Abstract
Background
Tricuspid annular plane systolic excursion (TAPSE) is a useful estimate of right ventricular function in humans. Reference intervals for dogs have been generated, but the value of measuring TAPSE in other diseases, or investigating the association between TAPSE and outcome, is unknown.
Hypothesis
TAPSE is lower in Boxer dogs with ≥50 VPCs/24 h on Holter than in dogs with fewer ventricular ectopics, and lower TAPSE is associated with a shorter survival time.
Animals
Fifty Boxer dogs that presented for investigation of syncope or suspected arrhythmogenic right ventricular cardiomyopathy (ARVC) at a veterinary teaching hospital (2004–2011).
Methods
Retrospective study. Clinical records, Holter, and echocardiographic data were reviewed. TAPSE was measured in a blinded manner on stored echocardiographic cine‐loops using anatomic M‐mode. Outcome information was obtained and death was classified as cardiac or noncardiac. Survival analysis was performed using Kaplan‐Meier curves and Cox proportional hazards models.
Results
TAPSE was lower in Boxers with ≥50 VPCs/24 h (13.9 ± 4.04 mm) than Boxers with <50 VPCs/24 h (16.8 ± 3.21 mm; P < .001). TAPSE <15.1 mm was associated with shorter cardiac survival time in all dogs (P = .004) and also in dogs without left ventricular dysfunction (P = .035). When controlling for other variables, including ventricular tachycardia on Holter and left ventricular systolic dysfunction, multivariable analysis showed that TAPSE remained an independent predictor of time to cardiac death (HR >4.09, 95%CI 1.15–16.9, P < .029).
Conclusions and Clinical Importance
TAPSE offers prognostic value for Boxer dogs, including those with apparently normal systolic function and ≥50 VPCs/24 h on Holter analysis.
Keywords: Arrhythmia, Canine, Echocardiography, Holter, M‐mode
Abbreviations
- ARVC
arrhythmogenic right ventricular cardiomyopathy
- cMRI
cardiac magnetic resonance imaging
- VPC
ventricular premature complex
- LV
left ventricular
- VT
ventricular tachycardia
- RV
right ventricular
- TAPSE
tricuspid annular plane systolic excursion
- CHF
congestive heart failure
- LA
left atrium
- Ao
aorta
- LVIDs
left ventricular internal diameter in systole
- FS%
fractional shortening
- SD
systolic dysfunction
- EF%
ejection fraction
Introduction
Arrhythmogenic right ventricular cardiomyopathy (ARVC) is a primary myocardial disease characterized by fibro‐fatty infiltration of the right, and occasionally left, ventricle.
The diagnosis of ARVC in humans is based upon a combination of genetic screening, family history, ambulatory electrocardiography (Holter), and the detection of right ventricular adipose tissue on cardiac magnetic resonance imaging (cMRI).1 There is an extensive literature describing the genetic, clinical, echocardiographic, and electrophysiologic characteristics of ARVC in Boxer dogs.2, 3, 4, 5, 6
There are several prognostic indicators for Boxers dogs with ARVC. Shorter survival times are associated with echocardiographic evidence of left ventricular (LV) systolic dysfunction,5, 7 increased frequency and complexity of VPCs, including ventricular tachycardia (VT), and polymorphic VPCs.7
In human medicine, right ventricular (RV) function is commonly assessed by measurement of tricuspid annular plane systolic excursion (TAPSE) using M‐mode echocardiography.8, 9, 10, 11 TAPSE is a quantitative estimate of longitudinal RV shortening. It does not account for the radial and circumferential motion of the RV myocardial wall, but physiologic studies in humans have shown that RV shortening is greater longitudinally than radially and torsion contributes less to RV than LV output.12 TAPSE was significantly correlated with RV ejection fraction in validation studies against the gold standard of radio‐nuclide angiography,13 and reduced TAPSE is associated with a worse outcome in people with congenital heart disease, pulmonary hypertension, acute symptomatic pulmonary embolism, hypertrophic cardiomyopathy, and ARVC.8, 14, 15, 16 Several studies have also reported a correlation in humans between TAPSE and echocardiographic measures of LV systolic function,17 illustrating the interdependence between the left and right heart.
