Abstract
Background
A 5‐year‐old, healthy English Springer Spaniel died suddenly 4 months after delivering a litter of 7 puppies. Within 4 months of the dam's death, 3 offspring also died suddenly.
Hypothesis
Abnormal cardiac repolarization, caused by an inherited long QT syndrome, is thought to be responsible for arrhythmias leading to sudden death in this family.
Animals
Four remaining dogs from the affected litter and 11 related dogs.
Methods
Physical examination and resting ECG were done on the littermates and 9 related dogs. Additional tests on some or all littermates included echocardiogram with Doppler, Holter monitoring, and routine serum biochemistry. Blood for DNA sequencing was obtained from all 15 dogs.
Results
Three of 4 littermates examined, but no other dogs, had prolonged QT intervals with unique T‐wave morphology. DNA sequencing of the KCNQ1 gene identified a heterozygous single base pair mutation, unique to these 3 dogs, which changes a conserved amino acid from threonine to lysine and is predicted to change protein structure.
Conclusions and Clinical Importance
This family represents the first documentation in dogs of spontaneous familial QT prolongation, which was associated with a KCNQ1 gene mutation and sudden death. Although the final rhythm could not be documented in these dogs, their phenotypic manifestations of QT interval prolongation and abnormal ECG restitution suggested increased risk for sudden arrhythmic death. The KCNQ1 gene mutation identified is speculated to impair the cardiac repolarizing current I Ks, similar to KCNQ1 mutations causing long QT syndrome 1 in humans.
Keywords: Long QT Syndrome
Abbreviations
- AP
action potential
- QTc
corrected QT interval
- ESS
English Springer Spaniel
- HR
heart rate
- LQTS
long QT syndrome(s)
- LQT1
LQTS form 1
- SD
sudden death
- TdP
Torsades de Pointes
Sudden death (SD) is known to occur in dogs with previously undiagnosed cardiac diseases, including various cardiomyopathies, subaortic stenosis, myocarditis, and others.1, 2, 3, 4, 5, 6, 7 Although similar and several other abnormalities are associated with SD in people, a major cause is cardiac ion channel dysfunction (channelopathy) that adversely affects action potential (AP) generation or termination.8, 9 Half of all sudden arrhythmic cardiac deaths in people are thought to result from a channelopathy of some type, caused either by genetic mutation or acquired malfunction.10 Channelopathies causing long QT syndrome (LQTS) are most common.
The QT interval represents the time of ventricular depolarization and repolarization on the ECG. Abnormally prolonged QT interval, heterogeneous repolarization, or both predisposes to potentially fatal ventricular arrhythmias. Acquired QT prolongation can occur as a consequence of drugs and other conditions that alter ion channel function.10, 11, 12 In dogs, acquired QT prolongation has been reported with some drugs, electrolyte disturbances, toxins, infectious or inflammatory lesions, autonomic imbalance, and metabolic disease.13, 14, 15, 16, 17, 18 Congenital LQTS is the most common of many inherited channelopathies identified in people; mutations in the KCNQ1 gene underlie the largest number of cases.10, 19, 20 However, inherited LQTS has not previously been documented in dogs.
An apparently normal, 5‐year‐old female English Springer Spaniel (ESS) died suddenly after activity. Four months earlier, she had delivered her only litter, with 7 healthy pups. Within 3 months of her death, 2 offspring also had SD during activity. A third littermate was presented for evaluation; the only abnormality found on routine clinical testing was an unusually long QT interval for the heart rate (HR).
We hypothesized that a form of inherited LQTS was responsible for abnormal cardiac repolarization and sudden death in this family of dogs. Our objectives were to evaluate surviving littermates and related dogs for phenotypic evidence of LQTS and a causative mutation.
Materials and Methods
Study Design
Observational case‐control study.
Clinical Evaluations
The affected litter consisted of 5 males and 2 females. Data could be obtained on 2 females (pups “C” and “R”) and 2 males (pups “E” and “T”). Nine related dogs also were evaluated, including the litter's sire, the dam of the affected litter's dam (proband), 2 full‐ and 1 half‐sister to the proband, and other members of the dogs' extended family (Fig 1). Initially, a complete history, physical examination, resting ECG (30–40 second), thoracic radiographs, routine serum biochemistry, indirect blood pressure measurement, echocardiogram with Doppler, and subsequently, 24‐hour Holter recording were done on pup C. All of these tests, except radiographs, also were done on pup E.
Figure 1.
