Abbreviations
- CT
computed tomography
A 4‐year‐old, 30.6‐kg castrated male mixed‐breed dog was referred for evaluation of acute diarrhea, inappetence, weight loss, and lethargy. The dog had otherwise been healthy since being adopted from a Greek animal shelter 18 months before presentation. The only other household pet was its clinically healthy littermate. On examination, the dog was lethargic, hyperthermic (102.4°F), had a body condition score of 3/9, and was moderately painful upon palpation of the cranial abdomen.
Abnormal hematologic findings included a moderate leukocytosis (19.3 × 109/L; reference range [RR], 5.5–13.7 × 109/L) with neutrophilia (15.6 × 109/L; RR, 2.8–8.7 × 109/L) and a mild microcytic nonregenerative anemia (PCV, 35%; RR, 39–56%). Serum biochemistry profile results disclosed moderate hyperglobulinemia (56.4 g/L; RR, 22.9–35.6 g/L), hypoalbuminemia (19.9 g/L; RR, 29.6–37.0 g/L), and moderately increased alkaline phosphatase activity (169 U/L; RR, 0–130 U/L). Urinalysis was unremarkable.
Results of serologic assays for anti‐Leishmania infantum antibody and Dirofilaria immitis antigen were negative. Fecal flotation was negative. Thoracic radiographs were within normal limits, but abdominal radiographs showed marked loss of detail in the cranial abdomen caudal to the stomach with a mass effect causing caudal intestinal displacement.
Ultrasonographic examination of the abdomen (Fig 1) identified an elongated, well‐defined, multilobular, mixed echoic mass 5 cm in length within the cranial to midabdomen caudal to the pyloric antrum and porta hepatis, surrounded by hyperechoic mesentery and including the portal vein as well as parts of the mesenteric vein. Multifocal hypo‐ to anechoic areas and hyperechoic foci with acoustic shadowing were noted. The surrounding mesenteric fat had mildly increased echogenicity. The stomach and pancreas were normal. Multiple hypoechoic nodules of varying size were visible throughout the parenchyma of all liver lobes. Based on the ultrasonographic findings, the most likely origin of the abdominal mass was thought to be mesenteric and hepatic lymph nodes. A multicentric neoplastic process or granulomatous disease of the mesenteric and hepatic lymph nodes and liver was suspected. Ultrasound‐guided fine needle aspirates of the abdominal mass were stained using May‐Grünwald‐Giemsa stain and cytologic evaluation indicated mild cellular necrosis and moderate pyogranulomatous inflammation consisting of nondegenerate neutrophils, small mature lymphocytes, and activated macrophages containing nonstaining rods suspicious for acid‐fast bacteria. Because these bacteria were positive on Ziehl‐Neelsen staining, infection with Mycobacterium spp. was suspected. After consulting with the National Reference Center for Mycobacteria and Supranational Reference Laboratory of the World Health Organization (WHO, Borstel, Germany), 2 weeks after the 1st presentation a laparotomy was performed to obtain multiple diagnostic biopsy specimen samples. To determine the organ of origin, the extent of the disease throughout the abdomen, and possible involvement of the lungs and hilar lymph nodes, which would have led to euthanasia because of public health safety issues, a computed tomographic (CT) examination was performed before laparotomy.
Figure 1.
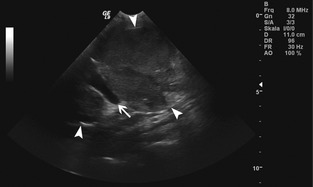
Longitudinal gray‐scale ultrasonographic image: An ovoid slightly heterogenous lobulated mass of low echogenicity (white arrowheads) containing a large vessel (white arrow) is visible. The cranial and caudal extent of the lesion is not included in the images.
