBL) at long‐term follow‐up.7, 9, 10 The microbiological short‐ and long‐term cure rates reported for SDS and LDBL in women are similar to their reported clinical cure rates. The lower long‐term clinical and microbiological cure rates in the dogs reported in this study may be attributable to differences in anatomy, endogenous estrogen concentrations, gut flora, perineal hygiene, degree of involvement of the upper urinary tract, or other factors between the 2 species.
Treatment failures occur most commonly in women with occult upper urinary tract infections.11 Although upper urinary tract involvement in the dogs in our study could not be completely ruled out, dogs with polyuria and polydipsia or azotemia and concurrent dilute urine were not eligible. Nine of 11 dogs in this study with clinical recurrence had concurrent bacteriuria. In this study, there was a subjective difference in the timing of clinical recurrence relative to completion of treatment between the 2 treatment groups, but in each case, the organism cultured at recurrence was the same bacterial species cultured at enrollment. This is in contrast to women with recurrent infections restricted to the bladder, which typically are caused by an organism different from the original isolate (ie, reinfection).11 However, without genotyping, discernment between a relapsing infection and reinfection is not possible in this group of dogs.
Factors that may contribute to antibiotic treatment failure in dogs with bacterial cystitis include antibiotic choice, duration of treatment, owner compliance, superficial versus deep bladder wall involvement, dysbiosis of the vaginal vault, or concurrent illness, even if subclinical. At study enrollment, all dogs in this study were screened for common complicating factors, and all bacterial isolates cultured had in vitro susceptibility to the study antibiotics. The high long‐term failure rates of both treatment groups suggest that in some female dogs, “uncomplicated” bacterial cystitis, diagnosed on the basis of acute onset of clinical signs in the absence of predisposing factors, may be more complicated than previously recognized.
The clinical use of and published reports describing short‐duration antimicrobial treatment for the treatment of cystitis in dogs are limited to a single experimental study and recently reported clinical study. In the small experimental study, bacterial cystitis was induced with pathogenic E. coli in dogs and after development of clinical signs, a 3‐day antimicrobial regimen was evaluated in 8 female dogs using either trimethoprim‐sulfadiazine (TMP‐SDZ) or amikacin.37 The 4 female dogs treated with TMP‐SDZ achieved microbiologic cures at 48 hours and 14 days after 3 days of treatment. A recent clinical study, published after initiation of this study, compared long‐duration beta‐lactam treatment to short‐duration enrofloxacin.38 The study found that high‐dose short‐duration enrofloxacin (20 mg/kg for 3 days) was not inferior to a 14‐day course of amoxicillin‐clavulanic acid in the treatment of male and female dogs with bacterial cystitis.38 Clinical cure rates of 77% (short‐duration enrofloxacin) versus 81% (long‐duration amoxicillin‐clavulanate) were based on short‐term follow‐up only (7 days after completion of treatment). Microbiological cure rates at the 7‐day follow‐up were 81% versus 80% for the short‐duration enrofloxacin and long‐duration amoxicillin‐clavulanate groups, respectively. Long‐term assessment of the clinical and microbiological cure rates of high‐dose short‐duration enrofloxacin is needed because long‐term cure also is a relevant clinical outcome, as demonstrated in this study.
Although short‐term clinical cure rates are comparable between the 2 short‐duration therapies (77% in the high‐dose short‐duration enrofloxacin group 7 days after treatment versus 85% in the SDS group 4 days after treatment reported here), the short‐term microbiological cure rate appears higher for short‐duration enrofloxacin than for SDS in this study (81% versus 59%, respectively). However, 76% (13 of 17) of the SDS dogs had microbiological cure 11 days after discontinuation of treatment (data not shown, but available because of the double‐blinded design of this study).
Fluoroquinolones are only considered first‐line treatment in women with uncomplicated bacterial cystitis in regions where bacterial sulfonamide resistance is high (eg, ≥20%), in women with known sulfonamide hypersensitivity, or in women in whom lower urinary tract signs persist or recur within a week or two of antibiotic treatment.39 In this study, dogs considered treatment failures that had positive bacterial urine culture were rescued with antibiotic treatment. Because these dogs were considered treatment failures, additional treatment for “uncomplicated” bacterial cystitis was not pursued. Eight of the 9 treatment failure culture‐positive dogs were treated with a 2‐week course of fluoroquinolone treatment. This treatment was thought to be a logical second‐tier intervention in the dogs in this study that failed traditional long‐duration beta‐lactam treatment or short‐duration sulfonamide treatment (treatment group was unknown to investigators and owners at the time of dispensing rescue antibiotics).
