Abstract
Background
Animals with chronic cough can have normal bronchoalveolar lavage fluid cytology when small airway disease is absent. Cytology of a tracheobronchial brushing can detect inflammation in larger airways; however, evaluation of this technique has been limited in veterinary medicine.
Objective
To compare airway brush cytology to bronchoalveolar lavage fluid analysis in dogs and cats with chronic cough.
Animals
Forty dogs and five cats undergoing bronchoscopic investigation of chronic cough.
Methods
Prospective study. Bronchoscopy and bronchoalveolar lavage were performed followed by tracheobronchial brushing of central airways. Results of cytologic assessment of BAL fluid and brush cytology were compared for the presence or absence of inflammation and concordance of inflammatory cell type.
Results
Brush cytology detected central airway inflammation in 34 of 40 (85%) dogs with inflammatory BAL fluid. However, the type of inflammation reported differed in 23 of 34 dogs. In five cats with inflammation in BAL fluid, brush cytology detected inflammation in four; the type of inflammation was discordant in all cats.
Conclusions and clinical relevance
Brush cytology has good agreement with BAL regarding the presence of inflammation, although the type of inflammation detected with the different sampling techniques commonly varies. Brush cytology can provide supplementary information to BAL, and additional studies will provide further information on the role of tracheobronchial brush cytology in the diagnosis and management of respiratory conditions.
Keywords: Bronchi, Cat, Dog, Endoscopy, Respiratory tract
Abbreviations
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- VMTH
Veterinary Medical Teaching Hospital
Bronchoscopy with airway sampling by bronchoalveolar lavage (BAL) is frequently employed for diagnosis of respiratory diseases in specialty practice. Common nonmalignant and noninfectious causes of chronic cough include airway collapse and inflammatory airway diseases, such as chronic bronchitis and eosinophilic bronchopneumopathy in the dog and asthma/chronic bronchitis in the cat. While airway collapse and bronchiectasis can be diagnosed visually during bronchoscopy, confirming a component of infectious versus inflammatory disease requires cytologic and microbiologic analyses of a fluid sample that has contacted the epithelial lining, a brush sample, fine needle aspirate, or biopsy specimen. In some animals, particularly cats, performing BAL can lead to complications,1 and variable BAL recovery could result in differential dilution of the epithelial lining fluid2, thus, impacting the diagnostic yield of the procedure. In clinical practice, some diseases can remain undiagnosed despite bronchoscopic assessment.
Bronchoscopic bronchoalveolar lavage provides a method for sampling a specific segment of lung that demonstrates radiographic or bronchoscopic evidence of disease. However, because types and presence of inflammation can differ between lung lobes in 37–42% of BALs,3, 4 pathology can be easily missed, particularly if only one lung segment is sampled. Bronchoalveolar lavage fluid (BALF) analysis does not contribute to the final diagnosis in 25% of canine patients presenting for signs of respiratory disease,3 indicating that additional tools are needed to confirm a diagnosis. Also, epithelial lining fluid collected via BAL is diluted to 2–3% of the retrieved volume,2 which could result in failure to obtain a representative sample. Further variation in retrieval amount because of technique or disease processes can make interpretation of these samples problematic.
Recent studies of dogs with cough have demonstrated normal BALF cytology in some dogs with large airway collapse.5, 6 This finding could reflect limitations of BAL in cases with disease affecting only large or central airways, and, collection of a sample within the area of interest could yield clinically important findings to guide therapy. The trachea and mainstem bronchial region represent a central site of exposure to all inhaled material, and addition of central airway sampling to BAL could enhance documentation of airway inflammation. Tracheobronchial brush cytology is a complementary airway sampling method that allows direct cytological evaluation of visible endobronchial lesions and has a sensitivity of up to 71% for the diagnosis of malignancy in human medicine.7 This is in contrast to BALF cytology, which is insensitive (37%) for the diagnosis of respiratory malignancy.7 In veterinary medicine, respiratory malignancies are rarely endobronchial, however, further evaluation of brushing samples in such cases would be of value.