Computed tomography examination (Fig 2) was performed using a 16‐slice helical scanner1 with a slice thickness of 3 mm. Pre‐ and postcontrast abdominal CT images after manual IV injection of nonionic iodinated contrast medium2 at a dosage of 600 mg/kg were acquired. An elongated, well‐defined, multilobulated, cavitary space‐occupying lesion 19 cm in length was noted, adjacent to the portal vein and caudodorsal to the gastric body and pylorus, extending caudally to the level of the caudal pole of the left kidney. Cranioventral displacement of the gastric body, pylorus, and left limb of the pancreas was present and the right limb of the pancreas and the duodenum were displaced to the right.
Figure 2.
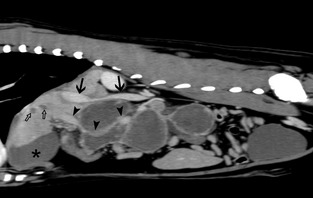
Postcontrast sagittal computed tomographic images of the abdominal cavity, soft‐tissue algorithm. The sagittally reformatted image is slightly to the right of the midline: Note the ring enhancement of the lesion while the center noncontrast enhancing and hypoattenuating (Mean 20 HU) indicating central necrosis or exudate. The caudal vena cava (arrows) is narrowed at the level of the cranial aspect of the space‐occupying lesion. Ventral displacement of the portal vein (arrowheads) caudal to the liver hilum is present. Several ovoid hypoattenuating noncontrast enhancing areas are visible within the liver parenchyma (open arrows). The asterisk (*) indicates the gallbladder.
Narrowing of the caudal vena cava was present at the level of the mass. The interior was heterogenous with attenuation values ranging from 15 to 45 Hounsfield Units (HU). Overall, the peripheral margins of the mass were slightly hyperattenuating, and the central aspects exhibited lower attenuation values but were interspersed by amorphous hyperattenuating foci (up to 82 HU). There was subtle hazy increased attenuation of the fat in the mesentery and liver hilum surrounding the lesion. On postcontrast CT images ring enhancement in the periphery of the subdivisions of the space‐occupying mass along with largely nonenhancing lesion centers resulted in a heterogenous appearance. Part of the portal vein including the junction with the splenic and gastroduodenal veins was localized centrally within the cranial aspect of the mass and appeared to be severely compressed and distorted in shape. The gastric and pancreaticoduodenal lymph nodes had mild spherical enlargement with a short‐to‐long axis ratio above the reported reference range of 0.5.1
Multiple round, well‐defined, noncontrast‐enhancing nodules 5–25 mm in diameter and with HU of 30 were present throughout the liver parenchyma. A CT diagnosis of necrotic mesenteric and hepatic lymphadenopathy with early calcification and hepatic nodules was made. The CT investigation of the thorax was unremarkable.
Laparotomy was performed to obtain biopsy samples and identified multifocal nodular lesions affecting the serous membranes of the diaphragm, stomach, intestines, and liver as well as liver parenchyma and multiple severely enlarged mesenteric lymph nodes. Biopsy specimens of abdominal lymph nodes were submitted to the National Reference Center for Mycobacteria in Borstel, Germany and processed according to WHO guidelines. The specimens were inoculated on 2 solid3 and 1 liquid4 media. There was only 1 strain grown, isolated from 2 different specimens. The strain was grown on all solid media with the typical morphology for M. tuberculosis. Differentiation was performed with a DNA strip assay, a molecular based technique (GenoType MTBC5) and Mycobacterium tuberculosis was identified. Drug susceptibility testing was carried out for first‐line drugs and performed using the Mycobacteria Growth Indicator Tube (MGIT) system.4
The owners were educated about the potential health risk and were asked to contact their physician. Furthermore, they were advised to avoid contact between their dog and young, old, or immunosuppressed people. The case was reported to the public health officer. Monthly evaluation of fecal smears from both the dog and its littermate using Ziehl‐Neelsen staining failed to detect any Mycobacteria spp.
