Abstract
Background
Short‐term intravenous co‐administration of famotidine and pantoprazole is used by some veterinarians to treat gastrointestinal bleeding in critically ill dogs. However, clinical studies have not evaluated the efficacy of combination acid suppressant treatment in dogs.
Hypothesis/Objectives
To compare the effect of intravenous co‐administration of famotidine and pantoprazole to monotherapy with pantoprazole on intragastric pH in dogs. We hypothesized that single agent pantoprazole would be more effective than combination with famotidine.
Animals
Twelve healthy adult colony dogs.
Methods
Randomized, 2‐way crossover design. All dogs received placebo (0.9% saline) for 24 hours followed by 1.0 mg/kg IV q12h pantoprazole or combination treatment with famotidine and pantoprazole for 3 consecutive days. Intragastric pH monitoring was used to continuously record intragastric pH for 96 hours beginning on day 0 of treatment. Mean percentage time (MPT) that intragastric pH was ≥3 and ≥4 were compared between groups using ANOVA with a posthoc Tukey‐Kramer test (α = 0.017).
Results
The MPT ± standard deviation intragastric pH was greater than ≥3 and 4 were 79 ± 17% and 68 ± 17% for pantoprazole and 74 ± 19% and 64 ± 23% for combination treatment, respectively. There were no significant differences in MPT intragastric pH was ≥3 and 4 between groups. Pantoprazole administered alone achieved pH goals established for humans with acid‐related disorders.
Conclusions and Clinical Importance
These results suggest that short‐term combination treatment with famotidine and pantoprazole is not superior to pantoprazole alone for increasing intragastric pH in dogs.
Keywords: Bravo monitoring, Canine, Famotidine, Pantoprazole, pH
Abbreviations
- H2RA
histamine 2 receptor antagonists
- MPT
mean percentage time
- PPIs
proton pump inhibitors
Gastrointestinal (GI) bleeding is a common complication in critically ill dogs with nonsteroidal anti‐inflammatory drug toxicosis, immune‐mediated thrombocytopenia, liver failure, and GI neoplasia among other diseases and might increase morbidity and case fatility.1, 2, 3 Despite a lack of studies to evaluate the correlation of increased intragastric pH to improved clinical outcome in dogs, treatment of GI bleeding with acid suppressants is considered a central treatment for many critically ill dogs. In human and small animal medicine, proton pump inhibitors (PPIs, eg, pantoprazole) have proven to be more effective at raising intragastric pH than histamine‐2 receptor antagonists (H2RAs, eg, famotidine) and are commonly used for prevention and treatment of acid‐related disorders.4, 5, 6
Pantoprazole is a substituted benzimidazole PPI that is available as an injectable IV drug (Protonix®). PPIs exist as weak bases and are therefore unprotonated in the physiologic pH of the blood. After cellular diffusion, PPIs become protonated and trapped in their active form in the acidic environment of parietal cells. After activation, PPIs form disulfide bonds with available active parietal cell H+˗K+‐adenosine triphosphatase (H+˗K+‐ATPase) enzymes.7 Recruitment and activation of previously inactive parietal cells follows initial PPI administration. Thus, PPIs need time to accumulate within newly recruited parietal cells and reach steady state.8 Inhibition of acid secretion after PPI administration is approximately 30% of maximal at 24 hours because not all H+˗K+‐ATPase enzymes are bound by the drug. Maximal inhibitory effect is thought to plateau at approximately 4 days.7, 9, 10, 11 In contrast, peak plasma concentrations of H2RAs occur within hours after a single dose.12 Many veterinarians administer the rapidly acting famotidine in the first few days of treatment in combination with the slower acting, but more effective pantoprazole to critically ill dogs with or at high risk for GI bleeding.13 Pharmacologists argue, however, that simultaneous treatment with an H2RA might decrease the efficacy of PPIs because of the requirement for PPI activation and trapping in the acidic canilicular environment of the parietal cell. This concept has been contested by intragastric pH studies in human subjects in which the combination of a PPI and H2RA in the first few days of treatment resulted in a more rapid time to raise intragastric pH > 4 and improved clinical signs compared to a PPI alone.14, 15
Despite their frequent use in combination, no studies have been undertaken in dogs to determine if there is a benefit to short‐term simultaneous treatment with PPIs and H2RAs. Accordingly, the objective of this study was to compare the immediate, short‐term effect of IV pantoprazole alone to combination treatment with IV pantoprazole and famotidine on canine intragastric pH.
