Abstract
Objective
To evaluate the effect of orally administered cisapride, bethanechol, and erythromycin on the absorption of colostral IgG in dairy calves.
Animals
Twenty‐four healthy neonatal Holstein‐Friesian calves.
Procedures
Calves were randomly assigned to one of the following treatments: 0.9% NaCl solution (2 mL, PO; negative control); erythromycin lactobionate (20 mg/kg BW, PO; anticipated to be a positive control); cisapride (0.5 mg/kg BW, PO); bethanechol chloride (0.5 mg/kg BW, PO). Calves were fed 3 L of pooled bovine colostrum containing acetaminophen (50 mg/kg) by suckling and oroesophageal intubation 30 minutes after each treatment was administered. Jugular venous blood samples were obtained periodically after the start of feeding and plasma total IgG, protein, acetaminophen, and glucose concentrations determined. Abomasal emptying rate was assessed by the time to maximal plasma acetaminophen concentration.
Results
Oral administration of cisapride facilitated the absorption of colostral IgG and protein. The effect of cisapride on abomasal emptying rate could not be evaluated because cisapride appeared to interfere with acetaminophen metabolism. Based on the total IgG and total protein concentration‐time relationships, the beneficial effects of cisapride appeared to occur early after oral administration and were transient.
Conclusions and Clinical Importance
Additional studies appear indicated to characterize the effect of cisapride dose on the magnitude and duration of its effect on facilitating the absorption of colostral IgG and protein. Identification of a nonantimicrobial method for increasing abomasal emptying rate, such as cisapride, will potentially provide a practical and effective method for facilitating transfer of passive immunity in colostrum‐fed dairy calves.
Keywords: IgG, Passive immunity, Prokinetic
Abbreviations
- AEA
apparent efficiency of absorption
- AUC24
area under the plasma IgG concentration‐versus‐time curve for 24 hours after colostrum ingestion
- AUC8
area under the plasma glucose concentration‐versus‐time curve for 8 hours after colostrum ingestion
- Cmax
maximal plasma concentration
- Tmax
time of maximal plasma concentration
Neonatal calves must ingest colostrum during the first 24 hours after birth to acquire passive immunity via the active uptake of maternal IgG across small intestinal epithelial cells.1, 2, 3 The mass of IgG absorbed from the small intestine of the colostrum‐fed calf depends on the IgG concentration in colostrum, the volume of colostrum administered, the apparent efficiency of absorption (AEA) of ingested IgG,1, 4 and the rate of abomasal emptying.5 Although the AEA ranges from 6 to 66% for calves <24 hours of age,3, 6, 7 an AEA of 33–45% appears to be near the practical biologic limit when 2 or more liter of colostrum is fed shortly after birth.6, 8, 9, 10, 11, 12, 13
Our laboratory has recently reported that the rate of abomasal emptying influences the rate at which colostral IgG is delivered to the site of IgG absorption, which is the small intestine.5 An increased rate of abomasal emptying results in an increased AEA because colostral IgG reaches the site of absorption in the small intestine earlier and at a higher luminal concentration.9 The most effective agent for increasing abomasal emptying rate in milk‐fed calves and adult cattle is the macrolide erythromycin14; however, it is inappropriate to administer an antimicrobial for a nonantimicrobial effect. We were, therefore, interested in determining the effect of potential nonantimicrobial prokinetic agents, such as cisapride and bethanechol, on abomasal emptying rate and AEA in neonatal calves, and in comparing their prokinetic effects to a positive control (erythromycin) and a negative control (0.9% NaCl solution). Erythromycin was selected as the positive control because it is known to be a strong prokinetic agent in milk‐fed calves and adult cattle.14, 15, 16, 17, 18, 19 Cisapride was investigated because it increases the rate of gastrointestinal motility in the cat, dog, and horse.20 Bethanechol was investigated because of reports documenting its accelerating effect on gastric and abomasal motility.21, 22, 23, 24
Material and Methods
Animals
The study was carried out on 24 male calves from the Foka dairy farm in Isfahan province. The study protocol was approved by the institutional animal care and use committee. Calves had an unassisted delivery and normal adaptation to extra‐uterine life. Calves were separated from their dam immediately after birth, weighed, and housed unrestrained in separate stalls that were bedded with wood shavings. Calves were fed fresh cow's milk twice a day (60 mL/kg) by a bottle with a nipple after their initial feeding of pooled colostrum on the first day life.
Experimental Design
Pooled colostrum was obtained by harvesting 2 L of first‐milking colostrum from 40 multiparous Holstein‐Friesian cows immediately after parturition, mixing the 2 L colostrum samples, and packaging the pooled colostrum in 1 L plastic bags before storing at −20°C.