Assessing RV function has received recent attention in animals. The index of myocardial performance (or TEI index) has been evaluated in Boxer dogs with ARVC, and measurements suggested worse global myocardial performance in affected dogs, independent of body weight and heart rate variability.18 Reference intervals for TAPSE in normal dogs of various breeds and body weights have been derived.19 In the same study, dogs with pulmonary hypertension had lower TAPSE than dogs without pulmonary hypertension. In addition, TAPSE is easily obtained and repeatable.1 This suggests that TAPSE could be a practical and clinically useful measurement in RV dysfunction in dogs, as has already been reported in humans. To the authors’ knowledge, the association between TAPSE and outcome has not been reported in dogs for any disease process.
The aim of this study was to evaluate the clinical utility of TAPSE in a cohort of Boxer dogs presented for evaluation of syncope or suspected ARVC. We hypothesized that TAPSE would be lower in dogs with ≥50 VPCs/24 h on Holter analysis compared to dogs with <50 VPCs/24 h. We also hypothesized that lower TAPSE would be significantly associated with a shorter time to an end‐point of cardiac death.
Materials and Methods
Dogs and Clinical Data
Records of client‐owned Boxer dogs that presented to a veterinary teaching hospital for investigation of suspected ARVC or other clinical signs of cardiac disease (2004–2011) were retrospectively reviewed. All dogs were assessed under the supervision of a Board‐certified cardiologist. Dogs were eligible for inclusion if they had at least 1 Holter recording with a minimum of 19 hours of valid data and an echocardiographic examination within 1 month of Holter available for review. Dogs with documented congenital heart disease were excluded from the study, including those with aortic blood flow velocities ≥2.25 m/s.20 Dogs were also excluded if stored echocardiographic images were not suitable for measurement of TAPSE by off‐line measurement software, or if they had a concurrent diagnosis of a potentially life‐limiting disease, such as malignant neoplasia. All Boxer dogs in this study were referred to the cardiology service of the Royal Veterinary College for the further assessment of clinical signs or physical exam findings including collapse or arrhythmia and were not screened for ARVC as part of a breed club program.
Baseline clinical details obtained from medical records at the time of the first Holter recording included age, sex, body weight, and presenting clinical signs, defined as: absent; a history of syncope, episodic weakness/collapse that was believed to be consistent with syncope; or the presence/absence of congestive heart failure (CHF) at or before initial presentation. CHF was considered present if radiographic evidence of cardiogenic pulmonary edema was detected or if the dog had clinical evidence of ascites with either concurrent jugular distension/pulsation or hepatic congestion, with a clinical response to diuretic treatment. Medication that dogs were receiving at the time of presentation was also documented.
Information on survival was obtained via several means; complete medical records, questionnaires mailed to owners, and telephone contact with clients and referring veterinarians. Documented outcome data included whether the dog was alive or dead, the date of death or euthanasia, and whether the death was cardiac related. Cardiac death was defined as death or euthanasia associated with clinical signs attributable to cardiac disease (uncontrolled CHF, worsening CHF, or unacceptable increase in the frequency of syncope) or an unexpected death without apparent clinical signs during the preceding 24‐hour period. Necropsy data were not available.