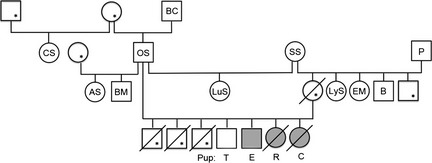
ESS family pedigree. Squares represent males; circles, females. Tested individuals identified by letters. Shaded symbols indicate LQTS‐affected dogs. Open symbols indicate normal QT duration—except those with an asterisk represent dogs of unknown phenotype. A diagonal line indicates sudden death.
Tests on other littermates and related dogs included, at minimum, a history, physical examination, and resting ECG. Pup T was directly examined by another veterinary cardiologist who performed a complete echocardiographic examination with Doppler. Pup R was evaluated by a local veterinarian; routine serum biochemistry also was performed. Holter recordings could be obtained on 5 dogs: pups C, E, R, T, and 1 related ESS (dog AS, Fig 1). Blood was collected on these 13 and 2 additional dogs (P, proband's sire and B, full brother; Fig 1) for DNA sequencing in search of a mutation in a candidate gene (KCNQ1) associated with a cardiac repolarizing current. DNA samples also were obtained from 99 unaffected, unrelated dogs (controls) of various breeds. After baseline data collection, pups C, R, and E were treated with a beta‐blocker (atenolol 12.5 mg PO q12h) because this treatment has been useful in some people with LQTS. All dogs were housed and cared for by their owners. All testing was done in accordance with accepted clinical practice and principles of the NIH Guide for Care and Use of Laboratory Animals.
Heart rate and standard complex measurements were determined from resting ECGs. Corrected QT intervals (QTc) were calculated using Van De Water's formula and average HR.21, 22 QT interval and preceding TQ interval durations were measured from hard‐copy resting ECGs at 50 mm/s. Ratios of QT to preceding TQ interval were calculated for each beat. To evaluate ECG restitution, mean QT/TQ ratio and the percentage of beats with QT/TQ ratio >1 were determined for each dog.23 In addition, resting ECGs were recorded from pup E after treatment (for >1 week) with atenolol at 0.7 mg/kg PO q12h (dose 1) and 1 mg/kg PO q12h (dose 2). QT and TQ intervals were measured by the same procedure, and results were compared with baseline values.
Statistical Analysis
Data for average HR and number of heartbeats in all dogs were normally distributed, and a t‐test for equal variance with unequal sample size was used to compare normal and affected ESS dogs. Data for QT duration and mean QT/TQ ratio were not normally distributed and the Mann‐Whitney test was used for between group comparisons. For pup E, comparisons of mean QT duration and QT/TQ ratio between baseline and the 2 atenolol doses were made using the Wilcoxon Signed Rank test when data were not normally distributed, and using a t‐test for comparisons where the test for normality was satisfied. Mean values ± standard deviation are reported. P < .05 was considered significant. Analyses were done by SAS 9.3.1
DNA Analysis
Sequences of exonic, untranslated, and splice site regions of the canine KCNQ1 gene (ENSCAFG00000010231) were obtained from the canine genome using the UCSC genome browser of the Broad CanFam3.1 assembly. Amplification primers were designed by Primer 3 software (http://frodo.wi.mit.edu). Amplification primers for exon 8 of the KCNQ1 gene, the exon containing the LQTS mutation, were as follows: forward 5′ catgcaggatggggtgtg; reverse 5′ ctccccacccactaatcact.
Standard PCR amplifications were carried out using AccuPrime GC‐rich buffer A,2 2 unit/μL AccuPrime GC‐rich DNA polymerase2, 20 mM of each amplification primer, and approximately 100 ng of template DNA. Samples were denatured for 3 minutes at 95°C followed by 30 cycles of 95°C for 30 seconds, 60°C for 30 seconds, 72°C for 30 seconds, and finally 72°C for 10 minutes. The annealing temperature was optimized (55–60°C) to accommodate the respective primer.
Residual amplification primers and dNTPs were removed from the PCR product using a single‐step enzymatic cleanup kit.3 Amplicons then were sequenced on an ABI 3730XL sequencer.4 The nucleotide sequences were evaluated for sequence change among ESS dogs, control dogs, and the published normal canine sequence (http://genome.ucsc.edu).
A base pair change was considered possibly causative for long QT syndrome if it: was present in dogs with QT prolongation but not other dogs or the published canine sequence, changed a conserved amino acid, and changed that amino acid to a different polarity, acid/base status, or structure. Any amino acid change identified was evaluated with the Polyphen2 (http://genetics.bwh.harvard.edu/pph2/) program to predict possible impact of the amino acid substitution on the protein. Polyphen2 also was used to determine level of conservation using the multiple sequence alignment function (UniProtKB/UniRef100 Release 2011_12). UniProtKB was utilized to evaluate the sequence annotation and determine which functional region of the protein the amino acid substitution affected (http://www.uniprot.org/).
