Abstract
Background
Reports of histiocytic sarcoma (HS) involving the central nervous system (CNS) are sparse and consist mainly of case reports describing 1–3 animals.
Objective
The objective of this study was to report the signalments, clinical signs, clinicopathologic and diagnostic imaging findings, treatment, and outcome of a series of dogs with HS and CNS involvement.
Animals
Nineteen dogs with HS examined at veterinary referral hospitals.
Methods
Retrospective case series. Medical records were reviewed and cases with a histopathological diagnosis of CNS HS were included in the study. Diagnostic imaging studies of the CNS were evaluated and histopathologic samples were reviewed to confirm the diagnosis.
Results
Retrievers and Pembroke Welsh Corgis were overrepresented in this cohort of dogs. Tumors involved the brain in 14 dogs and the spinal cord in 5. In 4 dogs, HS was part of a disseminated, multiorgan process whereas it appeared confined to the CNS in 15 dogs. Diagnostic imaging had variable appearances although extraaxial masses predominated in the brain. There was meningeal enhancement in all dogs that was often profound and remote from the primary mass lesion. Pleocytosis was present in all dogs with CSF evaluation. Median survival was 3 days.
Conclusions and Clinical Importance
Breed predispositions appear to vary from reports of HS in other organ systems. Some unique imaging and clinicopathologic characteristics, particularly brain herniation, profound meningeal enhancement, and pleocytosis in combination with 1 or more mass lesions, might help to differentiate this neoplasm from others involving the CNS, although this requires further study.
Keywords: Brain tumor, Malignant histiocytosis, Round cell tumor, Spinal cord tumor
Abbreviations
- CNS
central nervous system
- CSF
cerebrospinal fluid
- HS
histiocytic sarcoma
- MRI
magnetic resonance imaging
- NCSU
North Carolina State University
- PVSEC
Pittsburgh Veterinary Specialty & Emergency Center
Diseases affecting macrophage and dendritic cell populations include proliferative disorders (reactive histiocytoses), benign tumors (eg, cutaneous histiocytoma), and overtly malignant neoplasms known as histiocytic sarcomas (HS).1 Histiocytic sarcomas can occur as either localized tumors, which most commonly arise as soft tissue swellings on limbs, or can be disseminated, affecting multiple organ systems such as the spleen, liver, lungs, lymph nodes, bone marrow, and central nervous system (CNS). Most HS in dogs are considered to result from neoplastic transformation of interstitial dendritic cells with the exception of a hemophagocytic variant that occurs within splenic red pulp and arises from macrophages.1
Although CNS involvement has been recognized in dogs with disseminated (secondary) HS,2, 3 published reports of HS confined to the CNS (primary HS) are uncommon4, 5, 6, 7, 8, 9, 10, 11, 12 and most describe single cases or small numbers of dogs. The purpose of this report is to document the clinical, diagnostic, and pathologic findings as well as treatment and outcome in a larger case series of dogs with a confirmed diagnosis of HS involving the CNS.
Materials and Methods
The medical records of dogs with a diagnosis of HS involving the CNS were reviewed. Cases were identified by retrospectively searching hospital and pathology databases, as well as being prospectively identified by the authors. For inclusion, cases had to have histopathologic confirmation of HS involving the leptomeninges or parenchyma of the brain or spinal cord (ie, intradural location). Tumors arising outside the CNS with secondary compression or local invasion of nervous tissue were excluded. The signalments, presenting signs, neuroanatomic localization, results of clinicopathologic and diagnostic imaging tests, treatments, survival times, and results of necropsy and histopathology were retrieved from the medical records. In addition, all diagnostic imaging studies of the CNS were reviewed by 3 board‐certified radiologists (IDR, GSS, JCB) and 2 board‐certified neurologists (CLM, NJO) to generate a consensus opinion on the following parameters: tumor location within the neuraxis, tumor location relative to the CNS parenchyma, signal intensities of tumor(s) on acquired magnetic resonance imaging (MRI) sequences relative to normal gray matter tissue, enhancement of tumor and meninges after intravenous contrast medium administration, distinctness of tumor margins, degree of peritumoral edema, and the presence of a mass effect and herniation of CNS tissue (see Data S1). All MRI was performed on a single 1.5 Tesla unit1 before and after gadoversetamide2 administration (0.1 mmol/kg) except for 1 dog that was imaged with a 1.0 Tesla unit.3
All histopathologic material was reviewed by a board‐certified anatomic pathologist (LBB) to confirm the diagnosis. When survival data were not available from the record, follow‐up telephone calls were made to the referring veterinarian or owner to determine whether the dog was alive or the date and cause of death if the animal had died. The median survival time and other statistic data and comparisons were generated with a commercially available program.4 A Mann–Whitney test was used to compare the ages of dogs with primary versus secondary HS.