In a previous study of 10 dogs with chronic cough attributed to neutrophilic or eosinophilic bronchitis, tracheobronchial brush cytology failed to identify neutrophilic inflammation in 1/5 dogs and detected neutrophils in 4/5 cases in which BAL fluid cytology was eosinophilic or mixed.8 Brush cytology failed to identify eosinophils in 1/4 dogs with eosinophilic BAL, indicating that variable cytologic results can be encountered using the two sampling methodologies8 For this study, we hypothesized that proximal or central airway cytology collected by tracheobronchial brushing would detect inflammatory airway pathology in dogs and cats with various causes of chronic cough, even in the absence of inflammatory BALF cytology. Specifically, in dogs with airway collapse, we anticipated identification of central (large airway) inflammation through tracheobronchial brush cytology but not peripheral (small airway) inflammation through BAL cytology. Detection of any inflammatory pathology in the airways is useful in guiding treatment of patients with chronic cough, as untreated inflammation can contribute to airway remodeling and additional clinical signs.9, 10 Confirming the need for specific therapy directed at airway inflammation would reduce progression of disease and improve clinical management.
Materials and Methods
All dogs and cats undergoing bronchoscopy for the evaluation of chronic cough (longer than 2 months duration) at the William R. Pritchard Veterinary Medical Teaching Hospital (VMTH) between March 2012 and March 2014 were eligible for inclusion in this prospective study. The study was approved by the Institutional Animal Care and Use Committee and owner consent was obtained. Age, breed, sex, neutering status, body condition score, clinical signs, physical examination, and thoracic radiographic findings were recorded before entry into the study. As this study focused on dogs and cats with nonmalignant causes of chronic cough that did not have an obvious radiographic cause such as a mass effect or probable pneumonia, dogs and cats with significant alveolar pattern on radiographs or a high clinical suspicion for pneumonia, neoplasia or foreign bodies were excluded from evaluation.
Animals having bronchoscopy performed were all treated similarly, with preoxygenation and induction of general anesthesia according to the protocol deemed most appropriate by the Anesthesia Service. For animals large enough to accept a size 7 or greater endotracheal tube, one of four flexible bronchoscopes1 was passed through a special T adapter and gas anesthesia was employed. In smaller animals, anesthesia was maintained using a propofol infusion at 0.1–0.4 mg/kg/min, oxygen was provided by jet ventilation at 180 breaths/min, and a smaller flexible bronchoscope2 was employed. Pulse oximetry, ECG, and blood pressure were monitored throughout the procedure in all animals.
Bronchoscopic findings of tracheal or bronchial hyperemia, mucus accumulation, bronchiectasis, nodular irregularities and airway stenosis were recorded for each animal.11 The grade and extent of any tracheal or airway collapse was recorded according to a previously defined scheme based on percentage reduction in luminal size.5 Bronchial collapse was identified as a greater than 50% loss in luminal diameter of an individual bronchus. All examinations were digitally recorded for future review. After the assessment of all airways, the bronchoscope was removed from the airways, the outer surface was wiped with sterile saline, and the biopsy channel was rinsed with sterile saline to limit contamination before re‐entering a specific airway segment for lavage.
Bronchoalveolar lavage was performed by lodging the bronchoscope in a distal bronchus selected by the endoscopist. An aliquot of warmed, sterile saline solution was instilled by syringe and followed by approximately 5 mL of air to clear fluid from the endoscope channel before immediate retrieval by manual suction and gentle manipulation of the endoscope. Total aliquot volumes of 5–20 mLs/site were used depending on animal size, and two sites were lavaged unless the animal was considered unstable. Sites of lavage, volume of aliquots, number of aliquots instilled, and total volume of sterile saline utilized were determined by the endoscopist based on bronchoscopic findings and stability of the patient. Volumes instilled and retrieved were recorded.