Finally, the normal and altered sequences were evaluated for changes that occur in the secondary structure or function with 4 protein modeling software programs, GOR4 (http://npsapbil.ibcp.fr/cgibin/npsa_automat), SIFT (http://sift.jcvi.org), SWISS‐MODEL Workspace (http://swissmodel.expasy.org), and Protean 3D.5
Results
A month after the adult female ESS died, a male pup (5 months old) died suddenly after being released from its crate and running across the room. A second pup (male, 7 months old) died suddenly while playing outdoors. Pup C was evaluated at 7½ months of age; other available dogs were evaluated within the next 3 weeks. A third male (8 months old) had SD during play shortly before evaluation. There was no history of episodic weakness, syncope, or other abnormality in any dog, including those that died. Routine vaccinations and heartworm preventive had been given according to standard recommendations. Dogs with SD were not receiving other drugs at the time of death.
Physical examination findings on pups C, E, and R, and 7 related dogs were normal. A soft systolic left basilar murmur was heard in pup T. The litter's sire and one of the sire's half‐sisters also had soft systolic murmurs. Other physical findings were unremarkable. Blood pressure in pups C and E was normal (not available in others).
Resting ECGs showed normal sinus arrhythmia (9 dogs) or regular sinus rhythm (3 dogs) with HRs from 80 to 150 beats/min. Pup T had sinus tachycardia (180 beats/min). ECG measurements were normal with the exception of unusually long QT intervals for HRs of 110–120/min in pups C, E, and R (QT, 260–270 msec; QTc, 304–314 msec; Figs 2, 3). Other dogs had QT intervals ranging from 160 to 230 msec (QTc, 218–265 msec). Affected dogs (pups C, E, and R) also had large biphasic T waves not seen in the other dogs.
Figure 2.
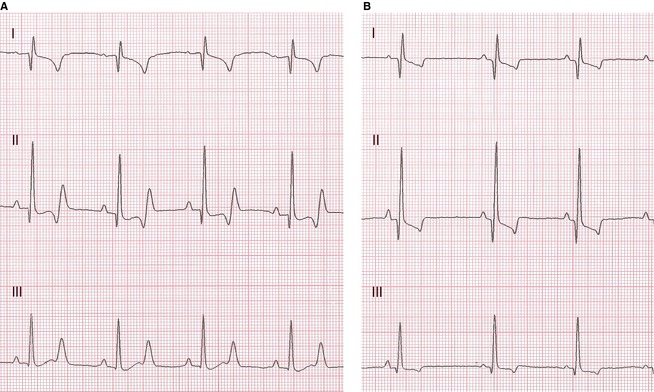
ECGs from an affected (A, pup E) and normal (B) ESS at HR of 120/min. Note prolonged QT duration (270 msec; QTc 310 msec) and large biphasic T waves in A. Leads marked, 50 mm/s, 1 cm = 1 mV.
Figure 3.
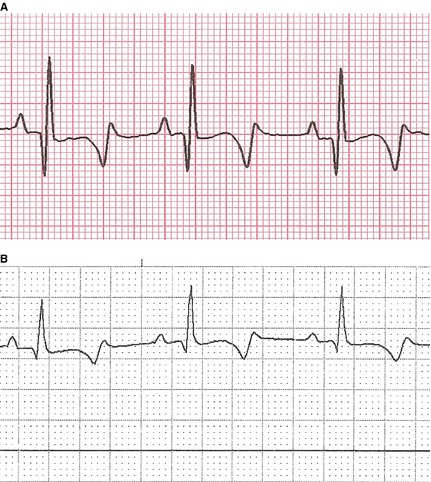
ECGs from affected pup C, QT duration 270 msec (A); and pup R, QT duration 260 msec (B). 50 mm/s.
Echocardiogram‐Doppler examinations on pups C, E, and T showed no abnormalities. Serum biochemistry results for pups C, E, and R were normal. All Holter recordings showed normal sinus rhythm or sinus arrhythmia with no ectopic complexes.
QT and TQ intervals, and QT/TQ ratios were obtained from 50 mm/s recordings of 9–10 second duration, during which 13–21 heartbeats occurred (mean 16.4 ± 2.6 beats for unaffected ESS and 19.7 ± 2.3 beats for affected dogs; nonsignificant difference, P = .077). Average HRs ranged from 80 to 160 beats/min and were not different between groups (mean 114 ± 25.0 for unaffected dogs and 123 ± 11.5 for affected dogs, P = .553). There was no overlap of mean QT interval duration between normal ESS (176–245 msec; mean, 209.9 ± 24.3 msec) and affected dogs (267–279 msec; mean, 272.0 ± 9.4 msec; P < .001).