Results
Clinical Findings
All cases were accrued between 2006 and 2012. Nineteen dogs were identified with HS involving the CNS. All tumors were confirmed histologically (15 after necropsy, 6 with surgical biopsy, 2 dogs had both). There were 11 spayed females, 7 castrated males and 1 intact male, with a median age of 8.0 years (range 4.0–11.6 years). Breeds represented included 5 Labrador retrievers, 4 Golden retrievers, 3 Pembroke Welsh Corgis, 2 mixed breed dogs, 2 Shetland Sheepdogs, 1 English springer spaniel, 1 Cavalier King Charles spaniel, and 1 Keeshond.
Presenting complaints included paresis (6 dogs), altered mentation or behavioral changes (5), seizures (2), ataxia (2), blindness (1), vomiting (1), and head tilt (1). One dog presented for paraplegia and had an acute disk herniation as well as an intramedullary mass identified with MR imaging. This dog improved after hemilaminectomy to remove the herniated disk, but subsequently worsened and repeated imaging revealed expansion of the intramedullary lesion. Neuroanatomic localization included forebrain (9 dogs), multifocal localization (3), T3‐L3 myelopathy (2), C1‐5 myelopathy (1), L4‐S3 myelopathy (1), medulla oblongata (1), and optic nerve (1). One dog that presented for vomiting and inappetence had a normal neurologic examination.
Clinicopathologic Findings
Complete blood count and serum biochemical evaluation was performed in all dogs. Notable abnormalities included mild thrombocytopenia in 4 dogs, a nonregenerative anemia in 1 dog, and increased liver enzyme activities in 5 dogs. Urinalysis was performed in 12 dogs and revealed evidence of a urinary tract infection (pyuria, bacteriuria or both) in 5 dogs and crystalluria in 2 dogs.
Cerebrospinal fluid (CSF) analysis was performed on 6 dogs with 4 samples collected from the cerebellomedullary cistern and 2 from the lumbar subarachnoid space. Pleocytosis (nucleated cell count >5/μL) was present in all dogs with a mean nucleated cell count of 1509 (median 254.5, range 10–8000 cells/μL). Small and large mononuclear cells predominated in all samples, although neutrophils were also noted in smaller numbers in all samples, 3 samples had eosinophils, and 2 samples had plasma cells. Overtly neoplastic cells were not noted in this cohort of dogs. The protein concentration was increased in all CSF samples (mean 411.9 mg/dL, median 540.4, range 34.1–677.0 mg/dL, reference range 0–25 mg/dL [cerebellomedullary cistern], or 0–40 mg/dL [lumbar subarachnoid space]). A fluoroscopic‐guided aspirate of a mass lesion involving the spinal cord was performed in 1 dog and revealed mesenchymal cell proliferation suggestive of neoplastic histiocytes.
Diagnostic Imaging Findings
Thoracic radiographs were obtained in 14 dogs. Abnormalities detected included single or multiple pulmonary nodules in 4 cases and tracheobronchial and sternal lymphadenopathy in 1 dog. Abdominal ultrasonography was performed in 11 dogs and identified splenic (4 dogs), liver (2), and renal (1) nodules and renal lymphadenopathy (1).
MRI of the CNS was performed in 12 dogs, including studies of the brain (8 dogs), thoracolumbar spinal cord (3), and cervical spinal cord (3). Two dogs had MR imaging of both the brain and cervical spinal cord performed. The results of the CNS imaging are summarized in Tables 1 and 2. Within the brain, solitary, extraaxial masses with moderate to marked enhancement after intravenous contrast medium administration predominated (Fig 1), and herniation of brain tissue with secondary syringohydromyelia was common (Fig 2). Tumors within the spinal cord appeared to be either intramedullary or intradural‐extramedullary in location, and enhanced after contrast medium administration (Fig 3). Two of the dogs had multifocal mass lesions involving the spinal cord. One striking observation was diffuse, marked enhancement of the meninges, even when there appeared to be a solitary, well‐defined mass lesion. This was particularly notable in the dogs with tumors involving the spinal cord (Fig 3).
