Abstract
Background
Cats with diabetes mellitus can have subclinical pancreatitis but prospective studies to confirm this are lacking. Metabolic control of diabetic cats with pancreatitis is difficult.
Hypothesis
Subclinical pancreatitis occurs in diabetic cats at the time diabetes is diagnosed or might develop during the follow‐up period, hampering diabetic remission.
Animals
Thirty cats with newly diagnosed diabetes without clinical signs of pancreatitis on admission.
Methods
Prospective study. On admission and 2 and 6 months later, serum Spec fPL and DGGR‐lipase were measured and the pancreas underwent ultrasonographic examination. Pancreatitis was suspected if serum markers were increased or ≥2 ultrasonographic abnormalities were detected. Cats were treated with insulin glargine and diabetic remission was defined as euglycemia ≥4 weeks after discontinuation of insulin. Nonparametric statistical tests were used for analysis.
Results
Subclinical pancreatitis at the time of diagnosis was suspected in 33, 50, and 31% of cats based on Spec fPL, DGGR‐lipase and ultrasonography, respectively; and in 60% when diagnostic criteria were combined. During the follow‐up period, suspected pancreatitis developed in additional 17–30% cats. Only 1 cat had transient clinical signs compatible with pancreatitis. Seventeen of the 30 cats (57%) achieved remission. Frequency of abnormal Spec fPL and DGGR‐lipase and abnormal ultrasonographic findings did not differ in cats achieving remission and those who did not. Cats achieving remission had significantly lower Spec fPL at 2 months (P < .001).
Conclusions and Clinical Importance
Based on laboratory and ultrasonographic measurements, many cats with diabetes might have pancreatitis, although without clinical signs. Cats with high Spec fPL might have a reduced chance of diabetic remission; however, this topic needs further studies in large cohorts of diabetic cats.
Keywords: DGGR‐lipase, Endocrinology, Feline, Gastroenterology, Spec fPL
Abbreviations
- DM
diabetes mellitus
- fPLI
feline pancreatic lipase immunoreactivity
- DGGR
1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester
Diabetes mellitus (DM) is one of the most common endocrinopathies in cats. The incidence of DM has increased in cats because of an increase in predisposing factors including obesity and physical inactivity.1, 2 It has been suggested that pancreatitis induces DM in cats,3 possibly because of the involvement of the islets of Langerhans in the inflamed exocrine tissue, leading to injury of the β‐cells. However, only a few studies have explored the association between pancreatitis and DM in cats and the effect of pancreatitis on glycemic control, and the results were controversial. One investigation showed histological signs of inflammation of the exocrine pancreas in 19 (51%) of 37 cats with DM examined postmortem; most cases were chronic but a few were acute to subacute.3 Moreover, achieving good glycemic control tended to be more difficult in cats in which pancreatic inflammation was documented, suggesting that pancreatitis might render insulin treatment less effective.3 In another study, 24 (83%) of 29 cats with long‐standing DM had increased serum feline pancreatic lipase immunoreactivity (fPLI), a marker for pancreatitis, compared with 15 (66%) of 23 control cats4; diabetic cats also had significantly higher fPLI, suggesting an association between pancreatitis and the endocrine disease.4 Moreover, there was a positive correlation between increased fPLI and serum fructosamine concentrations in diabetic cats, possibly suggesting an adverse effect of pancreatitis on glycemic control.4 An interesting aspect of both these studies was that clinical signs were uncommon or not obvious despite the increased frequency of pancreatitis. This suggests that exocrine disease is subclinical in the majority of cats with DM.3, 4
The histological prevalence of pancreatitis does not differ between diabetic cats (21 of 37, 57%) and control cats (12 of 20, 60%) examined postmortem.1 Moreover, cats with DM and pancreatitis, diagnosed in vivo based on serum lipase activity measured with the 1,2‐o‐dilaurylrac‐glycero‐3‐glutaric acid‐(60‐methylresorufin) ester (DGGR) assay, did not show less chances of diabetic remission.5 These findings question the association between pancreatitis and DM and might suggest that pancreatitis does not adversely affect metabolic control in diabetic cats.
Diagnosis of pancreatitis is difficult in cats, especially in subclinical cases.4 At present, the use of serum markers, such as fPLI or Spec fPL,2 combined with abdominal ultrasonography, are considered the most useful diagnostic parameters to suspect pancreatitis.6 The serum marker DGGR‐lipase activity has recently been shown to correlate well with Spec fPL concentration and has therefore been proposed as an additional diagnostic tool in cats with suspected pancreatitis.7 Therefore, the aims of this study were to use the above mentioned serum markers combined with ultrasonography to prospectively investigate the occurrence of subclinical pancreatitis in cats with newly diagnosed DM and to monitor the incidence of subclinical pancreatitis in the follow‐up period. In addition, the rate of diabetic remission was compared in cats with and without suspected pancreatitis.