The strengths of this study include the double‐blinded, placebo‐controlled experimental design and long‐term follow‐up. One limitation was the use of abdominal radiographs and not a complete ultrasound examination to rule out complicating factors at enrollment. However, full abdominal ultrasound examination is not performed routinely for first time or isolated urinary tract infections in dogs, and the study was designed to evaluate the efficacy of the 2 treatments in a clinically relevant setting.
Additional limitations of this study were single‐center recruitment and smaller than planned study population. Based on an interim analysis of clinical cure rates 2 years into enrollment, we closed the study because the results failed to show superior efficacy of TMP‐SMX at 4 or >30 days after discontinuation of treatment and our hypothesis was that clinical cure would be 20% higher for SDS. In addition, the rates of microbiological cure, which were higher in the SDS group at both short‐ and long‐term follow‐up, did not reach significance in this population of dogs. If present, this would be a clinically relevant difference, but based on a post hoc sample size calculation using our actual data, 80 dogs would have been needed in each treatment group to detect these differences, if real, as significant.
This relatively small canine patient population limited our ability to adequately evaluate the SDS‐treated dogs for sulfonamide immunoreactivity. In women, longer durations (eg, ≥5 days) of sulfonamide treatment are associated with more adverse effects compared with short‐duration (ie, single dose or 3‐day) protocols. However, published studies did not specifically evaluate the sulfonamide‐treated patients for a sulfonamide‐specific immune response.12, 40, 41 In this study, none of the dogs (n = 20) treated with TMP‐SMX for 3 days developed clinical signs consistent with a sulfonamide hypersensitivity reaction, and the subpopulation of dogs (n = 8) tested for antisulfonamide antibodies and sulfonamide‐specific T cells after 3 days of TMP‐SMX did not develop a sulfonamide‐specific immune response. These data only provide a pilot assessment of immunoreactivity in dogs treated with short‐duration sulfonamide treatment. Larger studies are needed to adequately determine if limiting a sulfonamide naive dog's exposure to 3 days is safe and avoids immune sensitization associated with longer duration protocols.31
In summary, this study did not demonstrate a difference in cure rates between SDS and long‐duration beta‐lactam treatment regimens for uncomplicated cystitis in female dogs. No serious clinical or biochemical adverse events were noted during the study in either treatment group. Given the increasing rates of fluoroquinolone resistance and the potential for interspecies spread of multi‐drug resistance among bacteria,5 future studies of adequate power are needed to determine if short‐duration potentiated sulfonamides and high‐dose short‐duration fluoroquinolones are comparable in safety outcomes and equivalent for long‐term clinical and microbiological cure of bacterial cystitis in female dogs.
Acknowledgments
The authors thank the Small Animal Internal Medicine technicians for their assistance in patient enrollment, appointment scheduling, and sample collection and handling and the UW Veterinary Care Pharmacy for providing patient randomization, preparing antibiotic and placebo capsules, and dispensing medications to owners. The authors also thank Kal Watson for assistance with ELISA assays.
Grant support: University of Wisconsin‐Madison, School of Veterinary Medicine, Companion Animal Grant.
Meetings: None.
Conflict of Interest: Authors disclose no conflict of interest.