Table 1.
Diagnostic imaging findings of dogs with intracranial histiocytic sarcomas (n = 7)
| Location relative to brain parenchyma | |
| Extraaxial | 6 |
| Intraaxial | 1 |
| Anatomic locationa | |
| Olfactory | 3 |
| Falcine | 1 |
| Cerebral convexity | 2 (parietal) |
| Cerebellopontomedullary angle | 1 |
| Cerebellum | 1 |
| Intensity of tumor (relative to normal gray matter) | |
| T1‐weighted | |
| Hypointense | 7 |
| T2‐weighted | |
| Hypointense | 1 |
| Isointense | 2 |
| Mixed | 4 |
| Proton density | |
| Isointense | 5 |
| Mixed | 1 |
| Not performed | 1 |
| FLAIR | |
| Hypointense | 1 |
| Isointense | 2 |
| Mixed | 4 |
| T2* susceptibility artifact | |
| Yes | 1 |
| No | 6 |
| Contrast enhancement of tumor | |
| Degree | |
| Moderate | 2 |
| Marked | 5 |
| Pattern | |
| Homogeneous | 3 |
| Heterogeneous | 4 |
| Ring enhancing | 1 |
| Patchy | 3 |
| Contrast enhancement of meninges | |
| Degree | |
| Mild | 1 |
| Moderate | 2 |
| Marked | 3 |
| Unclear | 1 |
| Pattern | |
| Pachymeningeal | 4 |
| Dural tail | 3 |
| Leptomeningeal | 2 |
| Unclear | 1 |
| Tumor margins | |
| Distinct | 2 |
| Indistinct | 2 |
| Mixed | 3 |
| Presence of mass effect | |
| Yes | 7 |
| Degree of peritumoral edema | |
| Mild | 3 |
| Moderate | 3 |
| Marked (hemispheric) | 1 |
| Presence of brain herniation | |
| None | 2 |
| Transtentorial | 4 |
| Foramen magnum | 4 |
| Other findings | |
| T2 hyperintensity in cervical spinal cord | 6 |
FLAIR, fluid attenuated inversion recovery.
Utilizing method of Sturges et al.,25 1 dog had 2 discrete mass lesions (olfactory and falcine).
Table 2.
Diagnostic imaging findings of dogs with histiocytic sarcomas involving the spinal cord (n = 5)
| Location relative to spinal cord parenchyma | |
| Intradural‐extramedullary | 2 |
| Intramedullary | 2 |
| Diffuse meningeal | 1 |
| Anatomic location of discrete masses | |
| Cervical | 1 |
| Thoracic | 2 |
| Lumbar/sacral | 1 |
| Intensity (relative to normal gray matter) | |
| T1‐weighted | |
| Hypointense | 1 |
| Isointense | 4 |
| T2‐weighted | |
| Hyperintense | 2 |
| Mixed | 3 |
| STIR | |
| Hyperintense | 4 |
| Not performed | 1 |
| T2* susceptibility artifact | |
| No | 1 |
| Not performed | 4 |
| Contrast enhancement of tumor | |
| Degree | |
| Mild | 1 |
| Moderate | 1 |
| Marked | 3 |
| Pattern | |
| Homogeneous | 2 |
| Heterogeneous (patchy) | 3 |
| Contrast enhancement of meninges | |
| Degree | |
| Mild | 1 |
| Marked | 4 |
| Pattern | |
| Localized | 1 |
| Diffuse | 4 |
| Tumor margins | |
| Distinct | 3 |
| Indistinct | 2 |
| Presence of mass effect | |
| Yes | 5 |
| Attenuation on HASTE images | |
| Yes | 4 |
| No | 1 |
| Degree of peritumoral edema | |
| Mild | 1 |
| Moderate | 1 |
| Marked | 2 |
| Unclear | 1 |
STIR, short tau inversion recovery; HASTE, half‐Fourier acquisition single‐shot turbo spin echo.