Echocardiography
Echocardiography2 was performed in unsedated dogs and all echocardiographic images were acquired by a cardiology diplomate or cardiology Resident under direct supervision. For calculation of left atrial to aortic root ratio (LA/Ao), diameters of the left atrium (LA) and aorta were measured using 2D echocardiography in a right parasternal short axis view of the heart base, from the first frame after aortic valve closure (end‐systolic).21 M‐mode echocardiography was used to measure (leading‐edge to leading‐edge) LV internal diameters in systole (LVIDs) and diastole, and to calculate fractional shortening (FS%).22, 23 Spectral Doppler echocardiography was used to measure aortic outflow velocity from a subcostal window. Left ventricular systolic dysfunction (SD) was deemed present where: LVIDs>35 mm,5, 7 FS%<25%, or ejection fraction (EF%) <50%.7 EF% was measured by the Modified Simpson's method.24
All TAPSE measurements were performed by a single observer (BK), blinded to the dog outcome. TAPSE was measured using 2D anatomic M‐mode on a suitable left apical 4‐chamber image as previously described in dogs.19 The initial measurement was taken at the onset of ventricular systole, defined as the onset of the QRS complex on simultaneous ECG. Cardiac cycles within 1 beat of an atrial or ventricular premature complex were not considered suitable for measurement. Where possible, 3 consecutive measurements of TAPSE were performed and the mean value recorded.
Intraobserver variability in TAPSE measurement was assessed using images from a randomly selected subset of 10 dogs, each measured 5 times. Coefficient of variation (CV) was calculated and defined as acceptable if CV<10%.
Holter ECG
Holter records were reviewed by a single operator (PM), blinded to outcome. Dogs were dichotomized into 2 groups based on Holter analysis: ≥50 VPCs/24 h and <50 VPCs/24 h. Ventricular tachycardia, defined as >3 consecutive VPCs with a sustained heart rate >100 beats/min, was also recorded as present/absent.7 Where multiple Holter ECGs were performed, the first Holter, which was placed within 1 month of echocardiography, was used for analysis.
Statistical Analysis
Statistical analyses were performed by commercially available software.3, 4 Normality was determined graphically and using the Shapiro‐Wilk test. Normally distributed data were represented as mean (±standard deviation) and compared using an independent samples t‐test. Nonnormally distributed data were represented as median (range) and compared using a Mann‐Whitney U‐test. Continuous variables were assessed for linear association using a Pearson's correlation test.
Survival analysis was performed using an end‐point of cardiac death. Animals dying of noncardiac disease or alive at the end of the study period were right‐censored. Continuous variables were explored by division into groups based on quartiles, and the effect of these variables on survival was evaluated using Kaplan‐Meier survival analysis and Log‐rank tests. Where 1 or more groups had disproportionately different hazards to the others, these variables were presumed to exhibit a threshold effect and were included in Cox models as categorical variables using a cut‐off established by the exceptional quartile/s. For example, LA:Ao was included in the analysis dichotomized either side of the median value.
Univariable time‐to‐event models were constructed using Cox proportional hazards analysis. Factors where P < .2 in the univariable analysis were included in the multivariable analysis. Multivariable Cox proportional hazards analysis was performed in a forward stepwise manner. The assumptions of Cox proportional hazards were tested using log cumulative hazard plots and Schoenfeld residuals. Overall model fit was evaluated using Cox‐Snell residuals. Statistical significance was set at the 5% level.
Results
Population
Eighty‐one eligible Boxer dogs were evaluated for ARVC over the period (2004–2011). Thirty‐one were excluded because of inadequate quality, poor alignment of echocardiographic images for measurement of TAPSE, or both; leaving 50 dogs with complete data on echocardiography, 24 hours Holter and outcome where TAPSE could be appropriately measured.
Fifty percent of the population studied was male. Median age was 6.0 years (0.5–12.5 years) and mean weight was 30.2 kg (±6.2 kg). Males were significantly heavier (34.2 ± 4.7 kg) than female dogs (26.3 ± 4.7 kg; P < .001). Syncope was the primary presenting sign in 39/50 (78%) dogs. Exactly, 7/50 (14%) dogs presented with or had a history of CHF. Medication histories were available for all Boxer dogs (Table 1). Thirty‐five dogs were not receiving any medication, 7 dogs were receiving antiarrhythmic medication, either alone or in combination with other therapeutics, and 10 dogs were receiving a diuretic, either alone or in combination with other therapeutics.
Table 1.