After BAL, a protected specimen brush on a catheter was passed through the biopsy channel of the endoscope. One of two different brushes3 (1.2 mm or 1.7 mm in diameter) was selected for use depending on compatibility with the endoscope. The brush was extruded from the guard and gently rotated and moved back and forth against airway mucosa to collect epithelial and inflammatory cells. The larger proximal airway mucosa (distal trachea, carina and mainstem bronchi) was brushed for at least 30 seconds in each patient.8 Visibly abnormal regions of mucosa were targeted to maximize yield, with attempts to avoid accumulations of mucus and pus. The site of brush cytology and visual abnormalities were digitally recorded for review. The brush was retracted into the guard and removed from the endoscope. Material collected from the extruded brush was immediately smeared onto glass slides.12 Direct smears of brush cytology samples were used in this study because a higher yield of cells was achieved with this method in a preliminary comparison of direct smears and saline suspended samples in three dogs (data not shown). Slides were stained with Wright Giemsa using an automated spray stainer4 and post‐4 minute manual Giemsa staining5. Two hundred cell differential cell counts were performed by certified laboratory technicians. Differential cell counts were verified and cytology assessed by a board‐certified veterinary clinical pathologist (WV) who was masked to results of BALF analysis.
BALF from individual sites was submitted to the VMTH hematology laboratory for total nucleated cell counts and cytologic assessment by the board‐certified clinical pathologist on clinical duty. Samples were processed within 30 minutes of submission. An automated cell counter6 was used to determine the total cell count per μL. A 250 μL sample was aliquoted for cytocentrifugation7. If the total nucleated cell count was >5000/μL, an additional 250 μL cytofuge preparation was made using a 1 : 10 dilution with saline. Slides were stained with the same method used to stain brush preparations and a 200 differential cell count was done by a certified laboratory technician. Normal cell counts were considered to be 400–600 cells/μL in dogs and 400 cells/μL in cats,13 BAL cytology was considered hypocellular if <300 cells/μL were obtained. Previously established normal values for differential cytology in dogs and cats were used for interpretation of results. Normal BALF cytology was defined as comprised primarily of macrophages, with the upper limit of other inflammatory cell populations based on previous mean values from healthy dogs and cats described as 7% lymphocytes, 5% neutrophils, 6% eosinophils, 1% mast cells and 1% epithelial cells in dogs and primarily macrophages, with 5% lymphocytes, 7% neutrophils, and 16% eosinophils in cats.13 Mean values were used as the upper limit of normal to enhance the ability of BALF cytology to identify inflammation.
BAL cytology was characterized as normal, neutrophilic (>8% neutrophils with eosinophils and lymphocytes within reference range, or >50% neutrophils), lymphocytic (>8% lymphocytes with neutrophils and eosinophils within reference range, or >50% lymphocytes), eosinophilic (>6% eosinophils in the dog or >16% eosinophils in the cat with neutrophils and lymphocytes within reference range, or >50% eosinophils) or as mixed inflammation (concurrent elevations in neutrophil, eosinophil and/or lymphocyte percentages) based on differential cytology. Histiocytic inflammation was defined on the basis of increased numbers of macrophages based on cell counts or a majority of macrophages that had increased volumes of foamy, vacuolated cytoplasm, indicating activation or reactivity. While it is widely accepted that BAL samples often have a subpopulation of macrophages with increased volumes of foamy, vacuolated cytoplasm, if the majority have this morphology, it is suggestive of a degree of stimulus beyond normal and at our institution, this is termed histiocytic inflammation. Animals with intracellular bacteria visualized on BALF cytology or those that had a pathogenic organism cultured from BALF had a final diagnosis of infectious bronchopulmonary disease and were not excluded from the study retrospectively.
Tracheobronchial cytology in normal dogs is comprised almost exclusively of epithelial cells (ciliated columnar, goblet cells, nonciliated cells).8 Occasional macrophages and rare other leukocytes can be expected. Brush cytology results were categorized as normal if there were no or very rare inflammatory cells present based on the differential cell count and assessment of the entire slide or slides by a board‐certified veterinary clinical pathologist (WV). When present, the type of inflammation was classified as neutrophilic, eosinophilic, lymphocytic or mixed.