A 16‐ or 18‐gauge catheter (5 cm in length) was inserted in the jugular vein as previously described.5 An extension set was attached to the catheter, and the catheter and extension set were secured to the calf's neck. Calves were weighed after IV catheterization was completed and randomly assigned to receive 1 of 4 treatments by using a random number generator1 ; the treatments were 2 mL of 0.9% NaCl solution2 PO (control treatment; n = 6), 2 mL of water containing erythromycin lactobionate3 (20 mg/kg BW, PO), 2 mL of water containing cisapride4 (0.5 mg/kg BW, PO), or 2 mL of water containing bethanechol chloride5 (0.5 mg/kg BW, PO). Oral administration, instead of parenteral administration, was selected for investigation to develop a practical treatment protocol for dairy producers and to minimize potential tissue inflammation or prolonged withdrawal times for slaughter related to intramuscular or subcutaneous injections. Three liter of pooled colostrum was thawed at 37°C in a water bath and acetaminophen (50 mg/kg BW) added to the colostrum; acetaminophen was added to colostrum because it provides a well described and validated method to estimate abomasal emptying rate in milk‐fed calves.25, 26 Calves were administered colostrum 30 minutes after receiving their assigned treatment by permitting the calf to suckle. Calves that did not suckle 3 L of colostrum within 10 minutes had the remaining volume administered by esophageal intubation. The delay of 30 minutes between treatment and administration of colostrum was intended to facilitate absorption of the orally administered treatment.
Venous blood samples for determination of plasma IgG concentration were obtained immediately before colostrum administration (time = 0 hour) and at 1, 3, 6, 9, 12, 18, and 24 hours after the start of colostrum administration. Blood samples were collected into 6‐mL partially evacuated tubes containing sodium heparin and centrifuged at 1,000 × g for 15 minutes; 3 mL of plasma was harvested and stored at −20°C until the concentration of IgG was measured. The venous catheter was flushed every 12 hours with heparinized saline (0.9% NaCl) solution (40 U of heparin/mL).
Abomasal emptying rate was measured using acetaminophen and glucose absorption techniques as previously described.25, 26 Venous blood samples for determination of plasma acetaminophen and glucose concentrations were obtained at 0, 15, 30, 45, 60, 90, 120, 180, 210, 240, 300, 360, 420, and 480 minutes (start of suckling was designated as time 0 minute) in a similar method to the samples obtained to measure plasma IgG concentration. These time points for obtaining samples were selected in an attempt to provide at least 6 data points before and after the time to maximal (T max) of acetaminophen to facilitate nonlinear regression analysis for pharmacokinetic modeling. Venous blood samples for determination of plasma total protein concentrations were obtained at 0, 1, 2, 3, 4, 5, 6, 7, and 8 hours after the start of colostrum ingestion. Serum total protein concentration (g/dL) was measured using the biuret reaction.6
Plasma IgG Concentration‐Time Relationship
A commercial ELISA kit for bovine immunoglobulin assay7 was used to measure total IgG concentration in serum and colostrum, according to the manufacturer's guidelines. Briefly serum or colostrum were thawed at 19–22°C, and 100 μL of diluted samples then was transferred into wells of a template plate and 100 μL of diluted conjugate were added to each well. After 30 seconds of shaking of the dilution microplate, 100 μL of each well was transferred to a second microplate which was incubated for 1 hour at room temperature (19–22°C). The plate was then washed 3 times and 100 μL of chromogen added to each well and incubated at room temperature for 10 minutes. The reaction was stopped with the addition of 50 μL of stop solution and the optical density of each well was read with ELISA reader7 , 8 and a 450 nm filter. The IgG concentration was calculated according to the optical density of standards with a log/logit computer program.
Apparent Efficiency of Absorption of Colostral IgG
The IgG intake was calculated by multiplying the IgG concentration (g/L) in pooled colostrum by the volume of colostrum administered (L). The AEA of IgG absorption was calculated from the measured plasma total [IgG] in grams per liter at 24 hours, the estimated plasma volume (PV) in liters, and the IgG intake in grams as AEA = (Plasma [IgG]) × PV/(IgG intake). The PV was calculated as PV = 0.089 × (BW at birth, kg).27 This calculation assumed that IgG absorption from the intestinal tract was minimal after 24 hours and that PV expansion because of absorption of colostral constituents had stabilized at 24 hours.28 Plasma IgG concentration usually peaks by 24 hours of age in colostrum‐fed calves; the decrease in plasma IgG concentration after this time has been attributed to transfer to other pools and catabolism of IgG.4, 6, 29
Plasma Acetaminophen Concentration‐Time Relationship and Abomasal Emptying Rate
Plasma was thawed at 19–22°C and the acetaminophen concentration analyzed spectrophotometrically by use of a colorimetric nitration assay9 as described elsewhere.25 Actual maximal plasma concentration (C max) and actual T max were derived from a plot of the plasma acetaminophen concentration‐versus‐time data. The first derivative of Siegel's modified power exponential formula was used to model the acetaminophen time curve.25, 26 The equation was derived from the fact that the acetaminophen concentration‐versus‐time curve represented as a cumulative dose curve is an inverse analog of the scintigraphic curve with the following equation: C(t) = m × k × β × e−k×t × (1 − e−k×t)β−1, where C(t) is the acetaminophen concentration in plasma at a specified time point, t is the time, m [unit (μg/mL) × minutes] is the area under the acetaminophen concentration‐time curve when time is infinite, k (min−1) is an estimate of the rate constant for abomasal emptying, β is a constant that provides an estimate of the duration of the lag phase before an exponential rate of emptying is reached, and e is the natural logarithm. Nonlinear regression (PROC NLIN)10 was used to estimate values for m, k, and β as described.25 Values for model C max and model T max were obtained by fitting the estimated values for k, β, and m in the nonlinear equation to the cumulative dose curve equation for acetaminophen.