Medical treatment received by Boxer dogs at the time of examination
| Medication | Number Receiving |
|---|---|
| None | 35 |
| Furosemide | 10 |
| Pimobendan | 8 |
| Benazapril | 5 |
| Spironolactone | 4 |
| Enalapril | 4 |
| Digoxin | 4 |
| Sotalol | 3 |
| Amiloride | 1 |
| Amiodarone | 1 |
| Hydrochlorothiazide | 1 |
| Mexiletine | 1 |
| Phenobarbitone | 1 |
| Ramipril | 1 |
Fifty‐six percentage (28/50) of Boxer dogs had ≥50 VPCs on 24 hour Holter analysis. Of the remaining 22 dogs with <50 VPCs, 11 (50%) had echocardiographic evidence of systolic dysfunction. Of the 28 dogs with ≥50 VPCs, 20 (71%) had systolic dysfunction (Table 2). Among dogs presenting with syncope, ≥50 VPCs were detected in 22/39 (56%) cases. Six dogs that had ≥50 VPCs, had no history of syncope. Dogs with ≥50 VPCs were significantly older (7.7 years, range 0.5–12.5 years) than those with <50VPCs (4.9 years, range 0.9–10 years; P = .046). There was no significant difference in weight or sex between groups (Table 2).
Table 2.
Characteristics of the 50 Boxer dogs included in survival analysis: tricuspid annular plane systolic excursion (TAPSE) and age at diagnosis were significantly different between groups. Normally distributed data are represented mean (± standard deviation) and nonnormally distributed data are represented median (range)
| Factor | Non‐ARVC: <50 VPCs on Holter) | ARVC: ≥50 VPCs on Holter | P Value |
|---|---|---|---|
| Number | 22 | 28 | – |
| Age (years) | 4.9 (0.9–10.0) | 7.7 (0.5–12.5) | .046 |
| Male: number (%) | 9 (41%) | 16 (57%) | .39 |
| Weight (kg) | 28.8 (±5.57) | 31.4 (±6.48) | .14 |
| Systolic dysfunction: number (%) | 11 (50%) | 20 (71%) | .079 |
| TAPSE (mm) | 16.8 (±3.21) | 13.9 (±4.04) | .008 |
Tricuspid Annular Plane Systolic Excursion
A minimum of 2 consecutive TAPSE measurements was obtained in 45/50 (90%) of dogs, and 3 consecutive TAPSE measurements were achievable in 27/50 (54%) of stored echocardiographic studies. Intraobserver variability of TAPSE measurement was 8%. Mean TAPSE for the entire population was 15.2 mm (±3.9 mm). TAPSE exhibited a weak negative correlation with both absolute weight (r = −0.324, P = .022) and weight scaled to the 1/3 power (r = −0.295, P = .038; Fig 1).22 There was no significant difference in TAPSE between genders (P = .39). Because correlation between TAPSE and weight was weak and unlikely to be clinically relevant (Fig 1), TAPSE was evaluated as an absolute measurement, rather than an indexed value. Dogs diagnosed with ≥50 VPCs/24 h had a significantly lower TAPSE (13.9 ± 4.04 mm) than the <50 VPCs/24 h group (16.8 ± 3.21 mm; P = .008, Table 2). TAPSE demonstrated weak to moderate correlation at the 5% significance level with the following variables: ejection fraction (r = 0.354, P = .017), fractional shortening (r = 0.424, P = .002), and LA:Ao (r = −0.448, P = .002). Dogs with LV systolic dysfunction had significantly lower TAPSE (14.1 ± 3.78 mm) than those with normal LV systolic function (16.8 ± 3.73 mm; P = .02). Also, dogs with detectable VT on Holter had lower TAPSE (12.9 ± 3.21 mm) than those without VT (16.1 ± 3.87 mm; P = .008; Fig 2).
Figure 1.
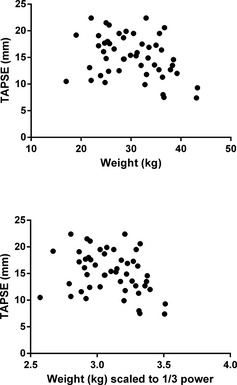
Scatter plots to show correlation between tricuspid annular plane systolic excursion (TAPSE) and weight. Both absolute weight (r = −0.324, P = .022) and weight scaled to the 1/3 power (r = −0.295, P = .038)22 exhibited a weak, negative correlation with TAPSE.