Figure 1.
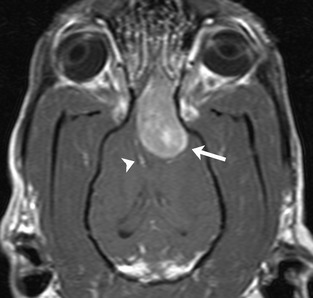
Dorsal postcontrast T1‐weighted magnetic resonance image showing a large, contrast‐enhancing extraaxial mass (arrow). The associated mass effect leads to deviation of the falx cerebri (arrowhead) and lateral ventricles.
Figure 2.
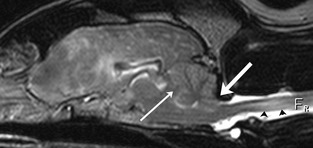
Sagittal T2‐weighted magnetic resonance image showing transtentorial (thin white arrow) and foramen magnum (thick white arrow) brain herniation and associated increased signal within the cervical spinal cord (black arrowheads).
Figure 3.
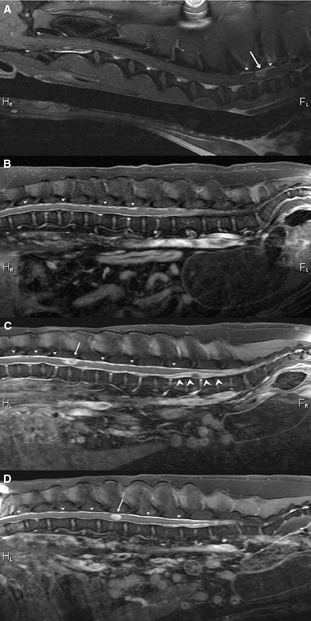
Sagittal postcontrast T1‐weighted images of 4 different dogs showing variation in imaging appearances of histiocytic sarcoma affecting the spinal cord. (A) Solitary, contrast‐enhancing mass lesion in the region of the T1 vertebra (arrow) with associated local meningeal enhancement (arrowheads), (B) diffuse, moderate meningeal enhancement (arrowheads), (C) diffuse, marked meningeal enhancement (small arrowheads) with 2 mass lesions in intradural‐extramedullary locations (arrow and large arrowheads), (D) marked diffuse enhancement of the meninges (arrowheads) accompanied by a contrast‐enhancing, seemingly intramedullary mass (arrow).
Histopathology and Immunohistochemistry
Antemortem histopathology was performed in 6 dogs, after tumor tissue was obtained via a craniectomy (3 dogs), dorsal cervical or thoracic laminectomy (2), or lumbar hemilaminectomy (1). Fourteen dogs underwent full necropsy examinations, including 2 with a previous biopsy, and in 1 dog, the brain was removed after euthanasia for histopathologic evaluation but the other organs were not examined microscopically. Histopathology confirmed HS in all dogs. Mass lesions were unencapsulated, with compression and frequent invasion of adjacent brain or spinal cord parenchyma. Meningeal involvement was a consistent feature and tumors that appeared to be in an intramedullary location on MRI or even with initial gross or histopathologic examination were determined to have some contact with the meninges after careful examination of all sections. When available for review, meninges that were noted to enhance dramatically on MRI studies consistently showed tumor cell invasion, even in locations remote from a distinct mass lesion. Neoplastic cells were round to polygonal or spindyloid with variably distinct cell borders and variable amounts of eosinophilic cytoplasm, which was occasionally vacuolated or foamy. Most cases had marked anisocytosis and anisokaryosis and multinucleated giant cells were common (Fig 4). Nucleoli were prominent and frequently multiple. Mitotic figures were also common, ranging from 2 to 34 per 10 high‐powered fields (400×) and often had bizarre morphology. In 7/19 dogs, neoplastic cells displayed erythrophagocytosis although this was an infrequently observed feature in these cases. Tumor necrosis was frequently noted. Neoplastic cells were admixed with variable numbers of lymphocytes, plasma cells, neutrophils and occasionally eosinophils, and lymphoplasmacytic perivascular cuffs were often noted in adjacent CNS tissue. A variety of immunohistochemical markers were used in select cases to further characterize the neoplasms. Neoplastic cells were CD18 and lysozyme positive but negative for CD3 and CD79a.