Materials and Methods
Study Animals
The Institutional Animal Care and Use Committee (IACUC) at the University of Tennessee approved the protocol for this study (Approval #2249‐0314). The subjects of this study were 12 healthy adult dogs (5 beagles, 7 mixed breed hound dogs) from a research colony at the University of Tennessee (7 neutered females, 5 neutered males), aged 2.5–13.0 years (median, 8 years) and weighing 9.7–23.6 kg (median, 14.9 kg). Dogs included in the study were deemed healthy on the basis of normal physical examination, normal CBC and biochemistry profile performed within 6 months of study entry. In addition, dogs had no evidence of GI disease (eg, vomiting, diarrhea, anorexia) and had a normal PCV, as well as normal total serum protein, blood urea nitrogen and blood glucose concentrations and urinalysis results at study entry. To ensure inclusion of healthy dogs and to comply with IACUC guidelines, dogs were excluded from the study if they developed inappetence for >24 hours, lost >10% of their body weight, had gross evidence of disease on gastroesophagoscopy during the study period or some combination of these. Dogs were walked 4 times per day during the study period.
Study Design
A randomized, open label, 2‐way crossover study was performed. All dogs were randomized to receive famotidine1 and pantoprazole2 in combination or pantoprazole alone. All dogs received 2 mL 0.9% saline q6 hours for the first 24 hours of intragastric pH monitoring (before administration of either treatment) to obtain baseline pH data. All treatments were dosed at 1.0 mg/kg IV q12h each for 3 consecutive days followed by a minimum 10‐day washout period. Each drug was administered as a slow IV bolus over a period of no less than 2 minutes. Dogs were randomized to a treatment group using a random number generator. Pantoprazole was resuspended to a concentration of 4 mg/mL immediately before administration according to manufacturer directions. Unused resuspended pantoprazole was discarded after 24 hours. Both famotidine and pantoprazole were stored at a controlled cold temperature (7°C) protected from light. The dogs' peripheral IV catheters were flushed with 2 mL of 0.9% saline every 6 hours throughout the study to maintain catheter patency. The dogs were medicated at 7:30 am and 5:30 pm daily and were fed a maintenance diet3 30 minutes after medicating at 8:00 am and 6:00 pm daily. Dogs had unlimited access to water during the pH monitoring period. Clinical signs, including changes in attitude, vomiting, number of defecations and fecal consistency were recorded twice daily. Feces were graded from 1 to 7 by a standardized fecal scoring system4 . For the purposes of this study, diarrhea was defined as a fecal score of ≥4.
Placement of Intragastric pH Monitor
On the morning of day 0 of each treatment period, the morning meal was withheld and dogs were anesthetized for gastroscopy‐assisted placement of a Bravo® pH capsule. Dogs were premedicated with dexmedetomidine5 (0.005 mg/kg) and butorphanol6 (0.4 mg/kg) IM. An IV catheter was placed and general anesthesia was induced with propofol7 to effect. Dogs were maintained with sevoflurane8 in 100% oxygen after endotracheal tube placement. Gastroscopy was performed with dogs in left lateral recumbency to aid in position and attachment of the Bravo® pH capsule to the fundic mucosa. At initial endoscopy for each dog, the entire stomach and esophagus were evaluated for evidence of gross disease. Bravo® pH capsules were calibrated and placed as previously described according to the manufacturer's instructions.4 Dogs were reversed with atipamezole9 (0.05 mg/kg IM) after the procedure.