In accordance with statutory provisions and the owners' wishes, treatment was initiated whereas susceptibility testing was pending with 3 antibiotics described for their use in M. tuberculosis infections2: rifampicin6 10 mg/kg administered PO q24h, clarithromycin7 12 mg/kg administered PO q12h, and enrofloxacin8 5 mg/kg administered PO q12h for the 1st 3 months. Results obtained 2 months after diagnosis indicated M. tuberculosis to be susceptible to rifampicin, isoniazid, ethambutol, and pyranzinamid, but resistant to streptomycin. Subsequently, rifampicin and clarithromycin were continued indefinitely.
The dog was reevaluated every 4 weeks for the next 6 months, and from then on monthly including physical examination, hematology, serum biochemistry, fecal smear examination using Ziehl‐Neelsen staining, thoracic and abdominal radiographs, and abdominal ultrasound and CT examinations at 7, 12, and 20 months postdiagnosis. Appetite was restored and diarrhea resolved 4 weeks after treatment was initiated, and all blood parameters except albumin (25.6 g/L) and alkaline phosphatase activity (143 U/L) were within reference intervals 7 months after diagnosis. Thoracic and abdominal radiographs were unremarkable.
CT examinations at 7, 12, and 20 months postdiagnosis (Fig 3A,B) identified progressive multifocal calcification and decreased size of the mesenteric and hepatic lymph nodes. At the time of writing (31 months postdiagnosis), the dog is clinically healthy.
Figure 3.
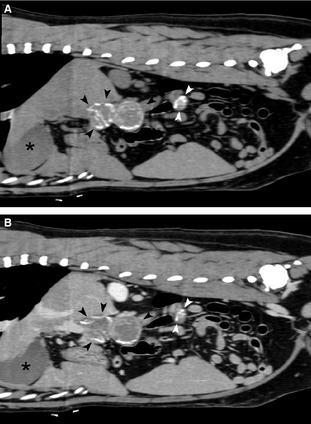
(A, B) Follow‐up CT 20 months after diagnosis: Precontrast (A) and corresponding postcontrast (B) sagittal computed tomographic images of the abdominal cavity, soft‐tissue algorithm, slightly to the right of the midline. A progressive decrease in size and increase in mineral content predominantly in the lesions peripheries is evident. Mineralization of the mass wall is visible (black arrows). The gallbladder is indicated by the asterisk (*). Because of reduction in size, the lesions comprise 2 separate entities (black and white arrowheads).
Canine M. tuberculosis infection is rarely reported, and so far an intra‐abdominal localization and successful treatment in a dog has not been documented. M. tuberculosis is a nonmotile, nonspore‐forming, obligate intracellular, gram‐positive aerobic bacillus that is characteristically acid‐alcohol fast. Culture is difficult and takes several weeks because the organism divides only once every 15–20 hours and needs special culture conditions such as Lowenstein‐Jensen Medium. Its unique cell wall composition (hydrophobic waxy mycolate layer and peptidoglycan layer held together by arabinogalactan) makes Mycobacterium spp. highly resistant to heat, pH changes, routine disinfection, and antibiotics.2
Humans are reservoir hosts for the organism and dogs have not been reported to spread M. tuberculosis infection to people.2 Thus, infections in dogs are considered an anthropozoonosis, with dogs becoming infected after prolonged exposition to human respiratory secretions. Transmission of M. tuberculosis to dogs almost always remains unclear. A case report proved positive by genotyping for anthropozoonosis from a woman to her dog.3 Furthermore in 2011, 3 veterinary pathologists with low risk before necropsy of a dog with disseminated clinical M. tuberculosis infection tested serologically positive a few weeks later.4
Considering that the owners of the present dog never showed any signs of tuberculosis, the Mediterranean background of the dog, and the long incubation period, it is likely that this dog became infected in Greece, possibly by ingestion of garbage contaminated by infected human sputum, before being obtained by the current owners.