Figure 2.
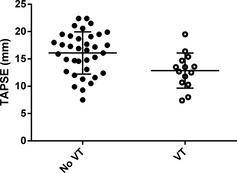
Scatter plots to illustrate the association of tricuspid annular plane systolic excursion (TAPSE) with Holter variables. TAPSE was significantly lower in dogs with ventricular tachycardia (VT) detected on Holter ECG (P = .008).
Survival Analysis
At the end of the study period, 26/50 (52%) Boxer dogs were dead; 20 dogs (40%) had suffered a cardiac death, 6 (12%) dogs died because of noncardiac causes, 20 (40%) remained alive, and 4 (8%) dogs were lost to follow‐up. Median survival time to cardiac death for all dogs was 440 days (6–2,083 days).
The presence of ≥50 VPCs on 24 hour Holter (yes versus no) and the presence of systolic dysfunction (present versus absent) were included in the Cox proportional hazards analysis as separate variables. The results of univariable Cox proportional hazards analysis are shown in Table 3. Lower TAPSE (<15.1 mm; cut‐off based upon a threshold effect seen when dichotomizing either side of median TAPSE value for the whole population, as described above) was significantly associated with a shorter time to cardiac death (hazard ratio 6.1, 95% confidence intervals 1.8–21; P = .004; Fig 3). In the 19 dogs without echocardiographic evidence of LV systolic dysfunction, TAPSE remained significantly associated with a shorter time to cardiac death (P = .035; Fig 4).
Table 3.
Results of univariable Cox proportional hazards analysis to evaluate factors associated with a shorter time to cardiac death
| Factor | Hazard Ratio | 95% Confidence Intervals | P Value |
|---|---|---|---|
| TAPSE (≥15.1 mm) | Ref. | ||
| TAPSE (<15.1 mm) | 6.1 | 1.8–21.0 | .004 |
| Weight: continuous per +1 kg | 1.1 | 1.0–1.2 | .028 |
| Sex: Male = No | Ref. | ||
| Sex: Male = Yes | 1.8 | 0.7–4.5 | .23 |
| ARVC diagnosis = no | Ref. | ||
| ARVC diagnosis = yes | 7.3 | 2.1–25.5 | .002 |
| LV systolic dysfunction = No | Ref. | ||
| LV systolic dysfunction = Yes | 5.9 | 1.3–26.1 | .019 |
| LA:Ao (<1.76) | Ref. | ||
| LA:Ao (≥1.76) | 2.4 | 0.9–6.8 | .088 |
| Presence of CHF = No | Ref. | ||
| Presence of CHF = Yes | 5.7 | 2.1–16.0 | .001 |
| Presence of VT = No | Ref. | ||
| Presence of VT = Yes | 5.6 | 2.2–14.2 | <.001 |
TAPSE, tricuspid annular plane systolic excursion; VPCs, ventricular premature complexes; LV, left ventricle; LA, left atrium; Ao, aorta; CHF, congestive heart failure; VT, ventricular tachycardia.
Figure 3.
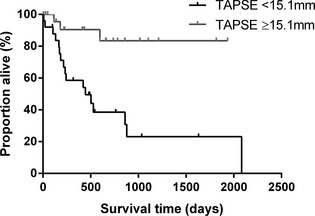
Kaplan‐Meier curve to show the effect of tricuspid annular plane systolic excursion (TAPSE) on survival time to cardiac death. Boxer dogs with TAPSE below the population median (<15.1 mm) had a significantly shorter survival time to cardiac death than those with higher TAPSE measurements (P < .001).
Figure 4.
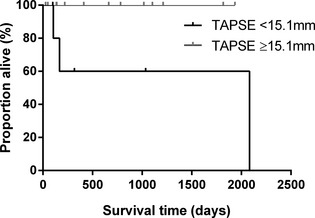
Kaplan‐Meier curve to show the effect of tricuspid annular plane systolic excursion (TAPSE) on survival time to cardiac death in dogs without echocardiographic evidence of left ventricular systolic dysfunction. Dogs with TAPSE below the population median (<15.1 mm) had a significantly shorter survival time to cardiac death than those with higher TAPSE measurements (P = .035).