Footnotes
Trek Diagnostic Systems, Westlake, OH
bioMérieux, Inc, Durham, NC
Remel, Lenexa, KS
Sigma Aldrich, St Louis, MO
SMX‐NO, Dalton Chemical, Toronto ON, Canada
PHA, 1:100, Invitrogen, Grand Island, NY
GraphPad Prism 6.0, GraphPad Software, Inc, San Diego, CA
SNAP 4Dx, IDEXX Laboratories Inc, Westbrook, ME
References
- 1. Chew DJ. Diagnosing Initial and Recurrent Urinary Tract Infections in Dogs. Orlando, FL: North American Veterinary Conference; 2001. [Google Scholar]
- 2. Drekonja DM, Johnson JR. Urinary tract infections. Prim Care 2008;35:345–367. [DOI] [PubMed] [Google Scholar]
- 3. Fang LS, Tolkoff‐Rubin NE, Rubin RH. Efficacy of single‐dose and conventional amoxicillin therapy in urinary‐tract infection localized by the antibody‐coated bacteria technique. N Engl J Med 1978;298:413–416. [DOI] [PubMed] [Google Scholar]
- 4. Nicolle LE. Urinary tract infection: Traditional pharmacologic therapies. Dis Mon 2003;49:111–128. [DOI] [PubMed] [Google Scholar]
- 5. Moura A, Nicolau A, Hooton T, et al. Antibiotherapy and pathogenesis of uncomplicated UTI: Difficult relationships. J Appl Microbiol 2009;106:1779–1791. [DOI] [PubMed] [Google Scholar]
- 6. Hooton TM, Winter C, Tiu F, et al. Randomized comparative trial and cost analysis of 3‐day antimicrobial regimens for treatment of acute cystitis in women. JAMA 1995;273:41–45. [PubMed] [Google Scholar]
- 7. McCarty JM, Richard G, Huck W, et al. A randomized trial of short‐course ciprofloxacin, ofloxacin, or trimethoprim/sulfamethoxazole for the treatment of acute urinary tract infection in women. Ciprofloxacin Urinary Tract Infection Group. Am J Med 1999;106:292–299. [DOI] [PubMed] [Google Scholar]
- 8. Iravani A, Tice AD, McCarty J, et al. Short‐course ciprofloxacin treatment of acute uncomplicated urinary tract infection in women. The minimum effective dose. The Urinary Tract Infection Study Group [corrected]. Arch Intern Med 1995;155:485–494. [PubMed] [Google Scholar]
- 9. Greenberg RN, Reilly PM, Luppen KL, et al. Randomized study of single‐dose, three‐day, and seven‐day treatment of cystitis in women. J Infect Dis 1986;153:277–282. [DOI] [PubMed] [Google Scholar]
- 10. Katchman EA, Milo G, Paul M, et al. Three‐day vs longer duration of antibiotic treatment for cystitis in women: Systematic review and meta‐analysis. Am J Med 2005;118:1196–1207. [DOI] [PubMed] [Google Scholar]
- 11. Fang LS, Tolkoff‐Rubin NE, Rubin RH. Clinical management of urinary tract infection. Pharmacotherapy 1982;2:91–99. [DOI] [PubMed] [Google Scholar]
- 12. Warren JW, Abrutyn E, Hebel JR, et al. Guidelines for antimicrobial treatment of uncomplicated acute bacterial cystitis and acute pyelonephritis in women. Infectious Diseases Society of America (IDSA). Clin Infect Dis 1999;29:745–758. [DOI] [PubMed] [Google Scholar]
- 13. Stamm WE, Hooton TM. Management of urinary tract infections in adults. N Engl J Med 1993;329:1328–1334. [DOI] [PubMed] [Google Scholar]
- 14. Czaja CA, Hooton TM. Update on acute uncomplicated urinary tract infection in women. Postgrad Med 2006;119:39–45. [DOI] [PubMed] [Google Scholar]
- 15. Bartges J. Bacterial urinary tract infections: Simple and complicated. Vet Med 2005;100:224–229. [Google Scholar]
- 16. Weese JS, Blondeau JM, Boothe DM, et al. Antimicrobial use guidelines for treatment of urinary tract disease in dogs and cats: Antimicrobial guidelines working group of the international society for companion animal infectious diseases. Vet Med Int 2011;2011:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Noli C, Koeman JP, Willemse T. A retrospective evaluation of adverse reactions to trimethoprim‐sulphonamide combinations in dogs and cats. Vet Q 1995;17:123–128. [DOI] [PubMed] [Google Scholar]
- 18. Trepanier LA, Danhof RS, Toll J, et al. Clinical findings in 40 dogs with hypersensitivity associated with administration of potentiated sulfonamides. J Vet Intern Med 2003;17:647–652. [DOI] [PubMed] [Google Scholar]
- 19. Cribb AE, Lee BL, Trepanier LA, et al. Adverse reactions to sulphonamide and sulphonamide‐trimethoprim antimicrobials: Clinical syndromes and pathogenesis. Adverse Drug React Toxicol Rev 1996;15:9–50. [PubMed] [Google Scholar]
- 20. Giger U, Werner LL, Millichamp NJ, et al. Sulfadiazine‐induced allergy in six Doberman Pinschers. J Am Vet Med Assoc 1985;186:479–484. [PubMed] [Google Scholar]