Statistical analysis
Brush and BAL samples were compared for the presence or absence of inflammation and agreement was calculated using StatXact 138. Confidence intervals for agreement were calculated using Exact Clopper‐Pearson mid‐P corrected method. When inflammation was present on both BAL and brush cytology, the type of inflammation was assessed for concordance. Samples were deemed to have identical inflammation type if the interpretation of brush cytology was the same as at least one BAL sample from the same animal. Samples were considered different if there was no overlap in the type of inflammation detected between each method. Samples were considered partially different if one sampling method detected additional inflammatory cell types compared to the other sampling technique.
Results
Between March 2012 and March 2014, bronchoscopy with BAL and tracheobronchial brushing was performed in 40 dogs and five cats. Dogs ranged in age from 10 to 16 years of age (median 9 years) and cats ranged from 5 to 13 years (median 10 years). Duration of cough ranged from 2 to 120 months (median 13 months) in dogs and 3–48 months (median 12 months) in cats.
BALF analysis detected inflammation in all 40 dogs and was characterized by neutrophilic (n = 13), lymphocytic (n = 11), or mixed (n = 16) inflammation. Mixed inflammation was neutrophilic and lymphocytic in seven dogs, neutrophilic and eosinophilic in seven dogs and lymphocytic and eosinophilic in two dogs. Brush cytology demonstrated inflammation in 34 dogs, and agreement on the presence of inflammation in brush cytology and BALF was 85% (71–94% confidence interval). In addition to cytologic findings, bronchoscopic evidence of airway collapse was noted in 21/40 dogs, bronchiectasis in 13/40 dogs, and tracheal collapse in 2/40 dogs.
Inflammation in brush cytology samples was initially categorized based on differential cell counts. However, cytologic assessment of all areas of all available slides often resulted in a different interpretation because of slide heterogeneity. Inflammation was frequently detected focally despite a normal differential cell count (Fig 1). Therefore, the clinical pathologist's cytologic interpretation of inflammation type was reported rather than differential cytology. Lymphocytic inflammation was difficult to detect in tracheobronchial brush cytology because small lymphocytes were often intercalated within clusters of epithelial cells, making accurate recognition problematic.
Figure 1.
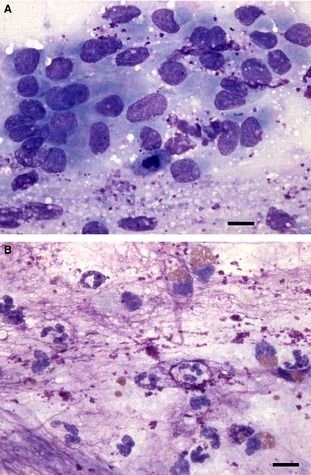
Tracheal brush cytology slide from a dog. In many areas, the slide consists of mildly hyperplastic epithelial cells with scattered mucus granules throughout the background (A). However, in other scattered, focal areas, there are clearly increased numbers of nondegenerate neutrophils and eosinophils (along with abundant background lytic mucus), indicative of neutrophilic and eosinophilic inflammation (B). 60× objective magnification, Wright Giemsa stain. Bar = 10 μm.
Inflammation type reported on brush cytology was identical to at least one BALF sample from the same dog in 11/34 (30%) dogs (Fig 2) while in 23 cases, some degree of discordance in the interpretation of brush cytology and BALF was observed. (Table 1) Of these 23 cases, two dogs had completely different inflammatory responses detected on brush cytology (neutrophilic) in comparison to BAL (lymphocytic). In 21 dogs, partially different inflammation was detected in the two sampling methods (Fig 3). Brush cytology identified one or more additional inflammatory cell types to that detected by BALF in 19 dogs, including combinations of histiocytic (9), neutrophilic (6), eosinophilic (6), lymphocytic (6), and mastocytic (3) inflammation. Fewer inflammatory cell types were found in brush cytology samples in comparison to BALF cytology in two dogs.
Figure 2.