Variables carried forward to the multivariable Cox analysis were: TAPSE (<15.1 mm), weight (continuous variable), presence of ≥50VPCs on 24 hour Holter, LV systolic dysfunction, LA:Ao ratio (≥1.76), CHF (yes) and VT (yes). Because 20 cardiac deaths were recorded, multivariable models were limited to a maximum of 2 factors. To avoid violation of the assumptions of Cox proportional hazards analysis, we chose not to include the combination of 2 echocardiographic variables (specifically LA size and LV systolic dysfunction) in the same model.
Multivariable analysis (Table 4) suggested that TAPSE was associated with a shorter time to cardiac death, independent of the presence of CHF, systolic dysfunction, and detectable VT. Weight and age each lost statistical significance when included with TAPSE <15.1 mm, which remained significantly associated with a shorter survival time.
Table 4.
Results of multivariable Cox proportional hazards analysis, performed to evaluate the association of tricuspid annular plane systolic excursion (TAPSE) on time to cardiac death when controlling for the effect of other measured variables. Reduced TAPSE lost statistical significance when patients had R‐on‐T phenomenon detected on Holter, but did demonstrate a significant association with shorter survival time when controlling for the presence of congestive heart failure (CHF) and detection of ventricular tachycardia (VT)
| Parameter | Hazard Ratio | 95% Confidence Intervals | P Value | |
|---|---|---|---|---|
| Model 1 | TAPSE <15.1 mm | 4.09 | 1.15–14.5 | .029 |
| ARVC diagnosis | 5.18 | 1.43–18.7 | .012 | |
| Model 2 | TAPSE <15.1 mm | 4.64 | 1.27–16.9 | .02 |
| CHF (yes) | 3.21 | 1.11–9.29 | .031 | |
| Model 3 | TAPSE <15.1 mm | 4.37 | 1.23–15.5 | .023 |
| LV systolic dysfunction | 3.69 | 0.81–16.9 | .093 | |
| Model 4 | TAPSE <15.1 mm | 4.36 | 1.22–15.6 | .023 |
| VT detected | 4.05 | 1.54–10.7 | .005 |
Discussion
Our results show that measurement of TAPSE in Boxer dogs with suspected ARVC provides useful prognostic information, in addition to a 24‐hour Holter recording. Multivariable analysis showed that TAPSE <15.1 mm remained significantly associated with a shorter time to an end‐point of cardiac death, even when controlling for the presence of CHF, Holter detection of VT, and echocardiographic evidence of LV systolic dysfunction. This is similar to findings in humans, where an absolute TAPSE measurement <18.1 mm is associated with a greater incidence of cardiac mortality in dogs with heart failure.25 Although our data suggested that TAPSE exhibited weak to moderate correlation with echocardiographic estimates of LV systolic function, when dogs with identifiable LV systolic dysfunction were excluded from survival analysis, dogs with TAPSE <15.1 mm still had a significantly shorter survival time than those with higher TAPSE. Despite a small sample size and low event rate in this group of dogs, our analysis did show a significant difference, suggesting that this is a genuine finding which merits further prospective study and validation in a larger cohort of Boxer dogs.
This study indicates that measurement of TAPSE is straightforward and repeatable, with acceptable intraobserver variability. However, during our inclusion of dogs for this study, we discovered that 31/81 echocardiographic studies (38%) from suitable Boxer dogs were excluded from analysis because of poor alignment or image quality. This is at least in part a consequence of the retrospective nature of this study using previously acquired images. Prospective acquisition of suitable images for TAPSE measurement was successfully performed in 80 dogs over a wide range of body weights and breeds in 1 recent study.1 That said, the acquisition of appropriate images for measurement of TAPSE might be prone to error if performed by an inexperienced operator or in a poorly compliant dog.
