Abstract
Background
Ehrlichia ewingii, which causes disease in dogs and people, is the most common Ehrlichia spp. infecting dogs in the United States, but little is known about how long E. ewingii infection persists in dogs.
Hypothesis/Objectives
To evaluate the persistence of natural infection with E. ewingii in dogs.
Animals
Four Class A Beagles; no previous exposure to ticks or tick‐borne infectious agents.
Methods
Dogs were exposed to ticks by weekly walks through tick habitat in north central Oklahoma; dogs positive for infection with Ehrlichia spp. by sequence‐confirmed PCR and peptide‐specific serology were evaluated for 733 days (D). Whole blood was collected once weekly for PCR, and serum was collected once monthly for detection of antibodies to Ehrlichia canis (peptide p16), Ehrlichia chaffeensis (indirect fluorescence antibody [IFA] and variable‐length PCR target [VLPT]), and E. ewingii (peptide p28).
Results
All dogs (4/4) became infected with Ehrlichia spp. as evidenced by seroconversion on IFA to E. chaffeensis (4/4); PCR detection of E. ewingii (4/4) and E. chaffeensis (2/4) DNA using both nested and real‐time assays; and presence of specific antibodies to E. ewingii (4/4) and E. chaffeensis (2/4). Infection with E. chaffeensis was not detected after D55. Intermittent E. ewingii rickettsemia persisted in 3 of 4 dogs for as long as 733 days.
Conclusions and Clinical Importance
Our data demonstrate that dogs infected with E. ewingii from tick feeding are capable of maintaining infection with this pathogen long‐term, and may serve as a reservoir host for the maintenance of E. ewingii in nature.
Keywords: Amblyomma americanum, Granulocytic ehrlichiosis, Reservoir host
Abbreviations
- PCR
polymerase chain reaction
- IFA
indirect fluorescence antibody test
- VLPT
variable‐length PCR target
- GMTMAX
maximum geometric mean titer
- IgG
immunoglobulin G
Ehrlichia spp. are obligate intracellular bacteria transmitted by ticks that often infect white blood cells of mammals.1 A number of Ehrlichia spp. infections have been reported in dogs from the United States, including E. canis, E. chaffeensis, E. ewingii, Panola Mountain Ehrlichia sp., and E. muris.2, 3, 4 E. canis infection in dogs can cause anorexia, fever, epistaxis, hemorrhage, and sometimes results in death.1, 2 E. ewingii also is an important pathogen in dogs. Fever, anorexia, thrombocytopenia, polyarthritis, and central nervous system abnormalities have been associated with E. ewingii infection in dogs.1, 2 Although there is little data to support E. chaffeensis causing disease in dogs, this species as well as several other canine Ehrlichia spp. are known to cause disease in people.1, 2, 5
Ehrlichia ewingii is the most prevalent Ehrlichia spp. detected by serology in dogs in the south central and south eastern United States.6 Infection is transmitted by Amblyomma americanum, the lone star tick.2 Infection with E. ewingii can cause clinically relevant disease in dogs, and dogs, in addition to white‐tailed deer, also may serve as a reservoir host for this agent.1, 7 Dogs are the primary reservoir host for E. canis, and infection can be maintained for several years.1, 2 There also is potential for dogs to serve as a reservoir host for E. chaffeensis, but their role appears to be less important than that of white‐tailed deer.1, 2 To characterize the persistence of infection with E. ewingii in dogs after natural tick exposure, we evaluated 4 dogs for 2 years after initial tick exposure.
Materials and Methods
Class A Beagle dogs (n = 4) infected with E. ewingii and E. chaffeensis as previously described8 were used for this study. All research was conducted under an Animal Care and Use Protocol approved by the Institutional Animal Care and Use Committee at Oklahoma State University. Briefly, dogs originally had been infested with ticks on 7 consecutive weekly walks and clinically monitored for evidence of tick‐borne infection as previously described.8 Whole blood was collected by jugular venipuncture twice weekly from study day (D) 0 through D121, and weekly from D256 to D733; serum was collected weekly from D0 to D121 and monthly from D256 to D712. Whole blood and serum were stored at −20°C until testing was performed.