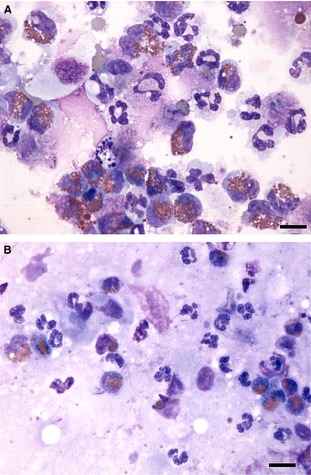
BALF cytospin cytology (A) and tracheal brush cytology (B) from a dog. There is complete concordance with increased numbers of neutrophils and eosinophils in both samples, indicative of neutrophilic and eosinophilic inflammation. Both samples also contain some background mucus and some lysed cells. 60× objective magnification, Wright Giemsa stain. Bar = 10 μm.
Table 1.
Concordance of inflammation presence and type between BAL fluid analysis and brush cytology in dogs
| Concordant inflammation (11) | Discordant inflammation (23) | |||
|---|---|---|---|---|
| Neutrophilic/Lymphocytic | 2 | Partially Different Inflammation Type (21) | Completely Different Inflammation Type (2) | |
| Neutrophilic | 5 | Brush had more inflammatory cell types compared to BAL | 19 |
Brush – Neutrophilic BAL – Lymphocytic |
| Neutrophilic/Eosinophilic | 4 | Brush had fewer inflammatory cell types than BAL | 2 | |
Figure 3.
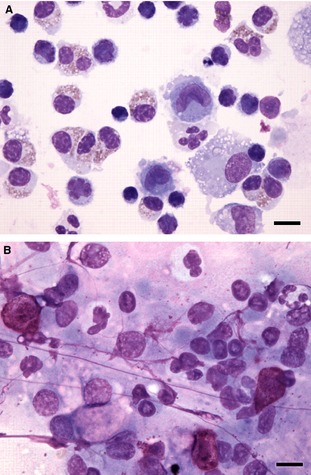
BALF cytospin cytology (A) and tracheal brush cytology (B) from a dog. There is discordance in the types of inflammation present. The BALF cytology has increased numbers of eosinophils and small mature lymphocytes, indicative of eosinophilic and lymphocytic inflammation. The tracheal brush cytology has increased numbers of mast cells (upper left, bottom right) and increased numbers of neutrophils (upper left, center and right), indicative of mastocytic and neutrophilic inflammation. There was also eosinophilic inflammation elsewhere in the brush cytology but lymphocytic inflammation was not detected. Note the brush cytology sample has some streaming nuclear material that is common in this sample type. Both samples also contain some background mucus and some lysed cells. 60× objective magnification, Wright Giemsa stain. Bar = 10 μm.
Five dogs were diagnosed with infectious bronchopulmonary disease based on the presence of BALF septic inflammation (3 of 5 dogs) or isolation of pathogenic bacteria on culture (5 of 5 dogs). Brush cytology showed evidence of septic inflammation in two of these five dogs, both of which had septic inflammation in BALF. Three dogs that did not have evidence of septic inflammation on brush cytology had mixed inflammation but bacteria were not seen.
Multiple BAL samples were collected in 36 of 40 dogs. In eight dogs, at least one BALF was described as hypocellular (<300 cells/μL) and in 2 of 8, both BAL samples were hypocellular. Cytologic assessment of these hypocellular samples revealed neutrophilic, lymphocytic and histiocytic inflammation. Brush cytology also detected inflammation in seven of the eight dogs with hypocellular BALF. Of these seven dogs, inflammation type was concordant in two, partially different in four and completely different in one.
In one dog, bronchoalveolar lavage cytology confirmed the presence of neoplasia (carcinoma), in association with mixed eosinophilic and neutrophilic inflammation. Brush cytology revealed epithelial hyperplasia and dysplasia in conjunction with neutrophilic, histiocytic and mastocytic inflammation but no obvious neoplastic cells.
Inflammation was detected in BALF of all cats, and brush cytology detected inflammation in 4 of 5 cats. Cytologic characterization of inflammation type on the brush sample was never identical to that found in BAL. In one cat, BAL from separate lung sites revealed lymphocytic or mastocytic inflammation, while brush cytology was non‐inflammatory. In three cats, brush cytology detected additional inflammatory cell types (histiocytic and lymphocytic inflammation) compared to neutrophilic and neutrophilic/lymphocytic in BALF analysis. In one cat, eosinophilic inflammation was detected in BAL while brush cytology revealed eosinophilic and lymphocytic inflammation.