Materials and Methods
Animals
Cats with newly diagnosed DM were prospectively enrolled in the study between July 2008 and January 2011. Cats were excluded from the study if they had received insulin therapy for longer than 1 week before admission, and if glucocorticoids or progestagens had been administered during the previous 4 months. On admission, all cats underwent thorough evaluation including a physical examination, complete blood cell count, serum biochemistry including the measurement of fructosamine and total T4 concentrations, serum Spec fPL concentration and DGGR‐lipase activity, urinalysis with bacterial culture and urinary protein‐to‐creatinine ratio, blood pressure measurement, abdominal and thoracic radiography, and abdominal ultrasonography. Serum Spec fPL concentration was measured by IDEXX Laboratories,3 , 6 and serum DGGR‐lipase activity was measured in the clinical laboratory of the faculty using a commercial assay.4 , 7 The study was approved by the Veterinary Office of the Canton of Zurich and conducted in accordance with guidelines established by the Animal Welfare Act of Switzerland (permission no. 83/2008). The owners provided informed consent to participate in the study.
Pancreatic Ultrasonography
The pancreas was evaluated during each abdominal ultrasonographic examination by a board‐certified radiologist using a standardized protocol, which included assessment of pancreatic contour (regular, irregular) and echogenicity (normal, increased or decreased compared with liver), thickness of the left and right pancreatic ducts (normal, increased [>0.25 cm]), and the presence of free fluid or hyperechoic mesentery in the vicinity of the pancreas. Ultrasonographic findings of the pancreas that were considered abnormal included irregular contour, increased or decreased echogenicity, increased duct size, and adjacent free fluid or hyperechoic mesentery.8, 9, 10 The radiologist used the same ultrasonographic protocol in all cats to reduce bias. The radiologist was informed that the cat was diabetic but was unaware of the clinical signs and hematology results.
Concurrent Diseases at the Time of Diagnosis
Cats with a concurrent disease (eg, renal failure, gastrointestinal disorders, heart disease, other endocrinopathies, and neoplasia) were not included in the study. Diabetic cats with ketoacidosis were included if acidaemia resolved and the general condition improved within 48 hours of insulin therapy. Cats suspected of having clinically relevant pancreatitis were also excluded. Because results of serum Spec fPL concentration were not available at the time DM was diagnosed, pancreatitis was suspected and arbitrarily considered clinically relevant during the initial examination when at least 1 clinical sign, such as anorexia, vomiting, abdominal pain, lethargy or hypothermia occurred together with increased DGGR‐lipase activity (>26 U/L)7 or with at least 2 ultrasonographic abnormalities of the pancreas.
Diabetic Remission and Tests for Pancreatitis During the Follow‐Up Period
During the 6‐month follow‐up period, the cats were treated with insulin glargine5 and fed a high‐protein, low‐carbohydrate diet.6 Re‐evaluations were scheduled at 1, 2–3, 6–8, 12–16 and 24 weeks. Insulin dosage adjustments were based on clinical signs, results of physical examination, fructosamine concentration and blood glucose curves.11 Remission of DM was defined as the absence of associated clinical signs (eg, polyuria and polydipsia, polyphagia) accompanied by normal blood glucose (72–162 mg/dL) and fructosamine concentrations (<340 μmol/L) for at least 4 weeks after discontinuation of insulin treatment.12 The insulin dosage was gradually decreased in steps of 0.5 UI twice daily, each week whenever possible. The last dosage before discontinuation of insulin treatment was 0.5 UI once daily, for at least 1 week. Cats that required insulin throughout the study were defined as not being in diabetic remission. During follow‐up, diagnostic tests for the tentative diagnosis of pancreatitis included measurement of serum Spec fPL concentration and DGGR‐lipase activity, as well as standardized pancreatic ultrasonography. These tests were carried out 2 and 6 months after the time of diagnosis.
Criteria to Suspect Pancreatitis and Statistical Analysis
Subclinical pancreatitis was suspected at the time of diagnosis when no clinical signs compatible with pancreatitis were detected together with 1 or more of the following results: (1) increased Spec fPL concentration, (2) increased DGGR‐lipase activity, (3) at least 2 ultrasonographic abnormalities. A cut‐off value of >5.3 μg/L was used for Spec fPL concentration.2 , 7 For DGGR‐lipase activity, the cut‐off was set at >26 U/L based on the reference range previously established in healthy cats (8–26 U/L).7 The same criteria to suspect pancreatitis were also used at the 2‐ and 6‐month follow‐up examinations in cats. The term “suspected” pancreatitis was used throughout the manuscript because pancreatitis is difficult to diagnose in vivo in cats.
Frequency distributions were constructed for the occurrence of subclinical pancreatitis on admission and of any form of pancreatitis that developed during the follow‐up period, based on the aforementioned diagnostic criteria. Fisher's exact test was used to examine associations between suspected pancreatitis at the time of diagnosis or during follow‐up and remission of DM. The Mann–Whitney test followed by Bonferroni correction was used at each time point for comparison of Spec fPL concentration and DGGR‐lipase activity in cats that achieved diabetic remission and those that did not. If significant differences were observed, receiver operating characteristic (ROC) curves were calculated to identify the cut‐off value with highest sensitivity and specificity for remission of DM. Furthermore, the Friedman test followed by Dunn's multiple comparison was performed in all cats to verify whether a decline or increase in Spec fPL concentration and DGGR‐lipase activity occurred during follow‐up. A commercial software8 was used for all calculations. Results are presented as median and ranges, and the level of significance was set at P < .05.