Mean QT/TQ ratios ranged from 0.493 to 1.022 (mean: 0.686 ± 0.199) in unaffected ESS, and from 1.055 to 1.534 (mean: 1.365 ± 0.264) in affected dogs (P < .001). QT/TQ ratios >1 occurred in only 2 of 10 unaffected dogs. These dogs had the highest HRs, with 1 unaffected dog (HR, 150 beats/min) having QT/TQ ratios >1 for 45% of measured heartbeats, and the other (pup T, 160 beats/min) with QT/TQ ratios >1 for 7.1% of heartbeats. There were no cardiac cycles with QT/TQ ratios >1 in any of the 8 other unaffected ESS. In contrast, QT/TQ ratios >1 were present in 100% of beats for pups C and R and in 62.5% of beats for pup E.
For pup E, mean QT duration and QT/TQ ratio at baseline (untreated) were compared to those after >1 week of treatment at each dose of atenolol, using an equal number of sequential heartbeats for each treatment dose. Mean QT duration decreased nonsignificantly between baseline and dose 1 (279 ± 5.7 msec versus 273 ± 8.7 msec, P = .055) at a HR of 112 beats/min in both ECG recordings. Mean QT duration at the higher dose of atenolol was more prolonged (298 ± 17.6, P = .002 compared to untreated), but average HR had declined to 96 beats/min during the ECG segment used for comparison. Mean QT/TQ ratio in pup E decreased only at the higher atenolol dose compared to baseline (1.055 ± 0.09 untreated; 1.039 ± 0.14 dose 1, P = .713 versus untreated; 0.928 ± 0.09 dose 2, P = .028 versus untreated). The percentage of heartbeats with QT/TQ ratio >1 decreased markedly with increasing atenolol dose for the ECG complexes measured (62.5% untreated; 50%, dose 1; and 19%, dose 2).
Results of KCNQ1 gene sequencing showed that the 3 dogs with QT prolongation, but no other dogs, had a heterozygous single base pair change from cytosine to adenine in codon 1 of exon 8 (Fig 4). This changes a highly conserved amino acid in the protein transcript from threonine (T), a nonaromatic, neutral hydrophilic amino acid, to lysine (K), a basic positively charged amino acid at position 377 (Genbank KF439050 ; KCNQ1_T377K). Altered protein structure is predicted (Fig 5). The protein structure analysis program GOR4 predicted an increase in the extended strand and a decrease in the random coil within the domain containing the mutation. The SWISS‐MODEL Workspace program predicted a 3‐dimensional model of the normal and mutant KCNQ1 C‐terminus cytoplasmic protein region, which further confirmed reduction in random coil and increased extended strand structure. Commercial software (Protean 3D) was used to further analyze the SWISS‐MODEL output files with the following results: Chou‐Fasman analysis of secondary structure indicated removal of a beta region and extension of the neighboring alpha region in the mutant protein; Kyte‐Doolittle hydropathy analysis indicated decreased hydrophobicity in the mutant protein; and Lehninger charge density evaluation indicated creation of a positive charge region in the mutant protein.
Figure 4.
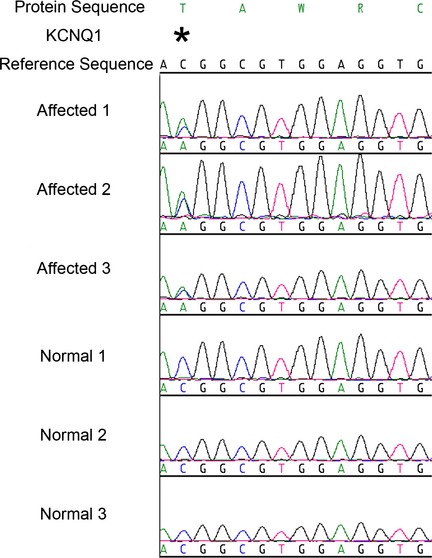
A heterozygous C to A nucleotide change was identified in the 3 dogs labeled “affected”. The 3 normal dogs match the reference sequence and are homozygous for the wild type C in position 2, codon 1, exon 8 of the KCNQ1 gene
Figure 5.
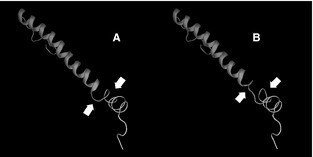
Protein models (SWISS‐MODEL) of the KCNQ1 gene, C‐terminus region, demonstrating normal protein sequence (A) and mutant protein sequence (B). Note loss of random coil and increased extended strand in (B) compared to normal (arrows).