Figure 4.
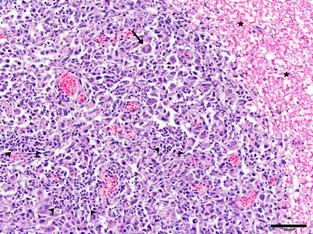
Photomicrograph of a histiocytic sarcoma mass compressing and invading the spinal cord (asterisks). Note marked anisocytosis and pleomorphic nature of neoplastic cells with multinucleated cells (arrow) and groups of inflammatory cells (arrowheads). Bar = 50 μm.
Primary versus Secondary CNS Histiocytic Sarcoma
Based on thoracic and abdominal imaging, necropsy examination, or both, HS appeared to be restricted to the CNS in 15 dogs, but was part of a disseminated, multiorgan process in 4 dogs. Organs involved in these latter dogs included the lungs (3 dogs), liver (2), lymph nodes (2), spleen, pancreas, kidney, adrenal gland, and heart (1 each). Concurrent but distinct neoplasms were detected in 3 dogs, and included an atrial hemangiosarcoma, a testicular seminoma, and a trichoepithelioma involving the tail. Of the 4 dogs with disseminated HS, 2 were Golden retrievers and 2 were Labrador retrievers, and all were female. There was no difference in age (P = .80) between dogs with primary versus secondary CNS HS. Of the dogs with secondary CNS HS, only 1 had CSF evaluation and 2 had diagnostic imaging of the CNS, precluding meaningful comparisons with the primary CNS cases.
Treatment and Outcome
Surgical interventions included craniectomies (3 dogs) and laminectomies (3). Two dogs received fractionated external beam radiation therapy for spinal cord tumors after surgical debulking. Two dogs received lomustine5 and 1 dog received cytarabine.6 Glucocorticoids were administered to 10 dogs, antibiotics to 8 dogs, anticonvulsants to 3 dogs and nonsteroidal anti‐inflammatory drugs to 2 dogs. Euthanasia was performed in 16 dogs, while 2 dogs died spontaneously. In 1 dog, this information was not available. Median survival time for the entire cohort was 3 days (range 1–92 days).
Discussion
Here, we describe a series of dogs with HS affecting the CNS either exclusively or as part of a multisystemic process. Neoplasms were found in all regions of the neuraxis and had several patterns of distribution on MRI. Solitary extraaxial mass lesions were common in the brain, whereas single, intradural‐extramedullary, or apparently intramedullary lesions were frequent in the spinal cord. More complex neoplastic patterns were also noted, comprising multiple nodular masses, diffuse meningeal involvement, or both. CSF analysis revealed a pleocytosis with increased protein in all dogs sampled. Histopathologic examination revealed that tumors consistently involved the meninges and that the profound meningeal enhancement seen on MRI was related to neoplastic infiltration in these areas. Although no firm conclusions can be drawn regarding treatment from this case series, survival times were short in all dogs.
Although reported relatively infrequently in the veterinary literature,3, 4, 5, 6, 7, 8, 9, 10, 11, 12 HS with CNS involvement is being recognized more frequently. At our institution (NCSU), we were unable to document a case of HS with CNS involvement before 2006, but confirmed 19 cases from 2006 to 2012. The reason for this apparent increase in prevalence is unknown.