Results
Animals
During the study period, 50 cats with newly diagnosed DM were admitted. Twenty cats were excluded because of idiopathic hypercalcaemia (3 cats), diabetic ketoacidosis with delayed response to treatment (2 cats), suspected acromegaly (2 cats), pancreatic carcinoma (2 cats), and one each because of cutaneous mast cell tumor, FIV, heart failure, hypertension and hepatic cysts, hyperthyroidism, ileocolic abscess, injection‐site fibrosarcoma, suspected clinically relevant pancreatitis, plasmacellular pododermatitis, recent administration of corticosteroids, and fractious behavior. Thirty cats fulfilled the inclusion criteria and were enrolled in the study. Median age was 11.0 years (range: 7.0–17.0), and median body weight was 5.0 kg (range: 2.5–9.6). Twenty‐five were domestic shorthair or longhair cats and 5 were purebred cats, including one each of Abyssinian, Burmese, Ragdoll, Siamese and Norwegian forest cat. Seventeen cats were neutered males and 13 were spayed females. Two of the enrolled cats had ketoacidosis at presentation with acidaemia that resolved within 1 day of therapy. None of the cats had clinical signs compatible with pancreatic or gastrointestinal disease. The present study is part of a previously pubished study (Data S1).13
Suspected Pancreatitis at the Time of Diagnosis of DM
At the time of DM diagnosis, median Spec fPL concentration was 3.7 μg/L (range: 0.7–19.7) and median DGGR‐lipase activity was 27 U/L (range: 14–75). Ten to 15 of the 30 cats (33.3–50.0%) had suspected pancreatitis, based on Spec fPL > 5.3 μg/L, DGGR‐lipase > 26 U/L and their combination (Table 1).
Table 1.
Diabetic cats with suspected pancreatitis based on increased levels of pancreatic serum markers and ultrasonographic abnormalities of the pancreas at the time of diagnosis and during the follow‐up period. Cats listed under follow‐up did not have suspected pancreatitis at the time of diagnosis
| Variable | Diagnosis | Follow‐Up | |
|---|---|---|---|
| Cats with Suspected Pancreatitis (%) | Cats with Suspected Pancreatitis (%) | Number of Cats at 2 and 6 Months | |
| Spec fPL > 5.3 μg/L | 10 of 30 (33) | 5 of 30 (17) | 2 and 3 |
| DGGR‐lipase > 26 U/L | 15 of 30 (50) | 7 of 30 (23) | 2 and 5 |
| Spec fPL > 5.3 μg/L or DGGR‐lipase > 26 U/L | 15 of 30 (50) | 7 of 30 (23) | 2 and 5 |
| ≥2 ultrasonographic abnormalities | 9 of 29 (31) | 8 of 27 (30) | 4 and 4 |
| ≥2 ultrasonographic abnormalities or Spec fPL > 5.3 μg/L | 13 of 30 (43) | 8 of 29 (28) | 3 and 5 |
| ≥2 ultrasonographic abnormalities or DGGR‐lipase > 26 U/L | 18 of 30 (60) | 7 of 30 (23) | 2 and 5 |
| ≥2 ultrasonographic abnormalities or Spec fPL > 5.3 μg/L or DGGR‐lipase > 26 U/L | 18 of 30 (60) | 7 of 30 (23) | 2 and 5 |
Ultrasonography was carried out in 29 of the 30 cats. Nine of them (31.0%) had ≥2 abnormalities and were suspected of having pancreatitis (Table 1). Of these 9 cats, 8 had increased or decreased echogenicity of the pancreas, 4 each had free fluid or hyperechoic mesentery in the vicinity of the pancreas and 3 each had irregular pancreatic contours or increased size of the left pancreatic duct. Three of the 9 cats had 4 (1 cat) or 3 (2 cats) concurrent ultrasonographic abnormalities; none had the same combination of findings. Of the remaining 20 cats, all had 1 ultrasonographic abnormality of the pancreas, including increased or decreased echogenicity (17 cats), increased size of the right duct (2 cats) and adjacent hyperechoic mesentery (1 cat). Based on the documentation of ≥2 ultrasonographic abnormalities or on the increase of at least one of the serum markers, pancreatitis was suspected in 9–18 cats (31.0–60.0%) (Table 1). Three of the 9 cats with suspected pancreatitis based on ultrasonography had normal Spec fPL or DGGR‐lipase levels.