Plasma Glucose Concentration‐Time Relationship
Plasma glucose concentration was determined using an automatic analyzer.11 Actual C max and actual T max were derived from a plot of the plasma glucose concentration‐versus‐time data, and the area under the plasma glucose concentration–time curve was calculated from 0 to 8 hours by using the trapezoid method; this area provides a crude index of the amount of glucose absorbed for each treatment.26, 30, 31
Statistical Analysis
Data were expressed as mean ± SD, and a value of P < .05 was considered significant for all statistical analyses which were conducted using a statistical software program.10 The primary variables of interest were the plasma IgG concentration‐time relationship, the abomasal emptying rate as assessed by the mean value for T max calculated by modeling the acetaminophen concentration‐time relationship (model T max), and the AEA. An ANOVA was used to determine the main effects of treatment on AEA (PROC GLM). Repeated‐measures ANOVA was used to determine the main effects of treatment and time, and the interaction between treatment and time, using an autoregressive(1) covariance matrix (PROC MIXED). Variables were log transformed when needed for analysis by ANOVA and repeated‐measures ANOVA, whenever distributions were abnormal and variances were heterogeneous. Dunnett's test was used for post hoc tests when indicated in the analysis of variance. For the repeated‐measures ANOVA, appropriate post hoc tests were conducted (within group to time = 0 value; between groups to control group at the same time) when indicated by a significant P‐value for the appropriate F test using Bonferroni‐corrected P‐values. A previous study by the investigators5 indicated that 6 calves per group was sufficient to provide adequate statistical power to detect an 18% change in AEA from the control group at α = 0.05 and β = 0.80.
Results
Calves ranged in weight from 36 to 57 kg (mean 46 kg) immediately before treatment was administered and all stood within 3 hours of birth. Calves in the 4 groups were of similar body weight and were administered colostrum at the same time after birth (Table 1).
Table 1.
Body weight, age at colostrum ingestion, indices of absorption of colostral IgG, and abomasal emptying rate indices (mean ± SD or geometric mean and 95% CI in parentheses) of 24 Holstein‐Friesian calves receiving 3 L of pooled colostrum containing acetaminophen (50 mg/kg) by suckling and oroesophageal intubation. Treatments were: 2 mL of 0.9% NaCl solution PO (control treatment; n = 6); 2 mL of water containing erythromycin lactobionate (20 mg/kg BW, PO; n = 6); 2 mL of water containing cisapride (0.5 mg/kg BW, PO; n = 6); and 2 mL of water containing bethanechol chloride (0.5 mg/kg BW, PO; n = 6). P‐values in bold are significant (<.05)
| Factor | Control | Erythromycin | Cisapride | Bethanechol | P‐value: F Test Treatment |
|---|---|---|---|---|---|
| Body weight (kg) | 45.2 ± 3.6 | 46.0 ± 4.1 | 46.0 ± 4.6 | 46.2 ± 3.1 | .53 |
| Age at colostrum administration (hours) | 3.1 (1.3, 7.3) | 2.2 (0.6, 8.2) | 3.4 (1.6, 7.2) | 2.2 (0.6, 8.4) | .40 |
| Total protein and IgG absorption | |||||
| Mean total protein concentration for first 8 hours after colostrum administration (g/dL) | 4.3 ± 0.8 | 5.5 ± 1.1h | 5.0 ± 1.2h | 4.8 ± 0.9h | .0001 |
| IgG AUC24 (g/L) × hoursa | 306 ± 25 | 304 ± 29 | 368 ± 35h | 301 ± 24 | .0014 |
| Apparent efficiency of absorption of colostral IgG (%) | 31.3 ± 6.9 | 31.0 ± 5.1 | 33.0 ± 7.2 | 30.0 ± 4.0 | .77 |
| Acetaminophen absorption | |||||
| Actual C max (μg/mL)b | 17.7 ± 6.6 | 28.9 ± 10.9 | 36.1 ± 11.9h | 27.2 ± 10.2 | .039 |
| Actual T max (minutes)b | 178 ± 89 | 265 ± 55 | 360 ± 85h | 220 ± 80 | .0044 |
| Model C max (μg/mL)c | 16.7 ± 6.1 | 26.3 ± 9.7 | 31.8 ± 11.5 | 25.2 ± 8.0 | .11 |
| Model T max (minutes)c | 243 ± 78 | 245 ± 44 | 332 ± 54 | 254 ± 83 | .13 |
| AUC480 (mg/mL) × minutesd | 5.9 ± 2.1 | 10.0 ± 3.1 | 12.5 ± 3.9h | 9.2 ± 2.8 | .011 |
| k (min−1)e | 0.0030 (0.0011, 0.0079) | 0.0029 (0.0017, 0.0052) | 0.0019 (0.0011, 0.0034) | 0.0026 (0.0012, 0.0055) | .24 |
| βf | 2.24 (0.85, 5.93) | 2.09 (1.30, 3.38) | 1.91 (1.42, 2.57) | 1.98 (1.17, 3.34) | .86 |
| m (mg/mL) × minutesg | 10.5 (3.6, 30.7) | 16.9 (7.7, 37.2) | 30.2 (11.2, 81.1)h | 18.0 (13.3, 24.3) | .011 |
| Glucose absorption | |||||
| Actual C max (mg/dL)b | 107 ± 16 | 135 ± 54 | 125 ± 43 | 125 ± 33 | .76 |
| Actual T max (minutes)b | 95 (19, 474) | 79 (12, 519) | 51 (10, 260) | 131 (37, 473) | .28 |
| AUC480 (g × min/dL)d | 39.8 (27.1, 59.0) | 34.0 (21.2, 54.7) | 39.5 (22.0, 71.1) | 39.4 (26.8, 57.1) | .64 |
AUC24 is the area under the plasma IgG concentration‐time curve for the first 24 hours after colostrum administration. Abomasal emptying rate was assessed by acetaminophen absorption and glucose absorption.