Certain breeds are at greater risk for the development of HS, including the Bernese mountain dog, Golden retriever, Labrador retriever, Flat coated retriever and Rottweiler.13, 14, 15, 16, 17 Despite its frequent occurrence in other organ systems, CNS HS has been rarely reported in Bernese mountain dogs and Rottweilers.3, 12, 18 Conversely, although not considered an at‐risk breed for HS in other body regions, the Pembroke Welsh Corgi comprised 7/15 (47%) cases of CNS HS in a previous report.12 In our study, retrievers, Pembroke Welsh Corgis, and possibly Shetland sheepdogs were overrepresented, together comprising 14/19 (73.7%) of dogs. Total hospital admissions of Labrador retrievers, Golden retrievers, Shetland sheepdogs, and Pembroke Welsh Corgis were 13.8, 5.9, 1.2, and 0.7% of all dogs, respectively for the time period in which these CNS HS cases were admitted, but represented 27.8, 22.2, 11.1, and 16.7% of dogs in this case series (NCSU cases only; the single dog from PVSEC was omitted from these calculations). All of the dogs with documented secondary HS involving the CNS were retrievers. Interestingly, we had no Bernese mountain dogs or Rottweilers in this cohort. These breed discrepancies are intriguing and suggest the possibility of discrete mechanisms of tumorigenesis or multiple cells of origin within different organ systems that are influenced strongly by genetic factors. Aside from the splenic hemophagocytic variant, HS in dogs is believed to arise primarily from interstitial dendritic cells.1 Within the CNS, these cells are considered to be restricted to the meninges and choroid plexus.19 Our data are consistent with these views as tumors in all dogs were observed to have some contact with the meninges on histologic examination, even those that appeared to be in an intramedullary location based on diagnostic imaging studies. Further study is warranted in order to better define the mechanisms of HS tumorigenesis and how this process is altered by genetic influences in different breeds.
There are few reports describing the imaging findings of HS involving the CNS in dogs.5, 6, 8, 9, 11, 20 These describe single or multiple intraparenchymal mass lesions,6, 8, 9, 11 diffuse or multifocal meningeal involvement,9, 20 or a single extraaxial mass lesion resembling a meningioma.8 MRI of several dogs in our cohort revealed extraaxial, contrast‐enhancing mass lesions within the brain or spinal cord that were suggestive of meningiomas (Figs 1, 3). Enhancement of adjacent meninges was noted, and a dural tail was observed in several dogs. Thus, the differentiation of HS from meningioma can be difficult based on imaging findings alone. Of note, however, 4/5 dogs with brain imaging and a forebrain tumor had transtentorial herniation with or without foramen magnum herniation, and 6/7 with intracranial tumors had increased signal on T2‐weighted images of the cervical spinal cord suggestive of syringohydromyelia secondary to crowding of the foramen magnum. These proportions are greater than would be expected for meningiomas and are perhaps indicative of the rapidly growing, aggressive nature of HS.21 We also noted animals with what appeared to be solitary intraparenchymal tumors as well as some with multifocal masses or diffuse meningeal involvement. Another notable feature in our cohort was meningeal enhancement, which was not just seen adjacent to mass lesions, but often in noncontiguous regions of the CNS. This was noted in dogs with tumors involving both the brain and spinal cord, and in several cases diffuse, marked enhancement of the meninges involving the entire CNS region imaged was observed (Fig 3).
In this study, all dogs that had CSF evaluation had a pleocytosis along with increased CSF protein, which is consistent with previous reports of CNS HS.6, 8, 9, 10, 11, 20 An inflammatory response was considered likely to account for this pleocytosis in most of our cases as overtly neoplastic cells were not identified in this case series. Several previous reports have described neoplastic histiocytes on CSF evaluation, often with the aid of immunocytochemistry.8, 9, 11, 20, 22 The neoplastic histiocytes might be driving an inflammatory response, reflected in the CSF evaluation, perhaps through the secretion of proinflammatory cytokines and chemokines. It is also possible that a proportion of the mononuclear cells within the CSF in many cases represent an unrecognized neoplastic cell population and additional studies investigating this hypothesis might be warranted.
HS can manifest in several different ways within the CNS. One report described 2 distinct patterns of distribution, a focal mass lesion and a diffuse leptomeningeal manifestation.12 In our cohort, we had cases that fit both of these patterns. However, we also noted cases with well‐circumscribed, discrete mass lesions that also had profound, diffuse meningeal enhancement or more limited meningeal enhancement remote from the mass lesions on MRI. In cases where histopathologic examination of this remote meningeal enhancement was possible (ie, those with necropsy examination), neoplastic meningeal invasion was a consistent finding. This, together with the observation in other reports of neoplastic cells on CSF evaluation, suggests that dissemination of HS through the subarachnoid space occurs more commonly than with most other neoplasms, similar to CNS lymphoma. Another similarity between these tumor types is the diversity of potential CNS manifestations, as both HS and lymphoma can be found in extraaxial or intra‐axial/intramedullary locations, with a multifocal distribution or as a diffuse meningeal lesion.23, 24 The combination of mass lesions in conjunction with diffuse or remote meningeal enhancement, pleocytosis and potentially brain herniation is quite characteristic, and should prompt clinicians to consider HS as a potential diagnosis.