Suspected Pancreatitis That Developed During the Follow‐Up Period
Two months after diagnosis of DM, median Spec fPL concentration was 2.6 μg/L (range: 3.2–40.0) and median DGGR‐lipase activity was 24 U/L (range: 12–132); after 6 months, median Spec fPL concentration was 4.5 μg/L (range: 0.9–>50) and median DGGR‐lipase activity was 29 U/L (range: 10–116). Median Spec fPL and DGGR‐lipase did not differ between time of diagnosis and the 2 re‐evaluations, nor between the latter 2 time points. The frequency of suspected pancreatitis was calculated for cats that developed increased serum markers or abnormal ultrasonographic findings of the pancreas during the follow‐up period. Pancreatitis at follow‐up was suspected in 5–7 additional cats (16.7–23.3%) based on Spec fPL concentration, DGGR‐lipase activity and their combinations (Table 1). With regard to pancreatic ultrasonography, 8 additional cats (29.6%) had ≥2 abnormal findings and were thought to have developed pancreatitis during follow‐up (Table 1). Of these 8 cats, all had increased or decreased echogenicity of the pancreas, 4 each had irregular pancreatic contours or hyperechoic mesentery in the vicinity of the pancreas, 2 had increased left pancreatic duct size, and 1 had free fluid adjacent to the pancreas. Based on documentation of ≥2 ultrasonographic abnormalities or on increased Spec fPL concentration, DGGR‐lipase activity and their combinations, pancreatitis was suspected in 7–8 additional cats during the follow‐up period (23.3–29.6%) (Table 1).
Signs compatible with clinically relevant pancreatitis (anorexia and vomiting) occurred in only 1 cat, which was thought to have developed acute pancreatitis 3 days after diagnosis of DM. This cat had increased Spec fPL concentration and DGGR‐lipase activity at the time of diagnosis, but no clinical signs of pancreatitis and only 1 ultrasonographic pancreatic abnormality (hypoechoic pancreas). Pancreatic ultrasonography during the acute episode revealed a small amount of abdominal effusion and hyperechoic mesentery in the vicinity of the pancreas in addition to hypoechogenicity of the pancreas. Although the cat recovered clinically after 8 days, Spec fPL concentration and DGGR‐lipase activity remained increased and ≥2 ultrasonographic abnormalities were seen at 2 and 6 months. None of the remaining cats that were suspected of developing pancreatitis during follow‐up had clinical signs compatible with clinically relevant pancreatitis.
Follow‐Up of Cats with Suspected Pancreatitis at the Time of Diagnosis
Considering the entire study period, 50.0, 73.3 and 61.8% of cats had suspected pancreatitis based on Spec fPL, DGGR‐lipase and ultrasonography, respectively, and 86.7% based on any combination of pancreatic enzymes and ultrasonography (Table 1).
In 8 of 10 (80.0%) cats, in which Spec fPL concentration was increased, the concentration remained increased during the follow‐up period in 4 and normalized by 2 months but then increased again by 6 months in the other 4. In the remaining 2 cats with increased Spec fPL concentration (20.0%) the enzyme levels normalized within 2 months. In 13 of 15 (86.7%) cats, in which DGGR‐lipase was increased, the enzymatic activity remained elevated during the follow‐up period in 11 and normalized by 2 months but then increased again by 6 months in 2 others. In the remaining 2 cats with increased DGGR‐lipase activity (13.3%) the enzyme levels normalized within 2 months (Tables 1 and S3).
Four of the 9 cats (44.4%) with ≥2 ultrasonographic abnormalities of the pancreas continued to have abnormal findings during the follow‐up period. Two had normal findings at 6 months and 1 at 2 months, but the latter had abnormal results at 6 months. The remaining 5 cats (55.6%) with ≥2 pancreatic abnormalities at the time of diagnosis continued to have a single abnormal ultrasonographic finding during the follow‐up period (increased or decreased pancreas echogenicity).
Diabetic Remission and Suspected Pancreatitis
Diabetic remission occurred in 17 of the 30 cats (56.7%) during the study period. In 8 (47.1%) of these, remission had occurred by 2 months after the start of treatment, and in the remaining 9 (52.9%), it occurred between 2 and 6 months. Two cats in remission relapsed by 4 and 7 weeks after remission had been diagnosed, respectively, and the remaining cats remained in remission until the end of the study. There were no significant associations between diabetic remission and suspected pancreatitis based on increased serum markers and ≥2 ultrasonographic abnormalities at the time of diagnosis or during the follow‐up period. However, based on direct observation of raw data, cats that did not achieve diabetic remission appeared to have increased serum markers or ultrasonographic abnormalities compatible with suspected pancreatitis more often than cats that had diabetic remission (Tables 2 and 3).
Table 2.
Associations between diabetic remission and pancreatitis based on increased levels of pancreatic serum markers and ultrasonographic abnormalities of the pancreas at the time of diagnosis. Each parameter is considered dichotomous (pancreatitis is present or absent). Absolute numbers of cats and percentages are shown
| Variable | Remission (Number of Cats) | No Remission (Number of Cats) | |||
|---|---|---|---|---|---|
| Pancreatitis (%) | No Pancreatitis (%) | Pancreatitis (%) | No Pancreatitis (%) | P‐Value | |
| Spec fPL > 5.3 μg/L | 4 (24) | 13 (77) | 6 (46) | 7 (54) | .26 |
| DGGR‐lipase > 26 U/L | 8 (47) | 9 (53) | 7 (54) | 6 (46) | 1.00 |
| Spec fPL > 5.3 μg/L or DGGR‐lipase > 26 U/L | 8 (47) | 9 (53) | 7 (54) | 6 (46) | 1.00 |
| ≥2 ultrasonographic abnormalities | 3 (18) | 14 (82) | 6 (46) | 7 (54) | .12 |
| ≥2 ultrasonographic abnormalities or Spec fPL > 5.3 μg/L | 5 (29) | 12 (71) | 8 (62) | 5 (39) | .14 |
| ≥2 ultrasonographic abnormalities or DGGR‐lipase > 26 U/L | 9 (53) | 8 (47) | 9 (69) | 4 (31) | .47 |
| ≥2 ultrasonographic abnormalities or Spec fPL > 5.3 μg/L or DGGR‐lipase > 26 U/L | 9 (53) | 8 (47) | 9 (69) | 4 (31) | .47 |
Table 3.