Few cases of infection of dogs with Mycobacteria spp. are reported throughout the world. M. tuberculosis usually infects the respiratory system of mammals because it needs high oxygen tension for survival and replication. Thus, aerogen disease transmission by inhaled infectious sputum is the most common route of infection. Until now, all reported cases of infections with M. tuberculosis in dogs were localized in the pulmonary system.5, 6, 7, 8, 9 In a review published in 1962 of 48 dogs and cats with known exposure to human tuberculosis, 14.6% were culture positive for M. tuberculosis.10 Recent studies in a high‐risk setting in South Africa reported a prevalence of 1% of nonclinical tuberculosis in dogs which is similar to the prevalence in Europe in the early part of the 20th century when 0.1–6.7% of dogs were M. tuberculosis positive.11, 12 Rare cases of Mycobaterium spp. infection of the digestive tract in the dog described so far are caused by M. bovis (member of the Mycobacterium tuberculosis complex) or members of the M. avium complex.13, 14, 15, 16, 17, 18
Computed tomography is a useful tool for diagnosing M. tuberculosis infections in human patients.19 It helps in differentiating 3 types of M. tuberculosis infection: the “wet ascitic” type, the “dry‐plastic” type, and “fibrotic‐fixed” lesions.20 The most frequently encountered form is the “wet ascitic type”, which is characterized by a large amount of high‐density ascitic fluid because of its exudative content. The “dry‐plastic” type is difficult to diagnose on CT scans because of the bulky mesenteric lymph nodes, which can result in a misdiagnosis of abdominal lymphoma. The “fibrotic‐fixed” lesion is defined by distinctive abdominal or omental masses.20, 21 In the present case, CT findings were consistent with the “dry‐plastic” type (ie, enlarged mesenteric lymph nodes, well‐circumscribed areas with central low density, and peripheral enhancement with low‐attenuation centers).19, 20, 21 This enhancement is highly suggestive, but not pathognomonic of M. tuberculosis infection.19, 21 The central low density areas indicate central caseation necrosis.19, 20, 21 Advanced lesions such as peripheral calcification, seen in the CT examination performed after 7 months, have only been described in M. tuberculosis infection in the liver or spleen.19
The hepatic hypoattenuating foci seen in this patient are similar to lesions described as a micronodular form of hepatosplenic tuberculosis (diameter of 1–3 cm) in human patients. This is caused by a secondary hematogenous dissemination of the primary form of the disease. Although the micronodular form usually is accompanied by miliary pulmonary tuberculosis, the CT findings of the thorax in the present dog were inconspicuous.19, 20
Treatment options in human and small animal patients offer few choices. Standard treatment of uncomplicated M. tuberculosis infections in human patients consists a minimum of 4 antibiotics (ie, isoniazid, rifampicin, ethambutol, and pyrazinamid for the initial treatment over a period of 2 months) followed by a maintenance treatment with 2 antibiotics (ie, isoniazid and rifampicin for several months).22 Streptomycin is an antibiotic of last resort and should only be administered after susceptibility testing.22 Options for treatment of multi‐drug‐resistant M. tuberculosis infection include fluoroquinolones, aminoglycosides, and thiamides over a period of at least 21 months starting with 5 antibiotics.22 Current recommendations for treatment of M. tuberculosis infections in small animals start with a minimum of 2 antibiotics (some combination of isoniazid, rifampicin, ethambutol, dihydrostreptomycin, and pyrazinamid).2 While susceptibility testing was not available, a combination of rifampicin, clarithromycin, and enrofloxacine was chosen for several reasons. Firstly, streptomycin should be kept as a last‐resort antibiotic for human patients and should not be used in dogs. Secondly, isoniazid can cause severe adverse neurological effects and has led to euthanasia in a case of pulmonary M. tuberculosis infection in a dog.6 Thirdly, pyrazinamid is useful in M. tuberculosis infections, but is ineffective in M. bovis infection,23 which initially was the presumed organism in this case because of its localization.15 Rifampicin, which later turned out to be effective according to susceptibility testing, has no antagonistic effect with other antibiotics but is potentially hepatotoxic. Therefore, liver enzyme activity was monitored monthly and showed no change over a 1‐year period. Enrofloxacin is an established and well‐tolerated antibiotic in small animal medicine. It was chosen as the 3rd antibiotic in case of multi‐drug‐resistant M. tuberculosis infection. Enrofloxacin was discontinued after 3 months and maintenance treatment was continued with rifampicin and clarithromycin.