pH Recordings
Intragastric pH recordings were obtained telemetrically at 6‐second sampling intervals for 96 hours (24 hours of baseline data followed by 3 treatment days) after capsule placement. Data receivers were kept on a hook within each respective dog's run during the data acquisition phase. The receivers remained on the caretaker during times when dogs were walked. Data was uploaded every 24 hours for the 96 hour monitoring period to the computer using manufacturer software (Polygram Net10). After data upload, the receiver was reset and was used to acquire the subsequent 24 hours of data. The percentage of time that intragastric pH was ≥3 and ≥4 and in 1 of each of 8 categories (pH 0–1, 1–2, 2–3, 3–4, 4–5, 5–6, 6–7, 7–8) was calculated by the manufacturer software.
Statistical Analysis
Intragastric pH measurements, fecal scores, and percentage food consumption were compared by treatment. For pH, the effect of day of treatment was also assessed. The pH data were analyzed with a cross‐over design analysis of variance (ANOVA). The Shapiro‐Wilk test was used to test the ANOVA assumption of normally distributed errors, and the Levene's F test was used to test the assumption of homogeniety of variance. When necessary, data were logarithmically (normal) transformed. Both assumptions were met with transformed data. Continuous variables (percentage food consumption, mean fecal score and change in fecal scores) were compared using an AB‐BA cross‐over design. Descriptive data (number of vomiting episodes and number of episodes with a fecal score ≥4) were reported as frequency of occurrences. To minimize the probability of type I error, a Bonferroni adjusted alpha of 0.01 was used to evaluate test results. When a significant treatment effect was observed, a posthoc Tukey‐Kramer test was performed (to determine which groups were significantly different from each other) (protected α = 0.05). No significant period or sequence effects were found for any dependent variable. Commercially available statistical software was used to perform all data analysis and to produce all descriptive statistics.11
Results
Bravo pH Monitoring System Results
All 24 Bravo® pH capsules were successfully attached to the fundic mucosa without complications. The total procedure times for gastroscopy and capsule placement ranged from 5 to 15 minutes with most procedures taking less than 10 minutes. On 5 of 24 occasions, the Bravo® pH capsules detached and exited the stomach before the end of the pH monitoring period. This occurred in 1 dog while receiving pantoprazole and combination treatment, each on day 2 of treatment, and 2 dogs receiving pantoprazole and 1 dog receiving combination treatment on day 3 of treatment. Therefore, data from these dogs (n = 4) were not included in the ANOVA treatment comparisons. An ANOVA performed using available pH data from all dogs on days 2 and 3 of treatment (excluding data from 1 dog from each treatment group) yielded the same results as the aforementioned analyses. On 2 occasions, the previously placed Bravo® pH capsule remained in place at the time of next capsule placement and a new capsule was placed in the area immediately adjacent to the previous capsule.
Intragastric pH Recordings
Mean percentage time (MPT) intragastric pH was ≥3 and ≥4 is considered the ideal baseline for encouraging healing of GI ulceration and gastroesophageal reflux lesions in humans, respectively16 and was therefore used for comparative analyses of treatments. The MPT ± standard deviation intragastric pH was ≥3 and ≥4 were 79 ± 17% and 68 ± 17% for pantoprazole and 74 ± 19% and 64 ± 23% for combination treatment, respectively. The MPT intragastric pH was ≥3 and 4 over the 3‐day treatment period was higher for pantoprazole compared to combination treatment; however, this difference was not significant (P = .26 and P = .48, respectively; Fig 1). A similar trend of pantoprazole being higher than combination was noted for MPT intragastric ≥3 and ≥4 when data from individual treatment days 2 and 3 were evaluated. However, none of the differences were significant (P = .76 and P = .99, respectively; Fig 2). No differences were observed in the distribution of intragastric pH over pH categories 1–8 when comparing pantoprazole to combination treatment (P = .64). There were no significant differences in MPT intragastric pH ≥3 and ≥4 for the 24 hours baseline (placebo) day before pantoprazole compared to the baseline day before combination treatment (P = .27 and P = .31, respectively; Fig 1). The MPT intragastric pH was ≥3 and ≥4 was 21 ± 21% and 13 ± 15% for the 24 hours baseline period before pantoprazole administration and 29 ± 25% and 22 ± 26% for the 24 hours baseline period before combination. The baseline day was significantly different from all subsequent days regardless of treatment type (P < .001).