Associations between diabetic remission and pancreatitis based on increased levels of pancreatic serum markers and ultrasonographic abnormalities of the pancreas during the follow‐up period. Cats with suspected pancreatitis at the time of diagnosis are excluded. Results from 2 and 6 months are pooled. Each parameter is considered dichotomous (pancreatitis is present or absent). Absolute numbers of cats and percentages are shown
| Variable | Remission (Number of Cats) | No Remission (Number of Cats) | |||
|---|---|---|---|---|---|
| Pancreatitis (%) | No Pancreatitis (%) | Pancreatitis (%) | No Pancreatitis (%) | P‐Value | |
| Spec fPL > 5.3 μg/L | 3 (23) | 10 (77) | 2 (29) | 5 (71) | 1.00 |
| DGGR‐lipase > 26 U/L | 3 (33) | 6 (67) | 4 (67) | 2 (33) | .32 |
| Spec fPL > 5.3 μg/L or DGGR‐lipase > 26 U/L | 3 (33) | 6 (67) | 4 (67) | 2 (33) | .32 |
| ≥2 ultrasonographic abnormalities | 5 (42) | 7 (58) | 3 (50) | 3 (50) | 1.00 |
| ≥2 ultrasonographic abnormalities or Spec fPL > 5.3 μg/L | 5 (46) | 6 (55) | 3 (60) | 2 (40) | 1.00 |
| ≥2 ultrasonographic abnormalities or DGGR‐lipase > 26 U/L | 4 (50) | 4 (50) | 3 (75) | 1 (25) | .58 |
| ≥2 ultrasonographic abnormalities or Spec fPL > 5.3 μg/L or DGGR‐lipase > 26 U/L | 4 (50) | 4 (50) | 3 (75) | 1 (25) | .58 |
When absolute levels of Spec fPL and DGGR‐lipase were compared in cats achieving or not diabetic remission the difference was only significant for Spec fPL at 2 months from admission (Fig 1A,B). Of note, the best cut‐off value of Spec fPL to identify remission of DM at 2 months was ≤2.7 μg/L (area under the ROC curve: 0.91; 95% confidence interval: 0.74–0.98); the sensitivity and specificity of Spec fPL ≤ 2.7 μg/L were 88.2 and 76.9%, respectively.
Figure 1.
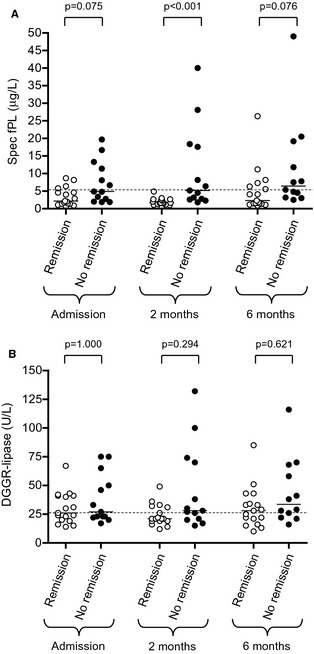
Scatter plots of Spec fPL serum concentration (A) and DGGR‐lipase activity (B) in diabetic cats with (white dots) and without remission (black dots) at the time of diagnosis and at 2 and 6 months later. Medians are shown. Dashed lines mark the upper cut‐off values (5.3 μg/L for Spec fPL, 26 U/L for DGGR‐lipase).
Increased Spec fPL concentration was accompanied by increased DGGR‐lipase activity in all but 1 of the 29 cats; 1 cat had increased Spec fPL concentration and normal DGGR‐lipase activity (Table S3). Conversely, 17 of the 45 cats with increased DGGR‐lipase activity had normal Spec fPL concentrations. These 17 cats had median DGGR‐lipase of 32 U/L (range 27–67 U/L); 16 of these cats underwent ultrasonographic examination of the pancreas, which revealed normal findings in 15 cats and findings compatible with pancreatitis in 1.