Our results showed a weak negative correlation between TAPSE and weight (absolute and scaled to the 1/3 power, Fig 1). Interestingly, a previous study reported a positive correlation in a population of normal dogs of different breeds and a greater weight range.22 This discrepancy between our data and theirs is difficult to explain but might reflect the different populations used and in particular the fact that our population consisted of dogs with myocardial disease. Our study design does not allow us to explain why heavier dogs in this population might have had lower TAPSE values. Despite this correlation reaching statistical significance, we elected to analyze TAPSE as an absolute measurement rather than indexing for body weight as previously described.22 This decision was made because the correlation was weak and the scatter plots of this data suggested that it was unlikely to be clinically relevant. We also felt that an absolute measurement would have greater clinical utility than a calculated indexed value. We believe that this is justified in Boxer dogs, because the range of body sizes among dogs of 1 breed is more limited than that evaluated by a previous study across different breeds.22 Another study used a single cut‐off value of LVIDs >35 mm to quantify systolic dysfunction in Boxers,5 and similar single measurements are commonly used among veterinary cardiologists without indexing to a measure of body size.5, 8, 10, 26 Body weight was also not significantly different between the ≥50 and <50 VPCs/24 h groups in our study, and although body weight was significantly associated with outcome at the univariable level, this association did not remain present at the multivariable level.
There is currently no definitive consensus among veterinary cardiologists as to how ARVC in Boxer dogs is best diagnosed.3, 7, 27, 28, 29 We acknowledge that our cut‐off value of 50 VPCs on 24‐hour Holter analysis is a low threshold for ARVC diagnosis. However, it is more likely to include Boxer dogs that are in the early stage of the disease. Evidence suggests that arrhythmias may precede the development morphologic abnormalities in dogs with ARVC.30 Therefore, in Boxer dogs that present for investigation of clinical signs, our TAPSE findings should provide clinicians with a more powerful prognostic indicator of survival, irrespective of VPC number. Our results suggest that TAPSE is a clinically useful measurement, giving additional relevant information whichever cut‐off value the clinician should choose to diagnose ARVC.
Day‐to‐day variation of VPC number on Holter can be >80% in dogs with ARVC,31 and there can be significant annual variation in the number of VPCs detected.3 Some dogs with a diagnosis of ARVC have fewer than 50 VPCs the year after a Holter showing ≥300 VPCs, despite receiving no antiarrhythmic medication whatsoever.3 It would therefore be interesting to evaluate TAPSE in a longitudinal fashion in a similar cohort of dogs.
Other limitations of this study are typical for any retrospective data analysis. Boxer dogs had diagnostic tests and treatment provided based upon individual decision making of the primary clinician managing each case at the time it is presented. Differences in treatment and diagnostic protocol could not be accounted for in our analysis. An inability to obtain 3 consecutive TAPSE measurements in 46% of stored images was the result of frequent ventricular arrhythmias in many of the Boxer dogs and could have impacted on the accuracy of the TAPSE measurement. However despite this, TAPSE remained significant following multivariable analysis when applied to affected dogs in a working clinical environment. Misclassification of clinical status, such as the presence/absence of CHF, and outcome (cardiac versus noncardiac death) are possible, despite robust criteria. A further limitation is that these dogs were placed on multiple different treatment protocols, which may have altered over time as their disease progressed. The study was not designed to assess the effect of medication on TAPSE.
Because of its complex geometry with dense trabeculation and load dependence, the RV is challenging to measure accurately using echocardiography.12 Despite this, TAPSE appears to be a reasonable, indirect estimate of RV function and was significantly associated with outcome in our study. As further imaging techniques are studied to evaluate the right heart, including evaluating RV myocardial strain or RV geometrical changes by 3D ultrasound technology and MRI techniques, more accurate measures of RV function may be validated for use in dogs. However, TAPSE has the benefit of accessibility to a greater number of practitioners, being simple to perform with standard echocardiographic equipment and an appropriate level of operator experience.