Discussion
This study confirmed that brush cytology detects central airway inflammation in the majority of cases in which inflammatory BALF is found. Tracheobronchial brushing detected inflammation less frequently than BALF analysis, but the agreement on the presence of inflammation (85%, 71–94% confidence interval) was still high. Unfortunately, the ability of brush cytology to detect inflammation in cases with normal BALF cytology could not be addressed in this study because normal BAL samples were not found in any cases during the time frame of this study. Although we were unable to test our hypothesis that central airway inflammation would be present in some disease situations in the absence of lower airway inflammation, this study suggests a role for brush cytology in evaluation of animals with chronic cough.
In this study, 85% of brush cytology samples concurred with BALF detection of inflammation. Therefore, tracheobronchial brush cytology can be considered for the detection of inflammatory disease when logistics limit the performance of BAL, such as when neither an appropriate size of bronchoscope nor sterile tubing to pass through the bronchoscope is available. Tracheobronchial brushing could also provide adequate sampling when BAL is deemed inadvisable because of anesthetic instability, which can occur in cats undergoing bronchoscopic bronchoalveolar lavage,1 although, because of variability in cytology from multi‐segment BAL in the cat,4 further study will be needed to interpret brush cytologic findings. In addition, tracheobronchial brushing can be performed when a hypocellular sample is anticipated by the endoscopist because of low volume of return or minimal surfactant retrieval during BAL.
This study also found that the type of inflammation detected using brush cytology often differed from BALF, with additional inflammatory cell types found in brush cytology of 23/45 animals. This finding is in agreement with a previous study in which different cytological interpretation was noted in 5/10 sample pairs.8 Further evaluation of the types of inflammation present with different diseases could provide further insight into the pathogenesis of disease. In people with asthma, the presence of mast cells in tracheobronchial brushings and eosinophils in induced sputum correlates well with clinical severity and airway hyperresponsiveness, while bronchoalveolar lavage fluid cell counts have no relationship with clinical severity or airway hyperresponsiveness.14 Similar studies have not been performed in veterinary medicine, however collection of a brush cytology sample should be considered in any stable patient because of the likelihood of detecting supplementary information that could establish a diagnosis and guide therapy.
An unexpected finding in this study was that brush cytology required cytologic assessment of the entire slide or slides rather than only a differential cell count to obtain an appropriate interpretation of the presence and type of inflammation. Differential cell counts were sometimes misleading because of heterogeneity of cellularity across the slides. Another finding was that lymphocytic inflammation was difficult to detect in tracheobronchial brush cytology because of clustering with epithelial cells. Both of these issues might be overcome by suspending the cytology brushes in saline and performing a cytospin on the fluid, similar to preparation of a cytospin of BALF, provided that yield and cytomorphology could be optimized.8
No clinical complications were experienced in obtaining brush cytology samples in this study. Endobronchial brushings can cause epithelial injury resulting in granulation tissue that replaces normal anatomical structures under newly proliferated epithelium in sheep.15 However, those studies were performed in segmental bronchi and it is unknown whether airway structure and healing is equivalent between the central and lower airways, or whether these changes are permanent or clinically significant.15 Tracheal brushing is also used in rabbits as a model for tracheal stenosis.16 However, in that model, a 5 mm diameter brush was used in 5 kg rabbits to brush circumferentially around a single area in the trachea, mimicking traumatic tracheal irritation caused by transoral endotracheal tubing.16 Our study used a 2 mm diameter brush in dogs and cats under direct visualization and did not brush circumferentially in one region, making stenosis less likely. Also, bronchial brushings are performed in children using a blind, nonbronchoscopic approach without known adverse effects.17
Study limitations could impact results obtained here. All brush cytology samples were taken after BAL and there is potential for contamination of the larger airways with fluid from the smaller airways. However, given that brush cytology tended to detect more inflammatory cell types than BALF and that BAL was performed in a small area of lung, this was assessed to be unlikely. Another consideration is that brush cytology and BAL sample different regions in the airways. For disease that is localized to only the larger or smaller airways, one sampling method could be anticipated to provide more relevant information than the other. Thus, lack of inflammation on brush samples could reflect a lack of central airway pathology. Another limitation is that multiple clinicians collected samples for this study. Although all were trained in proper collection technique, variations in brush contact with the epithelial surface could alter cellular collection. An additional consideration was the use of single aliquot BAL in this study. It has been shown that sequential aliquots of BAL fluid aspirated from the same bronchial segment vary in cellular composition,18, 19 and use of a single aliquot for BAL could have impacted results. Cellularity of brush samples in this study clearly varied, although it is unknown if this resulted from variations in sampling technique or from differing pathologic conditions. Finally, evaluations of cytologic specimens from multi‐segment BAL have revealed marked differences between lung lobes in animals with apparently diffuse disease.3, 4 Perhaps it should be anticipated that central airway inflammation would differ from that found in one or more lung segments, however clinical correlation between cytologic findings and disease states is required.