Antibodies to Ehrlichia spp. were detected using indirect fluorescence antibody (IFA) tests and species‐specific peptide ELISA. Sera were tested for antibodies using commercially available E. chaffeensis IFA slides1 and fluorescein isothiocyanate (FITC)‐labeled goat‐anti‐dog IgG2 as previously described.8 Sera also were analyzed for presence of antibodies against E. canis (p16), E. chaffeensis (VLPT), and E. ewingii (p28), with results measured by densigraph as previously described.8
Nested polymerase chain reaction (PCR) assays were performed on DNA extracted from 200 μL of whole blood. To independently confirm the nested PCR results, real‐time PCR also was performed on a subset of aliquots of samples collected every 2 weeks from D0 to D121 and every other month from D256 to D712. DNA for nested PCR was extracted using a commercial kit3 according to the manufacturer's instructions. Extraction of DNA for real‐time PCR utilized the High Pure PCR Template Preparation Kit4 according to manufacturer's instructions. Extracted DNA was stored at −20°C until testing. Nested PCR was employed to amplify species‐specific 16S ribosomal DNA fragments using external primers ECC/ECB and internal primers ECA/HE3 (E. canis), HE1/HE3 (E. chaffeensis), and EE72/HE3 (E. ewingii) as previously described, with representative amplicons directly sequenced to confirm identity.7 Real‐time PCR hybridization probe assays were used for detection of the disulfide oxidoreductase gene of E. chaffeensis (AF403711) and E. ewingii (DQ902688) as previously described.8
Results
All 4 dogs developed antibodies (inverse titers ≥128) on IFA to E. chaffeensis; antibodies were first detected by IFA as early as D26 and continued to be detected through the final day of the study in 3 dogs (Fig 1). Maximal inverse titers during the study ranged from 1,024 to 32,768. Near the end of the study, by D712, 2 dogs had titers ≥4,096 (Fig 1). Specific antibodies to E. ewingii (p28) were absent at D33 for all dogs, detected in 3 dogs by D65, and detected in 1 dog by D89; E. ewingii specific antibodies persisted in all 4 dogs through D649 and in 3 dogs through D712. Maximal peptide values ranged from 0.25 to 1.08 as read by a densigraph (Fig 1). Specific antibodies to E. chaffeensis (VLPT) were absent at D33 but detected in 2 dogs by D65 and persisted through D712 with maximal peptide values ranging from 0.18 to 0.45 (Fig 1). E. canis specific antibodies (p16) were not detected in any dog.
Figure 1.
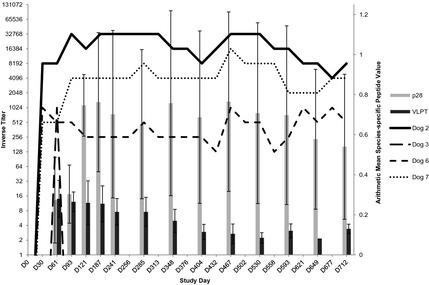
Inverse titers detected by IFA (lines; n = 4) and species‐specific mean peptide values to Ehrlichia ewingii/p28 and Ehrlichia chaffeensis/VLPT (bars with standard deviation) in dogs naturally infested with ticks. IFA, indirect fluorescence antibody.
Two of 4 dogs had intermittently detectable E. chaffeensis DNA using the nested PCR assay on samples collected on D23–D55 or D30–D51, respectively, but E. chaffeensis DNA was not detected in any dog after D55. Real‐time PCR did not detect E. chaffeensis DNA in any dog. All 4 dogs had detectable E. ewingii DNA by D59 using both PCR assays, with 2 dogs positive as early as D47 with nested PCR and by D51 with real‐time PCR. One dog was PCR positive for E. ewingii only briefly (D47–D61). Intermittent E. ewingii rickettsemia persisted long‐term in 3 dogs. Two dogs were PCR positive D47–D460 and the third dog was PCR positive D59–D733. E. canis DNA was not detected by nested PCR in any dog. Development of clinical illness was not observed in any dog during the course of this study.