- 21. Hooton TM. The current management strategies for community‐acquired urinary tract infection. Infect Dis Clin North Am 2003;17:303–332. [DOI] [PubMed] [Google Scholar]
- 22. Nicolle LE, Bradley S, Colgan R, et al. Infectious Diseases Society of America guidelines for the diagnosis and treatment of asymptomatic bacteriuria in adults. Clin Infect Dis 2005;40:643–654. [DOI] [PubMed] [Google Scholar]
- 23. Carter JM, Klausner JS, Osborne CA, et al. Comparison of collection techniques for quantitative urine culture in dogs. J Am Vet Med Assoc 1978;173:296–298. [PubMed] [Google Scholar]
- 24. Comer KM, Ling GV. Results of urinalysis and bacterial culture of canine urine obtained by antepubic cystocentesis, catheterization, and the midstream voided methods. J Am Vet Med Assoc 1981;179:891–895. [PubMed] [Google Scholar]
- 25. Pezzlo M, Mary KC, Deirdre LC. Chapter 3.12 Urine cultures In: Isenberg HD, ed. Clinical Microbiology Procedures Handbook, 3rd ed Washington, DC: ASM Press; 2010: 3.12.1‐3.12‐19. [Google Scholar]
- 26. Sanderson SL. Urinary system introduction In: Kahn C, ed. The Merck Veterinary Manual, 10th ed Whitehouse Station, NJ: Merck & Co.; 2010:1380. [Google Scholar]
- 27. Stamm WE, Wagner KF, Amsel R, et al. Causes of the acute urethral syndrome in women. N Engl J Med 1980;303:409–415. [DOI] [PubMed] [Google Scholar]
- 28. Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. In: CLSI document VET01‐S2. Wayne, PA: CLSI; 2013. [Google Scholar]
- 29. Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. In: CLSI document M100‐23. Wayne, PA: CLSI; 2013. [Google Scholar]
- 30. Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Disk and Dilution Susceptibility Tests for Bacteria Isolated from Animals. In: CLSI document VET01‐A4. Wayne, PA: CLSI; 2013. [Google Scholar]
- 31. Lavergne SN, Danhof RS, Volkman EM, et al. Association of drug‐serum protein adducts and anti‐drug antibodies in dogs with sulfonamide hypersensitivity: A naturally occurring model of idiosyncratic drug toxicity. Clin Exp Allergy 2006;36:907–915. [DOI] [PubMed] [Google Scholar]
- 32. Gruchalla RS, Sullivan TJ. Detection of human IgE to sulfamethoxazole by skin testing with sulfamethoxazoyl‐poly‐L‐tyrosine. J Allergy Clin Immunol 1991;88:784–792. [DOI] [PubMed] [Google Scholar]
- 33. Crowther J. The ELISA guidebook In: JM W, ed. Methods in Molecular Biology. Totowa, NJ: Humana Press; 2001:347–394. [Google Scholar]
- 34. Abouraya M, Sacco JC, Kahl BS, et al. Evaluation of sulfonamide detoxification pathways in haematologic malignancy patients prior to intermittent trimethoprim‐sulfamethoxazole prophylaxis. Br J Pharmacol 2011;71:566–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Farrell J, Naisbitt D, Drummond N, et al. Characterization of sulfamethoxazole and sulfamethoxazole metabolite‐specific T‐cell responses in animal and humans. Pharmacol Exp Ther 2003;306:229–237. [DOI] [PubMed] [Google Scholar]
- 36. Passmore CA, Sherington J, Stegemann MR. Efficacy and safety of cefovecin (Convenia) for the treatment of urinary tract infections in dogs. J Small Anim Pract 2007;48:139–144. [DOI] [PubMed] [Google Scholar]
- 37. Rogers KS, Lees GE, Simpson RB. Effects of single‐dose and three‐day trimethoprim‐sulfadiazine and amikacin treatment of induced Escherichia coli urinary tract infections in dogs. Am J Vet Res 1988;49:345–349. [PubMed] [Google Scholar]
- 38. Westropp JL, Sykes JE, Irom S, et al. Evaluation of the efficacy and safety of high dose short duration enrofloxacin treatment regimen for uncomplicated urinary tract infections in dogs. J Vet Intern Med 2012;26:506–512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hooton TM. Clinical practice. Uncomplicated urinary tract infection. N Engl J Med 2012;366:1028–1037. [DOI] [PubMed] [Google Scholar]
- 40. Gossius G, Vorland L. A randomised comparison of single‐dose vs. three‐day and ten‐day therapy with trimethoprim‐sulfamethoxazole for acute cystitis in women. Scand J Infect Dis 1984;16:373–379. [DOI] [PubMed] [Google Scholar]
- 41. Trienekens TA, Stobberingh EE, Winkens RA, et al. Different lengths of treatment with co‐trimoxazole for acute uncomplicated urinary tract infections in women. BMJ 1989;299:1319–1322. [DOI] [PMC free article] [PubMed] [Google Scholar]