Because of lack of inflammation on brush cytology in 6 of 45 cases, we recommend obtaining tracheobronchial brush cytology as an adjunct to BAL rather than as an alternative. BAL detected airway inflammation more reliably and appeared to be more successful in identifying bacterial infection and neoplasia, although case numbers were limited. Knowledge of the inflammatory types present throughout the airways will provide additional clinical information, although further research is required to elucidate the role of central airway cytology in various respiratory diseases. In addition, because of our failure to identify the target population of dogs with large airway collapse lacking evidence of inflammation in BALF, further study is needed to define the clinical utility of this airway sampling method in such cases.
Acknowledgments
The authors thank Dr. Philip H. Kass for statistical assistance.
Conflict of Interest Declaration: Johnson, Feline Advisory Board, speaker honoraria for various meetings.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This study was performed at the UC Davis VMTH.
This project was supported by grant 2011‐82R from the Center for Companion Animal Health and the Bailey Wrigley Fund, at the University of California, Davis School of Veterinary Medicine.
Footnotes
Olympus P180 5.0 mm × 55 cm videoendoscope and Olympus GIF N180 5.9 mm × 110 cm, Melville New York, Pentax FG16X or V 5.5 mm × 85 cm fiberoptic endoscope, Montvale, New Jersey.
Olympus BF3C160 3.8 mm × 55 cm video endoscope, Melville, New York.
Olympus Disposable Cytology Brush BC‐203D‐2006 (1.2 mm channel size, 2 mm brush diameter × 6 mm brush length), Hobbs Medical Bronchial Microbiology Brush REF 4320 (1.7 mm channel size, 2 mm brush diameter × 10 mm brush length).
Model 7151 Wescor Aerospray Hematology Pro, ELITech Biomedical Systems, Logan, Utah, USA.
Harleco Giemsa stain, EMD chemicals, Gibbstown, NJ, USA.
Advia 120, Siemens, Deerfield, IL, USA.
Cytospin3, ThermoShandon, Pittsburgh, PA.
Cytel Software Corporation, Cambridge, MA.