Discussion
This study demonstrated that at the time DM was diagnosed, up to 60% of cats had suspected subclinical pancreatitis based on increased serum markers of pancreatitis, the occurrence of at least 2 abnormal ultrasonographic pancreatic findings or combinations of these diagnostic criteria. Furthermore, 17–30% of diabetic cats were suspected to develop pancreatitis, mostly subclinical, during the 6‐month follow‐up period. The high rate of suspected pancreatitis in diabetic cats at the time of diagnosis and during the follow‐up period is in agreement with the observation that the frequency of pancreatitis is as high as 83% based on increased fPLI and 51% based on histological examination.3, 4 However, those retrospective studies included cats with long‐standing disease and did not report the prevalence of suspected pancreatitis at the time of initial examination. In this study, the prevalence of suspected pancreatitis at the time of diagnosis was 50% based on DGGR‐lipase activity, 33% based on Spec fPL concentration and 31% based on ultrasonography of the pancreas. When ultrasonography was combined with serum markers for assessment, the prevalence increased to 60%, suggesting that the former might lead to suspect pancreatitis in cats with normal serum enzymes. Indeed, 3 of the 9 cats with suspected pancreatitis based on ultrasonography did not have increased Spec fPL or DGGR‐lipase. Similar to our findings, only fair agreement between ultrasonographic abnormalities and the above serum markers for the diagnosis of pancreatitis was recently documented in cats.8 It is also important to note that the ultrasonographic abnormalities of the pancreas varied among cats with suspected inflammation. A variety of ultrasonographic abnormalities occurred singly or in combination including changes in echogenicity, the presence of free fluid or hyperechoic mesentery in the vicinity of the pancreas, and irregular margins. These features have been variably associated with elevated fPLI, Spec fPL, or DGGR‐lipase in recent studies.8, 14, 15
During the follow‐up period, an additional 17–23% of cats revealed increased pancreatic enzymes that led to suspect pancreatitis. On the basis of ultrasonographic examination, suspected pancreatitis was newly diagnosed during follow‐up in nearly 30% of cats. This observation indicates that cats with DM might develop pancreatitis at some point during the course of the disease. The relationship between DM and the development of pancreatitis is difficult to assess from the findings of this study because the number of diabetic cats was relatively small and there was no control group. Human beings with type‐2 DM had a 3‐fold increase in the risk of pancreatitis, although the cause has not yet been identified.16 Healthy cats that underwent experimental hyperglycemia for 10 days had neutrophilic infiltration of the exocrine pancreas, which suggests a direct effect of excess glucose on the pathogenesis of pancreatitis.17 However, reports on the risk of pancreatitis in cats with naturally occurring DM have been conflicting. We observed similar rates of histologically confirmed pancreatitis in diabetic cats and well matched control cats1 while others reported that diabetic cats had significantly higher fPLI than healthy controls.4
In approximately 80 and 87% of cats that had suspected pancreatitis at the time of diagnosis based on Spec fPL concentration and DGGR‐lipase activity, respectively, these variables remained increased during the follow‐up period, whereas abnormal pancreatic ultrasonographic findings persisted in only 44% of the cats. A possible explanation for this difference is a lower sensitivity of ultrasonography for diagnosis of chronic pancreatitis compared with pancreatic enzymes, but this conflicts with a study reporting that ultrasonography might be an equally suitable diagnostic method.18
Clinically relevant signs of pancreatitis were not apparent in any cat with suspected pancreatitis at the time of diagnosis or during the follow‐up period. Acute pancreatitis was suspected in 1 cat with anorexia and vomiting a few days after diagnosis of DM. Likewise, among the 20 diabetic cats not included in the study, only 1 had clinical signs suggestive of clinically relevant pancreatitis. Thus, in agreement with the results of previous studies,3, 4 we found that pancreatitis is clinically inapparent in the vast majority of diabetic cats.
It is not clear whether diabetic cats with pancreatitis have a decreased chance of diabetic remission. Good glycemic control tended to be more difficult to achieve in cats with pancreatitis.3 Another study showed a positive correlation between fPLI and serum fructosamine concentration,4 suggesting that pancreatitis might adversely affect β‐cells and thus reduces the likelihood of remission. However, a recent retrospective analysis in our clinic showed no association between increased serum DGGR‐lipase activity and the rate of diabetic remission.5
In the present prospective study, increased Spec fPL concentrations, DGGR‐lipase activities and ultrasonographic abnormalities indicative of pancreatitis tended to be less common in cats that achieved diabetic remission than in cats that did not, but the differences were not significant. Absolute concentrations of the pancreatic enzymes were also lower in cats that subsequently achieved diabetic remission, but the difference was significant only for Spec fPL concentration at 2 months from diagnosis. Spec fPL concentrations ≤2.7 μg/L yielded the best discriminating power to identify remission of DM if measured at 2 months. Of note, this value falls within the normal limits proposed by the laboratory.2 , 7 Therefore, the clinical relevance of the Spec fPL cut‐off calculated in this study might be questionable. Whether a lower Spec fPL cut‐off would be necessary to identify pancreatitis in diabetic cats deserves further studies. Taken together, evidence of a detrimental effect of pancreatitis on metabolic control in diabetic cats is currently weak. A recent meta‐analysis conducted in people showed that 15% of patients with acute pancreatitis developed DM within 12 months of the diagnosis, and another study revealed that 29% of people with chronic pancreatitis developed DM over a period of 8 years.19, 20 It is possible that a similar relationship exists between pancreatitis and DM in cats. One proposed cause of DM in people with pancreatitis is the spread of inflammation from the exocrine pancreas to the islets with subsequent β‐cell damage.19 It is not known whether a similar mechanism contributes to DM in cats.