After initial data collection and in addition to beta‐blocker treatment, avoidance of strenuous activity and excitement was recommended for the 3 affected dogs. Nevertheless, Pup C died suddenly after going outside within 2 months of initial evaluation (9 months of age). Pup R's owners decided to discontinue atenolol after a few weeks; SD occurred while running at 15 months of age. Pup E developed progressive unprovoked aggression toward children and was euthanized per owner request at 20 months of age. No cardiovascular abnormalities were found at necropsy.
Discussion
This study represents the first report of familial LQTS in the dog, with the identified KCNQ1 gene mutation apparently arising de novo in the dam of the affected litter. The clinical presentation was similar to LQTS in humans in which the first sign often is SD. It was characterized phenotypically by prolonged QT intervals in the absence of any pharmacologic manipulation or evidence of cardiac or other systemic disease. None of these ESS showed any indication of familial atrial standstill or ventricular dysfunction.
The QT interval duration, extending from the onset of the QRS complex to the end of the T wave, is determined by ventricular cell AP duration in aggregate. Factors that delay repolarization prolong the QT interval. Electrophysiologically, this includes reduction in outward K+ flux during phases 2 and 3, especially of either the slow (I Ks) or rapid (I Kr) components of the I K (delayed rectifier) current, or enhanced inward Na+ or Ca++ current during depolarization.10 Repolarization delay predisposes to potentially lethal arrhythmias from triggered activity, reentrant mechanisms, or both.24 Decreased outward K+ current, by prolonging phase 2 and allowing Ca++ channels to partially recover from inactivation, can facilitate development of early after‐depolarizations that may trigger premature ventricular depolarization. In addition, regional disparity in ventricular repolarization and refractoriness facilitates development of reentrant ventricular tachycardia. The polymorphic ventricular tachycardia known as Torsades de Pointes (TdP) is commonly associated with LQTS. TdP often degenerates to ventricular fibrillation.10, 24
QT duration varies with changes in HR, largely reflecting underlying cardiac autonomic influences. Therefore, QT duration is considered in the context of HR. Several equations have been developed to describe the QT‐HR relationship or to “correct” QT interval duration for HR, although these all have limitations. In people, QTc usually is calculated using the RR interval from the preceding beat (ie, instantaneous HR). In dogs, respiratory sinus arrhythmia generally produces marked variability in RR intervals. However, QT duration remains essentially unchanged during respiratory sinus arrhythmia despite large changes in beat‐to‐beat intervals because more beats than occur during a respiratory cycle are required for ionic conductance to reach a new steady state for the new HR (so‐called “QT memory”).21, 23 Although not perfect, we used Van de Water's correction formula, with mean RR interval calculated from mean HR (over 6–10 seconds), to normalize the QT interval to an RR interval of 1000 msec (HR of 60 beats/min): QTc = QT − 0.087(RR‐1000), with QT and RR in msec. Commonly used correction formulas in people (eg, Bazett's or Fridericia's) tend to overestimate QTc at higher HRs and underestimate QT prolongation at lower HRs.21, 22, 25, 26, 27 Although, as in people, there may be no clear cut‐off between normal and abnormal QTc in the larger canine population, the QTc calculations in these dogs showed a clear distinction between unaffected and affected dogs.
The utility of QTc formulas for distinguishing arrhythmogenic repolarization abnormalities from physiologic variation is limited however, because they do not account for physiologic changes in autonomic state or QT hysteresis on the QT‐RR relationship.10, 28 During increased vagal influence (eg, exhalation), there is little change in QT duration at long RR intervals (slow HR). However, as vagal tone decreases and sympathetic influence increases, the QT‐RR relationship becomes steeper. This creates a dynamic oscillation between flatter and steeper QT‐RR relationships, which QTc formulas cannot reliably predict.28
A newer technique for assessing arrhythmia vulnerability involves the generation of beat‐to‐beat QT‐RR plots (QT “clouds”) from 5‐ to 10‐minute ECG recordings to differentiate physiologic variation in the QT interval caused by changing HR and autonomic influence from impaired repolarization.28 This beat‐to‐beat method also is used to evaluate ECG restitution by plotting QT to preceding TQ intervals. ECG restitution is the ability of the heart to recover from 1 beat to the next. 28 It describes the “work” phase (QT interval) in relation to the preceding “rest” phase (TQ interval). This also is a dynamic relationship that varies with autonomic state as well as abnormal repolarization delay. The mean QT/TQ ratio and percent heartbeats with QT/TQ ratio >1 are also ECG biomarkers of restitution. In the normal unstressed heart, “rest” time exceeds “work” time and the QT/TQ ratio is <1. However with exercise (tachycardia) or other stress, this ratio may be >1 because the TQ shortens to a greater extent than does the QT. Sustained periods of QT/TQ >1 are thought to represent inadequate recovery time between beats and increased vulnerability to arrhythmia.23, 28 QT prolongation, especially with increased repolarization heterogeneity, magnifies this risk. The percentage of beats with QT/TQ >1 reflects the relative time spent on the restitution curve during which electrical stability is questionable. Healthy people have <25% of beats with QT/TQ >1; normal dogs appear to have fewer, and 5% was reported in 1 study.23, 28 An increase in mean QT/TQ ratio and higher percentage of beats with QT/TQ >1 have been correlated with increased arrhythmogenicity in people and have been observed experimentally in dogs after administration of arrhythmogenic substances.23, 28