Although low serum vitamin D concentrations have been described in cats with active Mycobacteria infections,24 the addition of vitamin D3 in M. tuberculosis‐infected patients had no clinically relevant effect on antigen‐induced lymphoproliferative response.25 Therefore, vitamin D3 concentrations were neither measured nor were vitamin D supplemented.
Treatment of M. tuberculosis infections in dogs is controversial, mainly because of the human health risk. Nevertheless, there are no documented cases of M. tuberculosis infection spreading from dogs to humans, thus this disease is known to be an anthropozoonosis. With that in mind, we tried to limit the potential risk by taking several measures: detailed owner education, isolation of the dog from immunosuppressed persons as well as young and elderly people, serial fecal examination for acid‐fast bacteria to detect if the dog was shedding organisms, and serial physical examinations and diagnostic imaging to document potential progression to the pulmonary system. In treating the M. tuberculosis infection in this dog, we walked a fine line between public health protection and saving a beloved companion animal.
Acknowledgments
Conflict of Interest: Authors disclose no conflict of interest.
Footnotes
Philips Brilliance, Philips Deutschland GmbH, Hamburg, Germany
Xenetix 300, Guerbet, Sulzbach, Germany
Löwenstein Jensen and Stonebrink, Becton Dickinson, Cockeysville, MD
MGIT 960 Becton Dickinson
Hain Lifescience, Nehren, Germany
Eremfat 300 mg, Riemser Arzneimittel AG, Greifswald, Germany
Clarithromycin HEXAL 500 mg, Holzkirchen, Germany
Baytril 150 mg, Bayer HealthCare, Leverkusen, Germany
References
- 1. De Swarte M, Alexander K, Rannou B, et al. Comparison of sonographic features of benign and neoplastic deep lymph nodes in dogs. Vet Radiol Ultrasound 2011;52:451–456. [DOI] [PubMed] [Google Scholar]
- 2. Greene CE, Gunn‐Moore DA. Infections caused by slow‐growing mycobacteria In: Greene CE, ed. Infectious Diseases of the Dog and Cat, 4th ed St. Louis, MO: Saunders, Elsevier; 2006:495–510. [Google Scholar]
- 3. Erwin PC, Bemis DA, Mawby DI, et al. Mycobacterium tuberculosis transmission from human to canine. Emerg Infect Dis 2004;10:2258–2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Posthaus HT, Bodmer T, Alves L, et al. Accidental infection of veterinary personnel with Mycobacterium tuberculosis at necropsy: A case study. Vet Microbiol 2011;149:374–380. [DOI] [PubMed] [Google Scholar]
- 5. Parsons SDC, Gous TA, Warren RM, van Heiden PD. Pulmonary Mycobacterium tuberculosis (Beijing strain) infection in a stray dog. J S Afr Vet Assoc 2008;79:95–98. [DOI] [PubMed] [Google Scholar]
- 6. Sykes JE, Cannon AB, Norris AJ, et al. Mycobacterium tuberculosis complex infection in a dog. J Vet Intern Med 2007;21:1108–1112. [DOI] [PubMed] [Google Scholar]
- 7. Deppenmeier SA, Schieszler A, Nolte I, et al. Pulmonary tuberculosis with evidence of Mycobacterium tuberculosis in a Golden Retriever. Tieraezrtl Prax K H 2007;35:111–115. [Google Scholar]
- 8. Hackendahl NC, Mawby DI, Bemis DA, Beazley SL. Putative transmission of Mycobacterium tuberculosis infection from a human to a dog. J Am Vet Med Assoc 2004;225:1573–1577. [DOI] [PubMed] [Google Scholar]