Pancreatitis is difficult to diagnose in cats and ideally the measurement of pancreatic serum markers and ultrasonographic examination should be supplemented by histological assessment of the pancreas. Unfortunately, pancreatic biopsy was not feasible in this longitudinal study of client‐owned cats. Diagnostic strategies in the absence of a gold standard have been reviewed,21, 22 but their use in this study was hampered by the relatively small number of cases and the fact that Spec fPL and DGGR‐lipase are dependent variables. Indeed, hierarchical models require 200–500 cases or more to estimate test accuracy, and the tests to be compared should be based on different physiological phenomena to avoid violation of the conditional independence assumption.21, 22
We used 2 serum markers for the tentative diagnosis of pancreatitis although until recently lipases other than Spec fPL were considered of limited diagnostic value in cats. However, it was recently established that the measurements of DGGR‐lipase activity and Spec fPL concentration essentially agree for cut‐off of the latter set >5.3 μg/L,7 and highly correlate.7 Nonetheless, in this study, increased DGGR‐lipase activity was not accompanied by increased Spec fPL concentration in all cats. A gray zone of measurements that are not very consistent with the occurrence of pancreatitis has been suggested for Spec fPL,2 , 7 and a similar gray zone might also exist for DGGR‐lipase. Indeed, fewer disagreements between the 2 lipases would have occurred if a higher cut‐off for DGGR‐lipase had been used, as demonstrated by the fact that 50% of cases in which DGGR‐lipase activity was increased and Spec fPL concentration was normal had DGGR‐lipase between 27 and 32 U/L, which was slightly above the reference range. Finally, because a control group was not included in this study, it is not known whether suspected pancreatitis was a primary event, or occurred secondary to DM. We find the latter possibility unlikely considering that histological signs of inflammation and fibrosis of the exocrine pancreas were detected in 45% of 41 healthy cats, especially older cats.23 It is therefore possible that DM was responsible for exocrine pancreatic disease in only some of the cats.
In summary, this prospective study showed that pancreatitis, based on currently used serum markers and ultrasonographic examination of the pancreas, is common in cats at the time of diagnosis of DM, and that an additional portion of the diabetic cats might develop pancreatitis during the following 6 months. Pancreatitis was subclinical in almost all cases, and therefore its clinical relevance is questionable. Increased concentrations of Spec fPL or DGGR‐lipase as well as abnormal pancreatic findings on ultrasonography might be associated with a lower chance of diabetic remission; however, the study did not reveal a clear statistical evidence.
Supporting information
Data S1. Information concerning grouping of cats.
Table S1 of Data S1. Activity of serum markers at 2 months after diagnosis.
Table S2 of Data S1. Activity of serum markers at 6 months after diagnosis.
Table S3. Serum Spec fPL and DGGR‐lipase, and pancreatic ultrasonography in cats with and without remission, at admission and at 2 and 6 months.
Acknowledgments
We are grateful to Dr Barbara Contiero for statistical advice. The study was supported in part by a grant from the Policlinico di Monza, Italy.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
The study was carried out at the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty, University of Zurich, Switzerland.
Footnotes
Zini E, Lunardi F, Zanetti R, et al. Histological investigation of endocrine and exocrine pancreas in cats with DM. J Vet Intern Med 2012;6:1519–1520 (abstract)
Forman MA, Shiroma J, Armstrong PJ, et al. Evaluation of feline pancreas‐specific lipase (Spec fPL™) for the diagnosis of feline pancreatitis. J Vet Intern Med 2009;23:733–734 (abstract)
IDEXX GmbH, Ludwigsburg, Germany
Lipase colorimetric for Roche Cobas Integra 800; Roche Diagnostics, Rotkreuz, Switzerland
Lantus, Sanofi Aventis, Meyrin, Switzerland
DM Purina Veterinary Diets; Medical solution, Steinhausen, Switzerland
GraphPad Prism version 5.0; GraphPad Software, La Jolla, CA
References
- 1. Prahl A, Guptill L, Glickman NW, et al. Time trends and risk factors for diabetes mellitus in cats presented to veterinary teaching hospitals. J Feline Med Surg 2007;9:351–358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Baral RM, Rand JS, Catt MJ, Farrow HA. Prevalence of feline diabetes mellitus in a feline private practice. J Vet Intern Med 2003;17:433–434. [Google Scholar]
- 3. Goossens MM, Nelson RW, Feldman EC, Griffey SM. Response to insulin treatment and survival in 104 cats with diabetes mellitus (1985–1995). J Vet Intern Med 1998;12:1–6. [DOI] [PubMed] [Google Scholar]