Although we were unable to apply beat‐to‐beat methods in our dogs because we lacked extended ECG recordings on all dogs, as well as the required software, we attempted to generate “snapshots” of ECG restitution using brief resting ECGs of consistent duration and recording conditions. We acknowledge that sinus arrhythmia might be a confounding factor in our brief recordings, however, most dogs had fairly regular sinus rhythm at the time. Furthermore, the ECGs would have been long enough to encompass 3–5 respiratory cycles, allowing measurements at different levels of autonomic tone. Although this approach has not been fully validated, and the duration of our ECGs was limited, it did identify clear differences between groups. The increased mean QT/TQ ratios and markedly increased percentage of heartbeats with QT/TQ >1 despite lower HRs in LQTS dogs is consistent with prolonged repolarization and increased risk for lethal arrhythmias. Indeed, the dogs that had 100% of heartbeats with QT/TQ >1 did experience SD.
Inherited long QT syndrome in people has a prevalence estimated at 1:2,500–5,000 individuals, with 13 LQTS forms now reported.10 About 70–75% of human LQTS cases result from mutations in 3 major genes: KCNQ1, KCNH2 (hERG), and SCN5A.9, 20 Respectively, these genes code for the alpha subunits of the slow‐activating delayed rectifier potassium current (I Ks), the rapid‐activating delayed rectifier potassium current (I Kr), and the fast inward sodium current (I Na). Approximately, 35% of positive LQTS genetic tests in people are caused by loss‐of‐function mutations in the KCNQ1 gene, causing LQT1.20 To the authors' knowledge, no examples of familial LQTS have been documented previously in dogs or other animals. The missense mutation in KCNQ1 discovered in these 3 affected ESS dogs, which changes a highly conserved amino acid, appears to have effects similar to those in people with LQT1, although this specific mutation has not been reported in people.
Common clinical manifestations in humans with LQTS include syncope and a high risk of TdP, which may spontaneously terminate or progress to ventricular fibrillation.10, 19 Sudden cardiac death often is the first sign of disease, as was observed in the dogs of this report. In humans with LQTS, the severity of clinical signs can vary even among people with the same mutation and within the same family. This clinical heterogeneity in phenotypic expression in patients with the same mutation may relate to the complex interactions between membrane ion channels and other membrane proteins as well as the intra‐ and extracellular environment.20
There are genotype‐specific triggers associated with LQTS cardiac events in people. Those with LQT1 are reportedly the most sensitive to sympathetic stimulation, and cardiac events most often occur during exercise. KCNQ1 mutations not only can cause a decrease in I Ks, but also decrease channel activation in response to beta‐adrenergic stimulation, which paradoxically increases QT duration (and the risk of life‐threatening arrhythmias) during exercise and during other times of sympathetic activation.29 The occurrence of SD in these ESS dogs while they were playing or running (and presumably excited) is consistent with the observations in people with LQT1.
A broad‐based, prolonged T‐wave morphology commonly is observed in people with LQT1.10, 19 In studies of a canine experimental model of LQT1 employing a I Ks blocker, the addition of the beta‐agonist isoproterenol induced preferential AP duration prolongation in mid‐myocardial (M) cells compared to cells near the epicardium and endocardium. The modified K+ current greatly increased the transmural heterogeneity of repolarization and resulted in large, broad‐based T waves.19, 30 The T waves of the affected ESS dogs in which ECGs were available all showed wide and pronounced negative‐positive biphasic configuration in Lead II. This was thought to reflect increased spatial heterogeneity of repolarization in these dogs. None of the other ESS ECGs had clearly biphasic T waves, although a few had subtle notching of a positive T wave.
Human males with LQT1 are more likely to experience a first cardiac event at an earlier age than females. However, the risk for SD in affected females becomes greater than for males during adulthood, with risk reversal occurring during the mid‐teens.19, 24 Although the number of dogs reported here is too small for statistical significance, the dam of the affected litter was ostensibly normal until SD at 5 years of age, whereas 3 male pups were the first to die.