Figure 1.
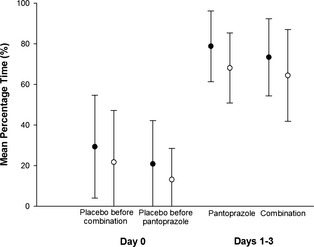
Comparison of the effect on intragastric pH of placebo before each treatment (day 0) and after 3 days of treatment (day 1–3) with IV administered pantoprazole alone (n = 9 dogs) or in combination with IV administered famotidine (n = 10 dogs). Circles represent the mean (± SD) percentage of time that intragastric pH was ≥3 (black circles) and ≥4 (open circles).
Figure 2.
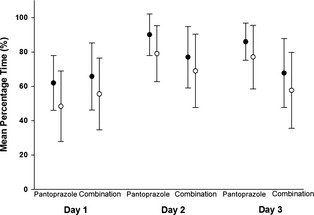
Comparison of the daily effect of IV administered pantoprazole alone (n = 9 dogs) and in combination with IV administered famotidine (n = 10 dogs) on individual treatment days 1, 2, and 3. Circles represent the mean ± SD percentage of time that intragastric pH was ≥3 (black bars) and 4 (open circles).
The MPT intragastric pH was ≥3 and ≥4 also was used to determine if there was an effect of order of treatment or day of treatment on intragastric pH between or within certain groups. For all dogs, the order of treatment did not significantly affect the percentage time intragastric pH was ≥3 and ≥4 (P = .09 and 0.1, respectively). No significant differences were identified for the MPT intragastric pH was ≥3 and ≥4 when comparing treatment days 1 through 3 between or within groups. The MPT intragastric pH was ≥3 and ≥4 was lower on day 1 compared to day 2 and 3 for pantoprazole (Fig 2); however, this effect was not significant (P = .07 and .05, respectively).
Both treatment groups resulted in a greater increase in the MPT that intragastric pH was ≥3 and ≥4 in nonbeagle dogs compared to beagle dogs on all days, however, this effect was not significant (P = .36 and P = .07, respectively). The MPT intragastric pH was ≥3 and ≥4 on day 2 (the day in which data were available from all dogs) was 69 ± 15% and 58 ± 19% for nonbeagle dogs and 53 ± 16% and 35 ± 19%, respectively for beagle dogs receiving pantoprazole treatment. The MPT intragastric pH was ≥3 and ≥4 on day 2 was 73 ± 22% and 64 ± 23% for nonbeagle dogs and 55 ± 14% and 44 ± 15%, respectively for beagle dogs receiving combination therapy.
Adverse Effects of Treatment
Vomiting was noted in 5 dogs with a total occurrence of 8 episodes of emesis. There were 5 episodes of vomiting in the pantoprazole group, 2 episodes in the combination group, and 1 episode in the placebo before pantoprazole group. There was no significant association with treatment received and appetite, mean fecal score or change in fecal consistency. The mean ± standard deviation fecal score was 3.0 ± 0.9 for the pantoprazole group, 2.6 ± 0.6 for the combination group, 3.3 ± 1.2 for placebo before combination, and 3.2 ± 1.3 for placebo before pantoprazole. There were 5 episodes of fecal scores ≥4 in the pantoprazole group, 3 episodes in the combination group, 3 episodes in the placebo before combination group and 3 episodes in the placebo before pantoprazole group.