References
- 1. Johnson LR, Drazenovich TL. Flexible bronchoscopy and bronchoalveolar lavage in 68 cats (2001–2006). J Vet Int MEd 2007;21:219–225. [DOI] [PubMed] [Google Scholar]
- 2. Melamies MA, Jarvinen AK, Seppala KM, et al. Comparison of results for weight‐adjusted and fixed‐amount bronchoalveolar lavage techniques in healthy Beagles. Am J Vet Res 2011;72:694–698. [DOI] [PubMed] [Google Scholar]
- 3. Hawkins EC, DeNicola DB, Plier ML. Cytological analysis of bronchoalveolar lavage fluid in the diagnosis of spontaneous respiratory tract disease in dogs: a retrospective study. J Vet Int Med 1995;9:386–392. [DOI] [PubMed] [Google Scholar]
- 4. Ybarra WL, Johnson LR, Drazenovich TL, et al. Interpretation of multisegment bronchoalveolar lavage in cats (1/2001–1/2011). J Vet Int Med 2012;26:1281–1287. [DOI] [PubMed] [Google Scholar]
- 5. Johnson LR, Pollard RE. Tracheal collapse and bronchomalacia in dogs: 58 cases (7/2001–1/2008). J Vet Int Med 2010;24:298–305. [DOI] [PubMed] [Google Scholar]
- 6. Bottero E, Bellino C, De Lorenzi D, et al. Clinical evaluation and endoscopic classification of bronchomalacia in dogs. J Vet Intern Med 2013;27:840–846. [DOI] [PubMed] [Google Scholar]
- 7. Garg S, Handa U, Mohan H, et al. Comparative analysis of various cytohistological techniques in diagnosis of lung diseases. Diagn Cytopathol 2007;35:26–31. [DOI] [PubMed] [Google Scholar]
- 8. Hawkins EC, Rogala AR, Large EE, et al. Cellular composition of bronchial brushings obtained from healthy dogs and dogs with chronic cough and cytologic composition of bronchoalveolar lavage fluid obtained from dogs with chronic cough. Am J Vet Res 2006;67:160–167. [DOI] [PubMed] [Google Scholar]
- 9. Flood‐Page P, Menzies‐Gow A, Phipps S, et al. Anti‐IL‐5 treatment reduces deposition of ECM proteins in the bronchial subepithelial basement membrane of mild atopic asthmatics. J Clin Invest 2003;112:1029–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Humbles AA, Lloyd CM, McMillan SJ, et al. A critical role for eosinophils in allergic airways remodeling. Science 2004;305:1776–1779. [DOI] [PubMed] [Google Scholar]
- 11. Johnson LR, Vernau W. Bronchoscopic findings in 48 cats with spontaneous lower respiratory tract disease (2002–2009). J Vet Int Med 2011;25:236–243. [DOI] [PubMed] [Google Scholar]
- 12. Kinnear WJ, Wilkinson MJ, James PD, et al. Comparison of the diagnostic yields of disposable and reusable cytology brushes in fibreoptic bronchoscopy. Thorax 1991;46:667–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hawkins EC, DeNicola DB, Kuehn NF. Bronchoalveolar lavage in the evaluation of pulmonary disease in the dog and cat State of the art. J Vet Int Med 1990;4:267–274. [DOI] [PubMed] [Google Scholar]
- 14. Gibson PG, Saltos N, Borgas T. Airway mast cells and eosinophils correlate with clinical severity and airway hyperresponsiveness in corticosteroid‐treated asthma. J Aller Clin Immunol 2000;105:752–759. [DOI] [PubMed] [Google Scholar]
- 15. Yahaya B, Baker A, Tennant P, et al. Analysis of airway epithelial regeneration and repair following endobronchial brush biopsy in sheep. Exp Lung Res 2011;37:519–535. [DOI] [PubMed] [Google Scholar]
- 16. Steehler MK, Hesham HN, Wycherly BJ, et al. Induction of tracheal stenosis in a rabbit model‐endoscopic versus open technique. Laryngoscope 2011;121:509–514. [DOI] [PubMed] [Google Scholar]
- 17. Doherty GM, Christie SN, Skibinski G, et al. Non‐bronchoscopic sampling and culture of bronchial epithelial cells in children. Clin Exp Allegry 2003;33:1221–1225. [DOI] [PubMed] [Google Scholar]
- 18. Rennard SI, Ghafouri M, Thompson AB, et al. Fractional processing of sequential bronchoalveolar lavage to separate bronchial and alveolar samples. Am Rev Respir Dis 1990;141(1):208–217. [DOI] [PubMed] [Google Scholar]
- 19. Pinsker KL, Norin AJ, Kamholz SL, et al. Cell content in repetitive canine bronchoalveolar lavage. Acta Cytol; 1980: 558–563. [PubMed] [Google Scholar]