Beta‐blockers are helpful in preventing syncope and sudden death in people with LQT1.19 Improved ECG restitution, as implied by the decreased percentage of heartbeats with QT/TQ >1 in pup E after atenolol, is thought to decrease arrhythmia risk. Other recommendations include exercise restriction, and avoidance of hypokalemia and drugs known to prolong the QT interval.
This study has a number of limitations, especially the fact that the dam and 3 pups were not available for study. Also, because ECG recordings were not available at the time of SD, we could not document an arrhythmia as the cause of death. Unfortunately, necropsy examinations were not available on dogs with SD. However, none of the 5 littermates with SD or their dam showed any evidence of decreased exercise tolerance or other signs of underlying myocardial disease. Likewise, clinical and echocardiographic findings on the pups examined did not indicate the presence of myocardial disease. Despite the large percentage of affected dogs in this litter, the total number of affected dogs was small. Nevertheless, the findings reported here are important because this family of ESS represents the first report of inherited LQTS in dogs, along with an associated, novel KCNQ1 mutation.
Acknowledgments
Lori Moran; ESS breeder and owners; Drs Robert Hamlin and Cecilia Marshall; Heritage Pet Hospital; Yaxuan Sun.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Work performed at Iowa State and North Carolina State Universities.
Abstract presented at the 2013 ACVIM Forum, Seattle, WA.
Footnotes
SAS Institute Inc, Cary, NC
Qiagen, Valencia, CA
ExoSAP‐IT, Affymetrix, Santa Clara, CA
Applied Biosystems, Foster City, CA
DNAStar, Madison, WI
References
- 1. Calvert CA, Hall G, Jacobs G, et al. Clinical and pathologic findings in Doberman pinschers with occult cardiomyopathy that died suddenly or developed congestive heart failure: 54 cases (1984–1991). J Am Vet Med Assoc 1997;210:505–511. [PubMed] [Google Scholar]
- 2. Alroy J, Rush JE, Freeman L, et al. Inherited infantile dilated cardiomyopathy in dogs: Genetic, clinical, biochemical, and morphologic findings. Am J Med Genet 2000;95:57–66. [PubMed] [Google Scholar]
- 3. Basso C, Fox PR, Meurs KM, et al. Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in boxer dogs: A new animal model of human disease. Circulation 2004;109:1180–1185. [DOI] [PubMed] [Google Scholar]
- 4. Schrope DP, Kelch WJ. Signalment, clinical signs, and prognostic indicators associated with high‐grade second‐ or third‐degree atrioventricular block in dogs: 124 cases (January 1, 1997–December 31, 1997). J Am Vet Med Assoc 2006;228:1710–1717. [DOI] [PubMed] [Google Scholar]
- 5. Hayes MA, Russell RG, Babiuk LA. Sudden death in young dogs with myocarditis caused by parvovirus. J Am Vet Med Assoc 1979;174:1197–1203. [PubMed] [Google Scholar]
- 6. Breitschwerdt EB, Atkins CE, Brown TT, et al. Bartonella vinsonii subsp. berkhoffii and related members of the alpha subdivision of the Proteobacteria in dogs with cardiac arrhythmias, endocarditis, or myocarditis. J Clin Microbiol 1999;37:3618–3626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Moise NS, Meyers‐Wallen V, Flahive WJ, et al. Inherited ventricular arrhythmias and sudden death in German shepherd dogs. J Am Coll Cardiol 1994;24:233–243. [DOI] [PubMed] [Google Scholar]
- 8. Chandra N, Bastiaenen R, Papadakis M, et al. Sudden cardiac death in young athletes: Practical challenges and diagnostic dilemmas. J Am Coll Cardiol 2013;61:1027–1040. [DOI] [PubMed] [Google Scholar]
- 9. Winkel BG, Larsen MK, Berge KE, et al. The prevalence of mutations in KCNQ1, KCNH2, and SCN5A in an unselected national cohort of young sudden unexplained death cases. J Cardiovasc Electrophysiol 2012;23:1092–1098. [DOI] [PubMed] [Google Scholar]
- 10. Abriel H, Zaklyazminskaya EV. Cardiac channelopathies: Genetic and molecular mechanisms. Gene 2013;517:1–11. [DOI] [PubMed] [Google Scholar]