Actual C max is the maximal plasma acetaminophen or glucose concentration, and Actual T max is the time at which Actual C max occurred.
Model C max and T max for acetaminophen were obtained by fitting a nonlinear equation to the first derivative of Siegel's modified power exponential formula for acetaminophen (see Materials and Methods for details).
AUC8 is the area under the acetaminophen concentration‐time curve, or the glucose concentration‐time curve, for the first 8 hours after colostrum administration.
k = an estimate of the rate constant for abomasal emptying.
β = constant that provides an estimate of the duration of the lag phase before an exponential rate of emptying is reached.
m = area under the acetaminophen concentration‐time curve when time is infinite. For glucose absorption, area under the curve is the area under the plasma glucose concentration‐time relationship for the 8 hours period after the start of colostrum ingestion.
Mean values within a row are significantly different from the control value using Dunnett's test.
Plasma Total Protein and IgG Concentration‐Time Relationship
The values for log10 plasma total protein concentration was influenced by the main effects of treatment (P = .0002) and time (P < .0001), but not by the interaction between treatment and time (P = .29). Mean plasma total protein concentration in the first 8 hours after colostrum administration was higher in calves administered erythromycin, cisapride, and bethanechol when compared to control calves (Table 1). Geometric mean plasma total protein concentration was higher in cisapride‐treated calves than in control calves at time = 1 hour (cisapride, 4.4 g/dL [3.0–5.7 g/dL, 95% CI for geometric mean]; control, 3.3 g/dL [1.8–4.7 g/dL]) and time = 6 hours (cisapride, 5.7 g/dL [4.0–7.3 g/dL]; control, 4.3 g/dL [2.7–6.0 g/dL]) after colostrum administration (Fig. 1). Geometric mean plasma total protein concentration in erythromycin treated calves was higher than that in control calves at time = 1 hour (4.6 g/dL [3.1–6.1 g/dL]), time = 6 hour (6.2 g/dL [4.9–7.6 g/dL]), and time = 7 hours (erythromycin, 6.1 g/dL [4.7–7.6 g/dL]; control, 4.6 g/dL [3.2–6.1 g/dL]) after colostrum administration.
Figure 1.
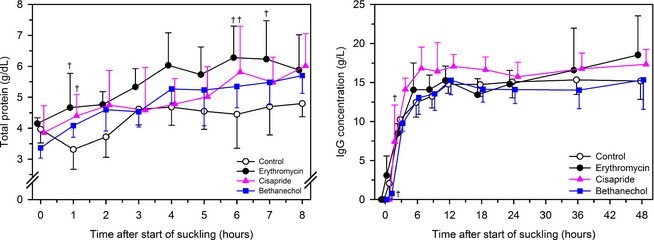
Mean ± SD plasma total protein concentration (left panel) and IgG concentration (right panel) in 24 Holstein‐Friesian calves receiving 3 L of pooled colostrum containing acetaminophen (50 mg/kg) by suckling and oroesophageal intubation. Treatments were: 2 mL of 0.9% NaCl solution PO (control treatment; n = 6, open circles); 2 mL of water containing erythromycin lactobionate (20 mg/kg BW, PO; n = 6, filled circles); 2 mL of water containing cisapride (0.5 mg/kg BW, PO; n = 6, filled triangles); and 2 mL of water containing bethanechol chloride (0.5 mg/kg BW, PO; n = 6, filled squares). Data are slightly offset for each group with respect to time to improve readability. †P < .0055 (Bonferroni corrected) for the specific treatment compared with control treatment at the same time.
The P‐values for the F test for the main effect of treatment and time, and for the interaction between treatment and time, on log10 of plasma total IgG concentration were .0012, <.0001, and <.0001, respectively. At time = 1 hour after colostrum ingestion, the plasma total IgG concentration was higher in cisapride‐treated calves (geometric mean, 7.1 g/L; 95% CI, 1.8–27.9 g/L), and lower in bethanechol treated calves (geometric mean, 1.7 g/L; 95% CI, 0.8–3.5 g/L), than control calves (geometric mean, 2.5 g/L; 95% CI, 0.6–9.9 g/L; Fig. 1). The area under the curve for the plasma total IgG concentration‐time relationship over the 24 hours after colostrum ingestion was higher in cisapride‐treated calves than control calves (Table 1).