Discussion
In the present study, we evaluated IV administered pantoprazole alone and in combination with famotidine in order to determine if combination treatment with pantoprazole and famotidine is more effective at raising canine intragastric pH compared to monotherapy with pantoprazole during the first few days of treatment. No significant differences were identified between treatments when comparing the MPT intragastric pH ≥3 and ≥4 over the 72 hours treatment period or on individual treatment days. Moreover, over the 72 hours monitoring period, the pantoprazole monotherapy group, but not the combination group, met clinical goals established for the treatment of gastroduodenal ulceration (intragastric pH of ≥3.0 for approximately 75% of the day) and gastroesophageal reflux disease (intragastric pH of ≥4.0 for approximately 67% of the day) in humans.16 Future studies using a larger sample size of dogs with GI bleeding are needed to address the clinical importance of this finding.
Pantoprazole resulted in a higher MPT intragastric pH was ≥3 and ≥4 on treatment days 2 and 3 compared to treatment day 1. This is expected based on the pharmacokinetics of the drug which require binding and inactivation of newly recruited H+˗K+‐ATPase enzymes.8 Despite the delay to peak effect, there was no significant difference in the efficacy of pantoprazole administered alone compared to combination treatment on day 1. Thus, there appears to be no benefit in the addition of IV administered famotidine to pantoprazole treatment even in the early stages of acid suppression in dogs.
Proton pump inhibitors have proven to be superior to H2RAs for raising canine intragastric pH and for prevention of exercise‐induced gastritis in dogs and are considered to be the standard of care for the treatment of GI bleeding in dogs at our hospital.4, 5, 6 For this reason, we did not include a treatment group that received famotidine alone. Additionally, we chose to evaluate twice daily administration of pantoprazole as a previous study performed in dogs demonstrated that once daily administration failed to meet clinical goals established for the treatment of people with gastroduodenal ulceration. In the same study, twice daily omeprazole administration significantly improved the MPT intragastric pH ≥3 and 4 compared to once daily therapy.5 In our study, twice daily pantoprazole achieved goals established for the treatment of acid‐related disorders in people.16
This study evaluated a small group of dogs with no history, physical exam or laboratory findings of GI disease. The crossover design of the study allowed for each dog to serve as its own control. The response to acid suppressant treatment in healthy dogs might not fully predict the response of dogs with GI bleeding to acid suppressant drugs; however, this study serves as a platform for future studies to address the utility of combination treatment in dogs with GI bleeding.
In conclusion, these results suggest that the combination of famotidine with pantoprazole administration is not more effective than pantoprazole administered alone for raising intragastric pH.
Acknowledgments
The authors thank Randy Buddington, Gina Galyon, Shanna Hillsman, and Frank Brown for their technical support.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Work for this manuscript was performed at the University of Tennessee, College of Veterinary Medicine in Knoxville, Tennessee.
This manuscript was presented in part in abstract form at the International Veterinary Emergency and Critical Care Symposium in Indianapolis in September of 2014.