Discussion
In the absence of transovarial transmission in ticks, maintenance of Ehrlichia spp. in nature requires persistently infected vertebrate hosts as reservoir hosts.5 The present study showed that some dogs maintain long‐term infection with E. ewingii after limited exposure to ticks. Three of the 4 dogs we followed maintained infections for 15 months, with 1 dog remaining infected for >2 years. Infection in this 1 dog continued until June 2014, more than 3 years after initial tick exposure (data not shown). Previous work has shown that infection with E. ewingii can be detected by PCR intermittently in dogs experimentally infected for at least 5 months after IV inoculation, and DNA also has been amplified from many apparently healthy client‐owned or shelter dogs with unknown durations of rickettsemia.6, 7, 9 Although many dogs die of serious disease, dogs have been shown to remain infected with E. canis for years while maintaining the ability to infect ticks, making them a key reservoir host.1, 2 The results of the present study suggest a similar situation may occur with E. ewingii in which dogs maintain long‐term infections, potentially serving as a reservoir host, while also occasionally developing clinical disease associated with the infection. Breed as well as co‐infection with multiple tick‐borne agents may play a role in persistence of infection. However, results of this study are not consistent in that the 2 Ehrlichia spp. co‐infected dogs maintained E. ewingii infection long‐term (460 days), whereas the singly E. ewingii infected dogs maintained infection either briefly (up to 61 days) or long‐term (>733 days). Moreover, no dog exhibited clinical signs consistent with tick‐borne illness.
The persistence of E. ewingii infection in the dogs in this study was documented by both serology and PCR. The inverse titers for the 3 persistently infected dogs exhibited small fluctuations throughout the 2‐year study period, remaining within 3‐fold from the lowest measured titer (Fig 1). The species‐specific average peptide values remained steady for E. ewingii (p28) throughout the study, whereas the E. chaffeensis (VLPT) average peptide values showed an overall gradual decrease during the 2‐year study period (Fig 1). Antibody titers to p28 remained stable in 2 dogs over 8 months after the last positive PCR (D460) for E. ewingii, and detectable antibodies to VLPT still were present in 2 dogs 22 months after the last positive PCR for E. chaffeensis. The E. chaffeensis IFA utilized in this study detected antibodies in the sera of 2 dogs consistently PCR‐negative for E. chaffeensis, suggesting detection of cross‐reactive antibodies generated against E. ewingii. In the present study, infection with E. ewingii was detected in all 4 dogs by both nested and real‐time PCR assays, whereas only nested PCR detected E. chaffeensis infection in 2 dogs. The reason for the occasional discordant results is not clear, but degradation of DNA could have occurred before sample processing and testing by real‐time PCR assays.10 Concurrent use of serologic and molecular diagnostic modalities likely would enhance detection of persistently infected animals.
Almost all of the known Ehrlichia spp. that infect dogs, including E. ewingii and E. chaffeensis, are zoonotic.1, 5 Although most infections occur by tick feeding, transmission of Ehrlichia spp. also has been reported through contaminated blood products.2 Dogs persistently infected with E. ewingii pose a potential infection risk to other animals by transfusion products and to veterinary staff members that may come into contact with infected blood.2 Persistently infected dogs also serve as a source of infection to ticks and may themselves acquire other tick‐borne infections, which can lead to more severe disease.2 The role that persistent infection with E. ewingii plays in the acquisition of and clinical signs associated with additional tick‐borne coinfections warrants further exploration.
Acknowledgments
This study was supported by funding from IDEXX Laboratories, Inc and the Krull‐Ewing endowment at Oklahoma State University.