With only 4 cases of secondary CNS HS, it is difficult to make conclusions as to how these cases might differ clinically and pathophysiologically from dogs with primary CNS HS. It is interesting to note that all dogs with secondary HS were retrievers, whereas Pembroke Welsh Corgis were only found in the primary HS cohort. It is possible that some dogs classified as primary HS had tumor lesions in other organs that went undetected with diagnostic imaging or necropsy examinations. Only 2 dogs with secondary HS had CNS imaging studies performed and only 1 had CSF evaluation. Therefore, conclusions regarding different patterns of disease manifestation on MRI or clinicopathologic testing between primary and secondary CNS HS must await further studies.
Therapeutic interventions in this study included surgical excision or debulking of neoplastic tissue, external beam radiation therapy, chemotherapy and palliation with glucocorticoids, anticonvulsants and other medications. However, too few dogs received definitive treatment to make any meaningful judgments or comparisons regarding treatment.
In conclusion, we describe here a series of HS cases with nervous system involvement, the majority of which were limited to the CNS. Neoplastic disease involved all regions of the neuraxis, with diffuse involvement, metastasis through the CSF, or both in several cases. Retrievers and Pembroke Welsh Corgis were overrepresented in this cohort of dogs. Imaging of the CNS disclosed several different patterns including extraaxial masses resembling meningiomas, apparent intra‐axial/intramedullary masses and diffuse or multifocal manifestations. Contrast enhancement of the meninges was often profound with extension a considerable distance from the obvious mass lesion. Evaluation of CSF revealed a pleocytosis with increased protein in all cases. A variety of treatments were administered but survival times were very short, and the prognosis for HS with CNS involvement seems to be poor with currently utilized therapies.
Supporting information
Data S1. MRI scoring sheet for CNS histiocytic sarcoma.
Acknowledgments
This study was supported by the Department of Clinical Sciences, North Carolina State University.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
This work was completed at the College of Veterinary Medicine, North Carolina State University.
The results of this study have not been previously presented.
Footnotes
MAGNETOM Symphony, Siemens Medical Solutions USA, Inc., Malvern, PA
Optimark, Mallinckrodt Inc., St. Louis, MO
GE 1.0T LX, 9.1 software, GE Healthcare, The General Electric Company, Fairfield, CT
Prism, Graphpad Software Inc., La Jolla, CA
CeeNU, Bristol‐Myers Squib Company, Princeton, NJ
Cytosar‐U, Hospira, Lake Forest, IL
References
- 1. Moore PF. A review of histiocytic diseases of dogs and cats. Vet Pathol 2014;51:167–184. [DOI] [PubMed] [Google Scholar]
- 2. Snyder JM, Lipitz L, Skorupski KA, et al. Secondary intracranial neoplasia in the dog: 177 cases (1986–2003). J Vet Intern Med 2008;22:172–177. [DOI] [PubMed] [Google Scholar]
- 3. Thio T, Hilbe M, Grest P, Pospischil A. Malignant histiocytosis of the brain in three dogs. J Comp Pathol 2006;134:241–244. [DOI] [PubMed] [Google Scholar]
- 4. Chandra AM, Ginn PE. Primary malignant histiocytosis of the brain in a dog. J Comp Pathol 1999;121:77–82. [DOI] [PubMed] [Google Scholar]
- 5. Kang BT, Park C, Yoo JH, et al. 18F‐fluorodeoxyglucose positron emission tomography and magnetic resonance imaging findings of primary intracranial histiocytic sarcoma in a dog. J Vet Med Sci 2009;71:1397–1401. [DOI] [PubMed] [Google Scholar]