- 4. Forcada Y, German AJ, Noble PJM, et al. Determination of serum fPLI concentrations in cats with diabetes mellitus. J Feline Med Surg 2008;10:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zini E, Hafner M, Osto M, et al. Predictors of clinical remission in cats with diabetes mellitus. J Vet Intern Med 2010;24:1314–1321. [DOI] [PubMed] [Google Scholar]
- 6. Xenoulis PG, Steiner JM. Canine and feline pancreatic lipase immunoreactivity. Vet Clin Pathol 2012;41:312–324. [DOI] [PubMed] [Google Scholar]
- 7. Oppliger S, Hartnack S, Riond B, et al. Agreement of the serum Spec fPL™ and 1,2‐o‐dilauryl‐rac‐glycero‐3‐glutaric acid‐(6′‐methylresorufin) ester lipase assay for the determination of serum lipase in cats with suspicion of pancreatitis. J Vet Intern Med 2013;27:1077–1082. [DOI] [PubMed] [Google Scholar]
- 8. Oppliger S, Hartnack S, Reusch CE, et al. Agreement of serum feline pancreas‐specific lipase and colorimetric lipase assays with pancreatic ultrasonographic findings in cats with suspicion of pancreatitis: 161 cases (2008–2012). J Am Vet Med Assoc 2014;244:1060–1065. [DOI] [PubMed] [Google Scholar]
- 9. Zimmermann E, Hittmair KM, Suchodolski JS, et al. Serum feline‐specific pancreatic lipase immunoreactivity concentrations and abdominal ultrasonographic findings in cats with trauma resulting from high‐rise syndrome. J Am Vet Med Assoc 2013;242:1238–1243. [DOI] [PubMed] [Google Scholar]
- 10. Larson MM, Panciera DL, Ward DL, et al. Age‐relatedchanges in the ultrasound appearance of the normal feline pancreas. Vet Radiol Ultrasound 2005;46:238–242. [DOI] [PubMed] [Google Scholar]
- 11. Reusch CE. Feline diabetes mellitus In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 7th ed St. Louis, MO: Saunders; 2010:1796–1816. [Google Scholar]
- 12. Sieber‐Ruckstuhl NS, Kley S, Tschuor F, et al. Remission of diabetes mellitus in cats with diabetic ketoacidosis. J Vet Intern Med 2008;22:1326–1332. [DOI] [PubMed] [Google Scholar]
- 13. Hafner M, Dietiker‐Moretti S, Kaufmann K, Mueller C, Lutz TA, Reusch CE, Zini E. Intensive intravenous infusion of insulin in diabetic cats. J Vet Intern Med 2014;28:1753–1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Armstrong PJ, Williams DA. Pancreatitis in cats. Top Companion Anim Med 2012;27:140–147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Williams JM, Panciera DL, Larson MM, Werre SR. Ultrasonographic findings of the pancreas in cats with elevated serum pancreatic lipase immunoreactivity. J Vet Intern Med 2013;27:913–918. [DOI] [PubMed] [Google Scholar]
- 16. Noel RA, Braun DK, Patterson RE, Bloomgren G. Increased risk of acute pancreatitis and biliary disease observed in patients with type 2 diabetes: A retrospective, cohort study. Diabetes Care 2009;32:834–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Zini E, Osto M, Moretti S, et al. Hyperglycaemia but not hyperlipidaemia decreases serum amylase and increases neutrophils in the exocrine pancreas of cats. Res Vet Sci 2010;89:20–26. [DOI] [PubMed] [Google Scholar]
- 18. Forman MA, Marks SL, De Cock HEV, et al. Evaluation of serum feline pancreatic lipase immunoreactivity and helical computed tomography versus conventional testing for the diagnosis of feline pancreatitis. J Vet Intern Med 2004;18:807–815. [DOI] [PubMed] [Google Scholar]
- 19. Das SL, Singh PP, Phillips AR, et al. Newly diagnosed diabetes mellitus after acute pancreatitis: A systematic review and meta‐analysis. Gut 2013;63:818–831. [DOI] [PubMed] [Google Scholar]
- 20. Ito T, Otsuki M, Itoi T, et al. Pancreatic diabetes in a follow‐up survey of chronic pancreatitis in Japan. J Gastroenterol 2007;42:291–297. [DOI] [PubMed] [Google Scholar]
- 21. Toft N, Jørgensen E, Højsgaard S. Diagnosing diagnostic tests: Evaluating the assumptions underlying the estimation of sensitivity and specificity in the absence of a gold standard. Prev Vet Med 2005;68:19–33. [DOI] [PubMed] [Google Scholar]
- 22. Hanson T, Johnson WO, Gardner IA. Hierarchical models for estimating herd prevalence and test accuracy in the absence of a gold standard. J Agric Biol Environ Stat 2003;8:223–239. [Google Scholar]
- 23. De Cock HEV, Forman MA, Farver TB, Marks SL. Prevalence and histopathologic characteristics of pancreatitis in cats. Vet Pathol 2007;44:39–49. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Information concerning grouping of cats.
Table S1 of Data S1. Activity of serum markers at 2 months after diagnosis.
Table S2 of Data S1. Activity of serum markers at 6 months after diagnosis.
Table S3. Serum Spec fPL and DGGR‐lipase, and pancreatic ultrasonography in cats with and without remission, at admission and at 2 and 6 months.