Footnotes
10 mg/mL injection, APP Pharmaceuticals, LLC, Schaumburg, IL
Protonix IV (pantoprazole sodium for injection), NovaPlus, Konstanz, Germany
Hill's Science Diet Oral Care, Hill's Pet Nutrition, Inc, Topeka, KS
Fecal Scoring System, Nestlè Purina PetCare Company, St Louis, MO
Dexdomitor 0.5 mg/mL injection, Orion Pharma, Espoo, Finland
Torbugesic 10 mg/mL injection, Fort Dodge Animal Health, Fort Dodge, IA
PropoFlo 10 mg/mL injection, Abbott Laboratories, North Chicago, IL
SevoFlo, Abbott Laboratories, North Chicago, IL
Antisedan 5 mg/mL injection, Orion Pharma, Espoo, Finland
Polygram Net Software, Given Imaging, Yoqneam, Israel
SAS 9.2, SAS Institute Inc, Cary, NC
References
- 1. Luna SP, Basilio AC, Steagall PV, et al. Evaluation of adverse effects of long‐term oral administration of carprofen, etodolac, flunixin meglumine, ketoprofen, and meloxicam in dogs. Am J Vet Res 2007;68:258–264. [DOI] [PubMed] [Google Scholar]
- 2. Dunayer EK, Gwaltney‐Brant SM. Acute hepatic failure and coagulopathy associated with xylitol ingestion in eight dogs. J Am Vet Med Assoc 2006;229:1113–1117. [DOI] [PubMed] [Google Scholar]
- 3. O'Marra SK, Delaforcade AM, Shaw SP. Treatment and predictors of outcome in dogs with immune‐mediated thrombocytopenia. J Am Vet Med Assoc 2011;238:346–352. [DOI] [PubMed] [Google Scholar]
- 4. Tolbert K, Bissett S, King A, et al. Efficacy of oral famotidine and 2 omeprazole formulations for the control of intragastric pH in dogs. J Vet Intern Med 2011;25:47–54. [DOI] [PubMed] [Google Scholar]
- 5. Bersenas AM, Mathews KA, Allen DG, et al. Effects of ranitidine, famotidine, pantoprazole, and omeprazole on intragastric pH in dogs. Am J Vet Res 2005;66:425–431. [DOI] [PubMed] [Google Scholar]
- 6. Williamson KK, Willard MD, Payton ME, et al. Efficacy of omeprazole versus high‐dose famotidine for prevention of exercise‐induced gastritis in racing Alaskan sled dogs. J Vet Intern Med 2010;24:285–288. [DOI] [PubMed] [Google Scholar]
- 7. Kromer W, Postius S, Riedel R. Animal pharmacology of reversible antagonism of the gastric acid pump, compared to standard antisecretory principles. Pharmacology 2000;60:179–187. [DOI] [PubMed] [Google Scholar]
- 8. Vanderhoff BT, Tahboub RM. Proton pump inhibitors: An update. Am Fam Physician 2002;66:273–280. [PubMed] [Google Scholar]
- 9. Wallmark B, Larsson H, Humble L. The relationship between gastric acid secretion and gastric H+, K+‐ATPase activity. J Biol Chem 1985;260:13681–13684. [PubMed] [Google Scholar]
- 10. Abelo A, Holstein B, Eriksson UG, et al. Gastric acid secretion in the dog: A mechanism‐based pharmacodynamic model for histamine stimulation and irreversible inhibition by omeprazole. J Pharmacokinet Pharmacodyn 2002;29:365–382. [DOI] [PubMed] [Google Scholar]
- 11. Larsson H, Carlsson E, Junggren U, et al. Inhibition of gastric acid secretion by omeprazole in the dog and rat. Gastroenterology 1983;85:900–907. [PubMed] [Google Scholar]
- 12. Arnestad JS, Kleveland PM, Waldum HL. In single doses ranitidine effervescent is more effective than lansoprazole in decreasing gastric acidity. Aliment Pharmacol Ther 1997;11:355–358. [DOI] [PubMed] [Google Scholar]
- 13. Monnig AA, Prittie JE. A review of stress‐related mucosal disease. J Vet Emerg Crit Care 2011;21:484–495. [DOI] [PubMed] [Google Scholar]
- 14. Fandriks L, Lonroth H, Pettersson A, et al. Can famotidine and omeprazole be combined on a once‐daily basis? Scand J Gastroenterol 2007;42:689–694. [DOI] [PubMed] [Google Scholar]
- 15. Vakil N, Guda N, Partington S. The effect of over‐the‐counter ranitidine 75 mg on night‐time heartburn in patients with erosive oesophagitis on daily proton pump inhibitor maintenance therapy. Aliment Pharmacol Ther 2006;23:649–653. [DOI] [PubMed] [Google Scholar]
- 16. Bell NJ, Burget D, Howden CW, et al. Appropriate acid suppression for the management of gastro‐oesophageal reflux disease. Digestion 1992;51(Suppl 1):59–67. [DOI] [PubMed] [Google Scholar]