Apparent Efficiency of Absorption
The measured total IgG concentration in pooled colostrum was 65 g/L; therefore calves were administered a standardized IgG mass of 195 g by suckling and oroesophageal intubation. The AEA was similar for calves in all 4 groups (Table 1).
Plasma Acetaminophen Concentration‐Time Relationship and Abomasal Emptying Rate
The P‐values for the F test for the main effect of treatment and time, and for the interaction between treatment and time, on plasma acetaminophen concentration were .018, <.0001, and .077, respectively. Plasma acetaminophen concentration in cisapride‐treated calves was higher than that in control calves at a number of time points after colostrum administration (Fig. 2), consistent with cisapride alteration of metabolism or clearance of acetaminophen. Plasma acetaminophen concentration in erythromycin treated calves was higher than that in control calves at a small number of time points after colostrum administration.
Figure 2.
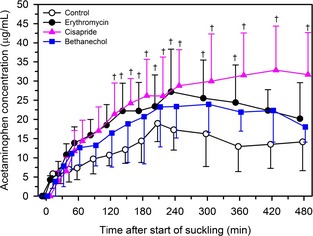
Mean ± SD plasma concentration of acetaminophen in 24 Holstein‐Friesian calves receiving 3 L of pooled colostrum containing acetaminophen (50 mg/kg) by suckling and oroesophageal intubation. Treatments were: 2 mL of 0.9% NaCl solution PO (control treatment; n = 6, open circles); 2 mL of water containing erythromycin lactobionate (20 mg/kg BW, PO; n = 6, filled circles); 2 mL of water containing cisapride (0.5 mg/kg BW, PO; n = 6, filled triangles); and 2 mL of water containing bethanechol chloride (0.5 mg/kg BW, PO; n = 6, filled squares). Data are slightly offset for each group with respect to time to improve readability. †P < .0167 (Bonferroni corrected) for the specific treatment compared to control treatment at the same time.
Plasma Glucose Concentration‐Time Relationship
There was no significant effect of treatment on the glucose absorption curve (Fig. 3, Table 1). The P‐values for the F test for the main effect of treatment and time, and for the interaction between treatment and time, on plasma glucose concentration were .99, <.0001, and .64, respectively.
Figure 3.
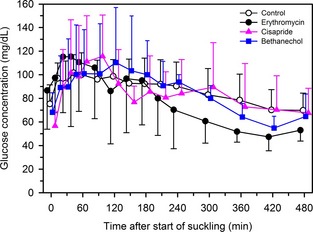
Mean ± SD plasma glucose concentration in 24 Holstein‐Friesian calves receiving 3 L of pooled colostrum containing acetaminophen (50 mg/kg) by suckling and oroesophageal intubation. Treatments were: 2 mL of 0.9% NaCl solution PO (control treatment; n = 6, open circles); 2 mL of water containing erythromycin lactobionate (20 mg/kg BW, PO; n = 6, filled circles); 2 mL of water containing cisapride (0.5 mg/kg BW, PO; n = 6, filled triangles); and 2 mL of water containing bethanechol chloride (0.5 mg/kg BW, PO; n = 6, filled squares). Data are slightly offset for each group with respect to time to improve readability.
Discussion
We believe that the study reported here is the first to investigate the effect of cisapride and bethanechol on the AEA of colostral IgG in neonatal calves. The results of this study indicated that the oral administration of cisapride (0.5 mg/kg BW) 30 minutes before colostrum administration facilitated the absorption of colostral IgG and protein. Based on the total IgG and total protein concentration‐time relationships, the beneficial effects of cisapride appeared to occur early after colostrum ingestion and were transient. This result was consistent with the findings of pharmacokinetic studies in ruminants that cisapride is rapidly cleared from the plasma of adult cattle, adult sheep, and lambs with elimination half‐lives of 1.9 hours,32 1.5 hours,33 and 1.4–1.8 hours,33 respectively. It is recommended that cisapride be administered 30 minutes before feeding, as in the study reported here, with the oral bioavailability in dogs being 53%.34 The oral dose rate of cisapride used in this study (0.5 mg/kg) was higher than that recommended for dogs, cats, and horses (0.1 mg/kg)20; however, higher oral doses of cisapride (0.2 and 0.5 mg/kg) that increased antral contractions have been safely administered to dogs.35 A relatively high oral dose was administered to the calves in this study because of the faster elimination half‐life of cisapride in cattle relative to dogs (4.0–8.1 hours)34 and unknown oral bioavailability. Additional studies appear indicated to characterize the effect of cisapride dose on the magnitude and duration of promoting absorption of colostral IgG and protein in calves.