The authors acknowledge laboratory animal resources at Oklahoma State University for the care of the animals used in this study and technical staff at IDEXX Laboratories, Inc for assistance with serologic and molecular testing. Dr Starkey is the Bayer Resident in Veterinary Parasitology, National Center for Veterinary Parasitology, Oklahoma State University.
Conflict of Interest Declaration: MB, RC, BT, and PT are employees of IDEXX Laboratories, Inc, which manufactures diagnostic assays for Ehrlichia ewingii. In the past 5 years, SL has received research funding, speaker honoraria, and travel reimbursement from IDEXX Laboratories, Inc. LS and AB have no conflicts to report.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Animal work was completed at Oklahoma State University in accordance with IACUC and ACUP standards. Real‐time PCR and peptide‐specific analyses were performed at IDEXX Laboratories, Inc, in Westbrook, Maine; all other laboratory procedures were performed at Oklahoma State University.
Findings from this study were presented at the American Association of Veterinary Parasitologists annual meeting, Chicago, Illinois, 2013 and the 2014 American College of Veterinary Internal Medicine Forum, Nashville, Tennessee.
Footnotes
Fuller Laboratories, Fullerton, CA
KPL, Gaithersburg, MD
Illustra blood genomic Prep Mini Spin Kit; GE Healthcare UK Limited, Buckinghamshire, UK
Roche Applied Science, Indianapolis, IN
References
- 1. Rar V, Golovljova I. Anaplasma, Ehrlichia, and “Candidatus Neoehrlichia” bacteria: Pathogenicity, biodiversity, and molecular genetic characteristics, a review. Infect Genet Evol 2011;11:1842–1861. [DOI] [PubMed] [Google Scholar]
- 2. Little SE. Ehrlichiosis and anaplasmosis in dogs and cats. Vet Clin North Am Small Anim Pract 2010;40:1121–1140. [DOI] [PubMed] [Google Scholar]
- 3. Hegarty BC, Maggi RG, Koskinen P, et al. Ehrlichia muris infection in a dog from Minnesota. J Vet Intern Med 2012;26:1217–1220. [DOI] [PubMed] [Google Scholar]
- 4. Qurollo BA, Davenport AC, Sherbert BM, et al. Infection with Panola Mountain Ehrlichia sp. in a dog with atypical lymphocytes and clonal T‐cell expansion. J Vet Intern Med 2013;27:1251–1255. [DOI] [PubMed] [Google Scholar]
- 5. Nicholson WL, Allen KE, McQuiston JH, et al. The increasing recognition of rickettsial pathogens in dogs and people. Trends Parasitol 2010;26:205–212. [DOI] [PubMed] [Google Scholar]
- 6. Beall MJ, Alleman AR, Breitschwerdt EB, et al. Seroprevalence of Ehrlichia canis, Ehrlichia chaffeensis and Ehrlichia ewingii in dogs in North America. Parasit Vectors 2012;5:29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Little SE, O'Connor TP, Hempstead J, et al. Ehrlichia ewingii infection and exposure rates in dogs from the southcentral United States. Vet Parasitol 2010;172:355–360. [DOI] [PubMed] [Google Scholar]
- 8. Starkey LA, Barrett A, Chandrashekar R, et al. Development of antibodies to and PCR detection of Ehrlichia spp. in dogs following natural tick exposure. Vet Microbiol 2014;173:379–384. [DOI] [PubMed] [Google Scholar]
- 9. Yabsley MJ, Adams DS, O'Connor TP, et al. Experimental primary and secondary infections of domestic dogs with Ehrlichia ewingii . Vet Microbiol 2011;150:315–321. [DOI] [PubMed] [Google Scholar]
- 10. Allison RW, Little SE. Diagnosis of rickettsial diseases in dogs and cats. Vet Clin Pathol 2013;42:127–144. [DOI] [PubMed] [Google Scholar]