- 6. Snyder JM, Shofer FS, Van Winkle TJ, Massicotte C. Canine intracranial primary neoplasia: 173 cases (1986–2003). J Vet Intern Med 2006;20:669–675. [DOI] [PubMed] [Google Scholar]
- 7. Suzuki M, Uchida K, Morozumi M, et al. A comparative pathological study on granulomatous meningoencephalomyelitis and central malignant histiocytosis in dogs. J Vet Med Sci 2003;65:1319–1324. [DOI] [PubMed] [Google Scholar]
- 8. Tamura S, Tamura Y, Nakamoto Y, et al. MR imaging of histiocytic sarcoma of the canine brain. Vet Radiol Ultrasound 2009;50:178–181. [DOI] [PubMed] [Google Scholar]
- 9. Tzipory L, Vernau KM, Sturges BK, et al. Antemortem diagnosis of localized central nervous system histiocytic sarcoma in 2 dogs. J Vet Intern Med 2009;23:369–374. [DOI] [PubMed] [Google Scholar]
- 10. Uchida K, Morozumi M, Yamaguchi R, Tateyama S. Diffuse leptomeningeal malignant histiocytosis in the brain and spinal cord of a Tibetan Terrier. Vet Pathol 2001;38:219–222. [DOI] [PubMed] [Google Scholar]
- 11. Zimmerman K, Almy F, Carter L, et al. Cerebrospinal fluid from a 10‐year‐old dog with a single seizure episode. Vet Clin Pathol 2006;35:127–131. [DOI] [PubMed] [Google Scholar]
- 12. Ide T, Uchida K, Kagawa Y, et al. Pathological and immunohistochemical features of subdural histiocytic sarcomas in 15 dogs. J Vet Diagn Invest 2011;23:127–132. [DOI] [PubMed] [Google Scholar]
- 13. Affolter VK, Moore PF. Localized and disseminated histiocytic sarcoma of dendritic cell origin in dogs. Vet Pathol 2002;39:74–83. [DOI] [PubMed] [Google Scholar]
- 14. Coomer AR, Liptak JM. Canine histiocytic diseases. Compend Contin Educ Vet 2008;30:202–204. [PubMed] [Google Scholar]
- 15. Nielsen L, Andreasen SN, Andersen SD, Kristensen AT. Malignant histiocytosis and other causes of death in Bernese mountain dogs in Denmark. Vet Rec 2010;166:199–202. [DOI] [PubMed] [Google Scholar]
- 16. Padgett GA, Madewell BR, Keller ET, et al. Inheritance of histiocytosis in Bernese mountain dogs. J Small Anim Pract 1995;36:93–98. [DOI] [PubMed] [Google Scholar]
- 17. Fidel J, Schiller I, Hauser B, et al. Histiocytic sarcomas in flat‐coated retrievers: A summary of 37 cases (November 1998–March 2005). Vet Comp Oncol 2006;4:63–74. [DOI] [PubMed] [Google Scholar]
- 18. Moore PF, Rosin A. Malignant histiocytosis of Bernese mountain dogs. Vet Pathol 1986;23:1–10. [DOI] [PubMed] [Google Scholar]
- 19. D'Agostino PM, Gottfried‐Blackmore A, Anandasabapathy N, Bulloch K. Brain dendritic cells: Biology and pathology. Acta Neuropathol 2012;124:599–614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Hay WH, Ogilvie GK, Parker AJ, et al. Disseminated meningeal tumor in a dog. J Am Vet Med Assoc 1987;191:692–694. [PubMed] [Google Scholar]
- 21. da Costa RC, Parent JM, Poma R, Duque MC. Cervical syringohydromyelia secondary to a brainstem tumor in a dog. J Am Vet Med Assoc 2004;225:1061–1064. [DOI] [PubMed] [Google Scholar]
- 22. Stowe DM, Escobar C, Neel JA. What is your diagnosis? Cerebrospinal fluid from a dog. Vet Clin Pathol 2012;41:429–430. [DOI] [PubMed] [Google Scholar]
- 23. Palus V, Volk HA, Lamb CR, et al. MRI features of CNS lymphoma in dogs and cats. Vet Radiol Ultrasound 2012;53:44–49. [DOI] [PubMed] [Google Scholar]
- 24. Wisner ER, Dickinson PJ, Higgins RJ. Magnetic resonance imaging features of canine intracranial neoplasia. Vet Radiol Ultrasound 2011;52:S52–S61. [DOI] [PubMed] [Google Scholar]
- 25. Sturges BK, Dickinson PJ, Bollen AW, et al. Magnetic resonance imaging and histological classification of intracranial meningiomas in 112 dogs. J Vet Intern Med 2008;22:586–595. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. MRI scoring sheet for CNS histiocytic sarcoma.