- 9. Turinelli V, Ledieu D, Guilbaud L, et al. Mycobacterium tuberculosis infection in a dog from Africa. Vet Clin Path 2004;33:177–181. [DOI] [PubMed] [Google Scholar]
- 10. Hawthorne VM, Lauder IM. Tuberculosis in man, dog, and cat. Am Rev Respir Dis 1962;85:858–869. [DOI] [PubMed] [Google Scholar]
- 11. Parsons SDC, Warren RM, Ottenhoff THM, et al. Detection of Mycobacterium tuberculosis infection in dogs in a high‐risk setting. Res Vet Sci 2012;92:414–419. [DOI] [PubMed] [Google Scholar]
- 12. Snider WR. Tuberculosis in canine and feline populations – Review of literature. Am Rev Respir Dis 1971;104:877–887. [DOI] [PubMed] [Google Scholar]
- 13. van der Burgt GM, Crawshaw T, Foster AP, et al. Mycobacterium bovis infection in dogs. Vet Rec 2009;165:634. [DOI] [PubMed] [Google Scholar]
- 14. Haist V, Seehusen F, Moser I, et al. Mycobacterium avium subsp hominissuis infection in 2 pet dogs. Germany. Emerg Infect Dis 2008;14:988–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ellis MD, Davies S, McCandlish IAP, et al. Mycobacterium bovis infection in a dog. Vet Rec 2006;159:46–48. [DOI] [PubMed] [Google Scholar]
- 16. Naughton JF, Mealey KL, Wardrop KJ, et al. Systemic Mycobacterium avium infection in a dog diagnosed by polymerase chain reaction analysis of buffy coat. J Am Anim Hosp Assoc 2005;41:128–132. [DOI] [PubMed] [Google Scholar]
- 17. O'Toole D, Tharp S, Thomsen BV, et al. Fatal mycobacteriosis with hepatosplenomegaly in a young dog due to Mycobacterium avium . J Vet Diagn Invest 2005;17:200–204. [DOI] [PubMed] [Google Scholar]
- 18. Bauer N, Burkhardt S, Kirsch A, et al. Lymphadenopathy and diarrhea in a Miniature Schnauzer. Vet Clin Path 2002;31:61–64. [DOI] [PubMed] [Google Scholar]
- 19. Harisinghani MG, McLoud TC, Shepard JAO, et al. Tuberculosis from head to toe. Radiographics 2000;20:449–470. [DOI] [PubMed] [Google Scholar]
- 20. Hanson RD, Hunter TB. Tuberculous peritonitis – CT‐appearance. Am J Roentgenol 1985;144:931–932. [DOI] [PubMed] [Google Scholar]
- 21. Engin G, Acunas B, Acunas G, Tunaci M. Imaging of extrapulmonary tuberculosis. Radiographics 2000;20:471–488. [DOI] [PubMed] [Google Scholar]
- 22. Schaberg T, Bauer T, Castell S, et al. Recommendations for therapy, chemoprevention and chemoprophylaxis of tuberculosis in adults and children. German Central Committee against Tuberculosis (DZK), German Respiratory Society (DGP). Pneumologie (Stuttgart, Germany) 2012;66:133–171. [DOI] [PubMed] [Google Scholar]
- 23. de Jong BC, Onipede A, Pym AS, et al. Does resistance to pyrazinamide accurately indicate the presence of Mycobacterium bovis? J Clin Microbiol 2005;43:3530–3532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lalor SM, Mellanby RJ, Friend EJ, et al. Domesticated cats with active Mycobacteria infections have low serum vitamin D (25(OH)D) concentrations. Transbound Emerg Dis 2012;59:279–281. [DOI] [PubMed] [Google Scholar]
- 25. Chandra G, Selvaraj P, Jawahar MS, et al. Effect of vitamin D‐3 on phagocytic potential of macrophages with live Mycobacterium tuberculosis and lymphoproliferative response in pulmonary tuberculosis. J Clin Immunol 2004;24:249–257. [DOI] [PubMed] [Google Scholar]