The beneficial effect of cisapride on increasing absorption of colostral IgG and protein was most likely because of cisapride‐induced increases in abomasal emptying rate. We were unable to confirm that cisapride increased the emptying rate, although the plasma acetaminophen concentration in cisapride‐treated calves was higher than that in control calves at a number of time points after colostrum administration. This result was most likely because of interference in cisapride metabolism by acetaminophen, as demonstrated in a study in humans,36 rather than indicating a prokinetic effect. Demonstration of a prokinetic effect in cisapride‐treated calves may therefore be best examined using ultrasonographic measurement of abomasal dimensions to calculate changes in abomasal volume over time.15 In comparison, erythromycin and bethanechol do not appear to interfere with acetaminophen metabolism. Consequently, the acetaminophen concentration‐time relationship provided a useful measure of abomasal emptying rate in calves in these two treatment groups.
A highly water‐soluble formulation of erythromycin (erythromycin lactobionate) was administered orally in the study reported here because other oral formulations were not readily available in Iran. An unexpected finding of this study was the minimal effect that oral erythromycin lactobionate (20 mg/kg BW) had on indices of abomasal emptying rate and the total IgG and total protein‐time relationships. Previous studies have consistently demonstrated that IM administration of erythromycin (8.8–10 mg/kg BW) produced a marked prokinetic effect in milk‐fed calves and adult cattle.5, 14, 15, 16, 17, 18, 19 Erythromycin base is not absorbed in preruminant calves when administered orally at 10 mg/kg; this result has been attributed to acid destruction of erythromycin in the abomasum.37 Moderately water‐soluble, acid stable esters of erythromycin (such as erythromycin thiocyanate, carbonate, ethyl succinate, and gluconate) have therefore been recommended for oral administration in milk‐fed calves, even though the mean oral bioavailability is low (25% at 20 mg/kg) with large calf to calf variability.37 Erythromycin esters do not dissolve readily in low pH solutions and are therefore believed to be minimally degraded in the calf's abomasum; esters dissociate in the small intestine because of the presence of nonspecific esterases, yielding free erythromycin base in an environment where the higher luminal pH facilitates stability.37 On the basis of the results of this study and those of a previous study,37 we speculate that orally administered erythromycin will have markedly reduced prokinetic effects relative to parenteral administration of an equivalent dose of erythromycin.
Compared to control calves, oral administration of bethanechol (0.5 mg/kg BW) did not appear to alter the abomasal emptying rate in the study reported here, based on acetaminophen pharmacokinetic indices and the plasma total IgG and total protein‐time relationships. Very few studies evaluating the effect of bethanechol on gastric emptying are available,38 and the authors of the manuscript reported here are not aware of any study in animals that demonstrates that the oral administration of bethanechol promotes gastric emptying. Oral administration of bethanechol (≈0.7 mg/kg BW) prolonged the gastric retention time of a large capsule in the stomach of adult humans, which is suggestive of an inhibition of gastric emptying of solids, and did not change mouth‐to‐cecum transit time.39 In a study of yearling cattle, administration of bethanechol (0.07 mg/kg, SC) increased the antral spike rate and total number of antegrade propagating spikes,23 which is suggestive of a prokinetic effect; however, because of a lack in coordination, bethanechol did not alter the abomasal emptying rate.
Although cisapride increased the AEA of colostral IgG by 5% compared to calves in the control group, the increase in AEA was not statistically significant, consistent with our calculation that the study had adequate power to detect an 18% difference in AEA. The AEA was lower than that in our previous study5 and the within group variability in AEA in this study was more than two times larger than that in our previous study.5 We attributed the lower AEA and larger variability in AEA in this study to most treatments being administered when calves were more than 2 hours of age, and to allowing calves to suckle a variable volume of colostrum within 10 minutes before the remainder was administered by oroesophageal intubation. In addition, the oral administration of small volumes of drug solution may have led to variable absorption, in part because of differences in inducing esophageal groove closure. Future studies characterizing the dose and time‐dependent relationships of cisapride with abomasal emptying rate should be conducted in milk‐fed calves, and the results of these studies used to identify a potential optimal treatment protocol for investigating the effect of oral cisapride on increasing the AEA in newborn calves. Additional studies examining the effect of treatment on AEA would benefit from administering colostrum within 2 hours of birth, using a standard method of colostrum administration such as oroesophageal intubation, or using a group size larger than 6 if calves are permitted to suckle colostrum.
In conclusion, these results suggest that oral cisapride administration may provide a practical nonantimicrobial treatment protocol for increasing the absorption of colostral IgG in calves. Based on this proof of concept, additional studies appear indicated using the related prokinetic drug mosapride which is more widely available and lacks the deleterious cardiovascular effects (QT prolongation, cardiac arrhythmias including ventricular arrhythmias such as torsades de pointes) that led to the voluntarily removal of cisapride from the US market for use in humans in 2000.
Acknowledgments
This study was supported, in part, by a grant from the University of Shahid Chamran, Ahvaz, Iran. We acknowledge the technical assistance of Dr M. Khosravi from Foka dairy farm in Isfahan city in completing this study.
Conflict of Interest Declaration: Peter Constable is a consulting editor for experimental design and statistics with the Journal of Veterinary Internal Medicine.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
Work done at the University of Shahid Chamran.