- 11. Said TH, Wilson LD, Jeyaraj D, et al. Transmural dispersion of repolarization as a preclinical marker of drug‐induced proarrhythmia. J Cardiovasc Pharmacol 2012;60:165–171. [DOI] [PubMed] [Google Scholar]
- 12. Redfern WS, Carlsson L, Davis AS, et al. Relationships between preclinical cardiac electrophysiology, clinical QT interval prolongation and torsade de pointes for a broad range of drugs: Evidence for a provisional safety margin in drug development. Cardiovasc Res 2003;58:32–45. [DOI] [PubMed] [Google Scholar]
- 13. Campbell FE, Atwell RB. Long QT syndrome in dogs with tick toxicity (Ixodes holocyclus). Aust Vet J 2002;80:611–616. [DOI] [PubMed] [Google Scholar]
- 14. Sugiyama A, Satoh Y, Shiina H, et al. Torsadegenic action of the antipsychotic drug sulpiride assessed using in vivo canine models. J Cardiovasc Pharmacol 2002;40:235–245. [DOI] [PubMed] [Google Scholar]
- 15. Finley MR, Lillich JD, Gilmour RF Jr, et al. Structural and functional basis for the long QT syndrome: Relevance to veterinary patients. J Vet Intern Med 2003;17:473–488. [DOI] [PubMed] [Google Scholar]
- 16. Harada T, Abe J, Shiotani M, et al. Effect of autonomic nervous function on QT interval in dogs. J Toxicol Sci 2005;30:229–237. [DOI] [PubMed] [Google Scholar]
- 17. Dennis SG, Wotton PR, Boswood A, et al. Comparison of the effects of thiopentone and propofol on the electrocardiogram of dogs. Vet Rec 2007;160:681–686. [DOI] [PubMed] [Google Scholar]
- 18. Hanton G, Yvon A, Racaud A. Temporal variability of QT interval and changes in T wave morphology in dogs as markers of the clinical risk of drug‐induced proarrhythmia. J Pharmacol Toxicol Methods 2008;57:194–201. [DOI] [PubMed] [Google Scholar]
- 19. Shimizu W, Horie M. Phenotypic manifestations of mutations in genes encoding subunits of cardiac potassium channels. Circ Res 2011;109:97–109. [DOI] [PubMed] [Google Scholar]
- 20. Webster G, Berul CI. An update on channelopathies: From mechanisms to management. Circulation 2013;127:126–140. [DOI] [PubMed] [Google Scholar]
- 21. Oguchi Y, Hamlin RL. Rate of change of QT interval in response to a sudden change in the heart rate in dogs. Am J Vet Res 1994;55:1618–1623. [PubMed] [Google Scholar]
- 22. Van de Water, A , Verheyen, J , Xhonnuex, R , Reneman, RS . An improved method to correct the QT interval of the electrocardiogram for changes in heart rate. J Pharmacol Methods 1989;22:207–217. [DOI] [PubMed] [Google Scholar]
- 23. Kijtawornrat A, Panyasing Y, Del Rio C, et al. Assessment of ECG interval and restitution parameters in the canine model of short QT syndrome. J Pharmacol Toxicol Methods 2010;61:231–237. [DOI] [PubMed] [Google Scholar]
- 24. Bastiaenen R, Behr ER. Sudden death and ion channel disease: Pathophysiology and implications for management. Heart 2011;97:1365–1372. [DOI] [PubMed] [Google Scholar]
- 25. Chiladakis J, Kalogeropoulos A, Arvanitis P, et al. Preferred QT correction formula for the assessment of drug‐induced QT interval prolongation. J Cardiovasc Electrophysiol 2010;21:905–913. [DOI] [PubMed] [Google Scholar]
- 26. Oguchi Y, Hamlin RL. Duration of QT interval in clinically normal dogs. Am J Vet Res 1993;54:2145–2149. [PubMed] [Google Scholar]
- 27. Tattersall ML, Dymond M, Hammond T, et al. Correction of QT values to allow for increases in heart rate in conscious Beagle dogs in toxicology assessment. J Pharmacol Toxicol Methods 2006;53:11–19. [DOI] [PubMed] [Google Scholar]
- 28. Fossa AA. Assessing QT prolongation and electrocardiography restitution using a beat‐to‐beat method. Cardiol J 2010;17:230–243. [PubMed] [Google Scholar]
- 29. Nademanee K. Exercise and vagal reflex in long QT syndrome type 1. J Am Coll Cardiol 2012;60:2525–2526. [DOI] [PubMed] [Google Scholar]
- 30. Shimizu W, Antzelevitch C. Cellular basis for the ECG features of the LQT1 form of the long‐QT syndrome: Effects of beta‐adrenergic agonists and antagonists and sodium channel blockers on transmural dispersion of repolarization and torsade de pointes. Circulation 1998;98:2314–2322. [DOI] [PubMed] [Google Scholar]