Footnotes
Excel spreadsheet, Microsoft Corp., Redmond, WA
Ramopharmin, Tehran, Iran
ShifaPharmed, Tehran, Iran
SobhanPhar, Tehran, Iran
Wockhardt Limited, Mumbai, India
Biuret method, Pars azmun, Iran
Bio‐X Diagnostics, Belgium
Dynatech, Netherlands
Jenway, Stone, UK
PROC NLIN, SAS, version 9.3, SAS Institute Inc, Cary, NC
Hexokinase assay; Hitachi 704 automatic analyzer, Hitachi, Tokyo, Japan
References
- 1. Stott GH, Marx DB, Menifee BE, et al. Colostral immunoglobulin transfer in calves I. period of absorption. J Dairy Sci 1979;62:1632–1638. [DOI] [PubMed] [Google Scholar]
- 2. Bush LJ, Staley TE. Absorption of colostral immunoglobulins in newborn calves. J Dairy Sci 1980;63:672–680. [DOI] [PubMed] [Google Scholar]
- 3. Matte JJ, Girard CL, Seoane JR, et al. Absorption of colostral immunoglobulin G in the newborn dairy calf. J Dairy Sci 1982;65:1765–1770. [DOI] [PubMed] [Google Scholar]
- 4. Rajala P, Castren H. Serum immunoglobulin concentrations and health of dairy calves in two management system from birth to 12 weeks of age. J Dairy Sci 1995;78:2737–2744. [DOI] [PubMed] [Google Scholar]
- 5. Mokhber‐Dezfooli MR, Nouri M, Rasekh M, et al. Effect of abomasal emptying rate on the apparent efficiency of colostral immunoglobulin G absorption in neonatal Holstein‐Friesian calves. J Dairy Sci 2012;95:6740–6749. [DOI] [PubMed] [Google Scholar]
- 6. Bush LJ, Aguilera MA, Adams GD, et al. Absorption of colostral immunoglobulins by newborn dairy calves. J Dairy Sci 1971;54:1547–1549. [DOI] [PubMed] [Google Scholar]
- 7. Stott GH, Menefee BE. Selective absorption of immunoglobulin IgM in the newborn calf. J Dairy Sci 1978;61:461–466. [DOI] [PubMed] [Google Scholar]
- 8. Baumwart AL, Bush LJ, Mungle M, et al. Effect of potassium isobutyrate on absorption of immunoglobulins from colostrum by calves. J Dairy Sci 1977;60:759–762. [DOI] [PubMed] [Google Scholar]
- 9. Stott GH, Fellah A. Colostral immunoglobulin absorption linearly related to concentration for calves. J Dairy Sci 1983;66:1319–1328. [DOI] [PubMed] [Google Scholar]
- 10. Besser TE, Garmedia AE, McGuire TC, et al. Effect of colostral immunoglobulin G1 and immunoglobulin M concentrations on immunoglobulin absorption in calves. J Dairy Sci 1985;68:2033–2037. [DOI] [PubMed] [Google Scholar]
- 11. Quigley JD, French P, James RE. Short communication: Effect of pH on absorption of immunoglobulin G in neonatal calves. J Dairy Sci 2000;83:1853–1855. [DOI] [PubMed] [Google Scholar]
- 12. Godden S, Haines D, Hagman D. Improving passive transfer of immunoglobulins in calves I: Dose effect of feeding acommercial colostrum replacer. J Dairy Sci 2009;92:1750–1757. [DOI] [PubMed] [Google Scholar]
- 13. Godden SM, Haines DM, Konkol K, et al. Improving passive transfer of immunoglobulins in calves. II: Interaction between feeding method and volume of colostrum fed. J Dairy Sci 2009;92:1758–1764. [DOI] [PubMed] [Google Scholar]
- 14. Constable PD, Nouri M, Sen I, et al. Evidence‐based use of prokinetic drugs for abomasal disorders in cattle. Vet Clin North Am Food Anim Pract 2012;28:51–70. [DOI] [PubMed] [Google Scholar]
- 15. Wittek T, Constable PD. Assessment of the effects of erythromycin, neostigmine, and metoclopramide on abomasal motility and emptying rate in calves. Am J Vet Res 2005;66:545–552. [DOI] [PubMed] [Google Scholar]
- 16. Wittek T, Tischer K, Korner I, et al. Effect of preoperative erythromycin or dexamethasone/vitamin C on postoperative abomasal emptying rate in dairy cows undergoing surgical correction of abomasal volvulus. Vet Surg 2008;37:537–544. [DOI] [PubMed] [Google Scholar]
- 17. Nouri M, Constable PD. Effect of parenteral administration of erythromycin, tilmicosin, and tylosin on abomasal emptying rate in suckling calves. Am J Vet Res 2007;68:1392–1398. [DOI] [PubMed] [Google Scholar]
- 18. Nouri M, Hajikolaee MR, Constable PD, et al. Effect of erythromycin and gentamicin on abomasal emptying rate in suckling calves. J Vet Intern Med 2008;22:196–201. [DOI] [PubMed] [Google Scholar]
- 19. Afshari GR, Nouri M, Hassan EB, et al. Effect of parenteral administration of ivermectin and erythromycin on abomasal emptying rate in suckling calves. Am J Vet Res 2009;70:527–531. [DOI] [PubMed] [Google Scholar]
- 20. Dowling PM. Prokinetic drugs: Metoclopramide and cisapride. Can Vet J 1995;36:115–116. [PMC free article] [PubMed] [Google Scholar]
- 21. Megens AA, Awouters FH, Niemegeers CJ. General pharmacology of the four gastrointestinal motility stimulants bethanechol, metoclopramide, trimebutine, and cisapride. Arzneimittelforschung 1991;41:631–634. [PubMed] [Google Scholar]
- 22. Michel A, Mevissen M, Burkhardt HW, et al. In vitro effects of cisapride, metoclopramide and bethanechol on smooth muscle preparations from abomasal antrum and duodenum of dairy cows. J Vet Pharmacol Ther 2003;26:413–420. [DOI] [PubMed] [Google Scholar]
- 23. Roussel AJ, Brumbaugh GW, Waldron RC, et al. Abomasal and duodenal motility in yearling cattle after administration of prokinetic drugs. Am J Vet Res 1994;55:111–115. [PubMed] [Google Scholar]
- 24. Buehler M, Steiner A, Meylan M, et al. In vitro effects of bethanechol on smooth muscle preparations from abomasal fundus, corpus, and antrum of dairy cows. Res Vet Sci 2008;84:444–451. [DOI] [PubMed] [Google Scholar]
- 25. Marshall TS, Constable PD, Crochik SS, et al. Determination of abomasal emptying rate in suckling calves by use of nuclear scintigraphy and acetaminophen absorption. Am J Vet Res 2005;66:364–374. [DOI] [PubMed] [Google Scholar]
- 26. Nouri M, Constable PD. Comparison of two oral electrolyte solutions and route of administration on the abomasal emptying rate of Holstein‐Friesian calves. J Vet Intern Med 2006;20:620–626. [DOI] [PubMed] [Google Scholar]
- 27. Quigley JD, Drewry JJ, Martin KR. Estimation of plasma volume in Holstein and Jersey calves. J Dairy Sci 1998;81:1308–1312. [DOI] [PubMed] [Google Scholar]
- 28. Sasaki M, Davis CL, Larson BL. Immunoglobulin IgG1 metabolism in new born calves. J Dairy Sci 1977;60:623–626. [DOI] [PubMed] [Google Scholar]
- 29. Grongnet JF, Grongnet‐Pinchon E, Levieux D, et al. Newborn calf intestinal absorption of immunoglobulins extracted from colostrum. Reprod Nutr Dev 1986;26:731–743. [DOI] [PubMed] [Google Scholar]
- 30. Sen I, Constable PD, Marshall TS. Effect of suckling isotonic or hypertonic solutions of sodium bicarbonate or glucose on abomasal emptying rate in calves. Am J Vet Res 2006;67:1377–1384. [DOI] [PubMed] [Google Scholar]
- 31. Constable PD, Grunberg W, Carstensen L. Comparative effects of two oral rehydration solutions on milk clotting, abomasal luminal pH, and abomasal emptying rate in suckling calves. J Dairy Sci 2009;92:296–312. [DOI] [PubMed] [Google Scholar]
- 32. Takemura N, Masuda H, Hirose H, Tagawa M. Pharmaco‐kinetics of cisapride in dairy cattle after intravenous administration. J Japan Vet Med Assoc 2002;55:77–79. [Google Scholar]
- 33. Veereman‐Wauters G, Monbaliu J, Meuldermans W, et al. Study of the placental transfer of cisapride in sheep. Plasma levels in the pregnant ewe, the fetus, and the lamb. Drug Metab Disp 1991;19:168–172. [PubMed] [Google Scholar]
- 34. Michiels M, Monbaliu J, Hendriks R, et al. Pharmacokinetics and tissue distribution of the new gastrokinetic agent cisapride in rat, rabbit, and dog. Arnzeimittel forschung 1987;37:1159–1167. [PubMed] [Google Scholar]
- 35. Burger DM, Wiestner T, Hubler M, et al. Effect of anticholinergics (atropine, glycopyrrolate) and prokinetics (metoclopramide, cisapride) on gastric motility in Beagles and Labrador Retrievers. J Vet Med A 2006;53:97–107. [DOI] [PubMed] [Google Scholar]
- 36. Itoh H, Nagano T, Takeyama M. Cisapride raises the bioavailability of paracetamol by inhibiting its glucuronidation in man. J Pharm Pharmacol 2001;53:1041–1045. [DOI] [PubMed] [Google Scholar]
- 37. Soback S, Ziv G, Kurtz B, Risenberg R. Age‐dependent oral biovailability of erythromycin thiocyanate in calves. J Vet Med A 1987;34:102–107. [DOI] [PubMed] [Google Scholar]
- 38. Ramirez B, Richter JE. Review article: Promotility drugs in the treatment of gastro‐oesophageal reflux disease. Aliment Pharmacol Ther 1993;7:5–20. [DOI] [PubMed] [Google Scholar]
- 39. Kirby MG, Dukes GE, Heizer WD, et al. Effect of metoclopramide, bethanechol, and loperamide on gastric residence time, gastric emptying, and mouth‐to‐cecum transit time. Pharmacotherapy 1989;9:226–231. [DOI] [PubMed] [Google Scholar]