In conclusion, our results indicate that TAPSE <15.1 mm in Boxer dogs with ≥50 VPCs/24 h on Holter analysis is associated with a shorter time to cardiac death, irrespective of the presence of CHF, echocardiographic evidence of LV systolic dysfunction, and Holter detection of VT. This easy to obtain and repeatable measurement therefore gives the clinician valuable prognostic information in addition to that provided by Holter analysis alone.
Acknowledgments
The authors thank Yu‐Mei Chang for her statistical advice.
Funding: This research was not supported by a grant or any other source of funding. This data have not been presented at any congress or meeting.
Conflict of Interest Declaration: Drs. Connolly, Luis Fuentes, and Borgeat have performed consultancy work for Boehringer Ingelheim, Novartis Animal Health, and Royal Canin.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Where the work was done: Royal Veterinary College.
Footnotes
Visser LC, Scansen BA, Brown NV, et al. ACVIM Forum Research Abstracts Program. J Vet Intern Med. 2014;28:1000. (abstract C.2).
Vivid 7 with EchoPac off‐line measurement software, GE systems, Hatfield, United Kingdom.
IBM® SPSS® Statistics Version 21; for Windows 7, IBM (UK) Ltd, Portsmouth, UK.
GraphPad Prism Version 6.0; for Windows 7, GraphPad Software Inc. Sandiego, CA, USA.
References
- 1. Marcus FI, McKenna WJ, Sherrill D, et al. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: Proposed modification of the task force criteria. Circulation 2010;121:1533–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Meurs KM, Stern JA, Sisson DD, et al. Association of dilated cardiomyopathy with the striatin mutation genotype in Boxer dogs. J Vet Intern Med 2013;27:1437–1440. [DOI] [PubMed] [Google Scholar]
- 3. Meurs KM, Stern JA, Reina‐Doreste Y, et al. Natural history of arrhythmogenic right ventricular cardiomyopathy in the Boxer dog: A prospective study. J Vet Intern Med 2014;28:1214–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Basso C, Fox PR, Meurs KM, et al. Arrhythmogenic right ventricular cardiomyopathy causing sudden cardiac death in Boxer dogs: A new animal model of human disease. Circulation 2004;109:1180–1185. [DOI] [PubMed] [Google Scholar]
- 5. Palermo V, Johnson MJS, Sala E, et al. Cardiomyopathy in Boxer dogs: A retrospective study of the clinical presentation, diagnostic findings and survival. J Vet Cardiol 2011;13:45–55. [DOI] [PubMed] [Google Scholar]
- 6. Harpster NK. Boxer Cardiomyopathy In: Kirk R, ed. Current Veterinary Therapy VIII. Philadelphia, PA: WB Saunders; 1983:329–337. [Google Scholar]
- 7. Mõtsküla PF, Linney C, Palermo V, et al. Prognostic value of 24‐hour ambulatory ECG (Holter) monitoring in Boxer dogs. J Vet Intern Med 2013;27:904–912. [DOI] [PubMed] [Google Scholar]
- 8. Saguner AM, Vecchiati A, Baldinger SH, et al. Different prognostic value of functional right ventricular parameters in arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circ Cardiovasc Imaging 2014;7:230–239. [DOI] [PubMed] [Google Scholar]
- 9. Dini FL, Conti U, Fontanive P, et al. Right ventricular dysfunction is a major predictor of outcome in patients with moderate to severe mitral regurgitation and left ventricular dysfunction. Am Heart J 2007;154:172–179. [DOI] [PubMed] [Google Scholar]
- 10. Gupta S, Khan F, Shapiro M, et al. The associations between tricuspid annular plane systolic excursion (TAPSE), ventricular dyssynchrony, and ventricular interaction in heart failure patients. Eur J Echocardiogr 2008;9:766–771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Mercer‐Rosa L, Parnell A, Forfia PR, et al. Tricuspid annular plane systolic excursion in the assessment of right ventricular function in children and adolescents after repair of tetralogy of Fallot. J Am Soc Echocardiogr 2013;26:1322–1329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Haddad F, Hunt SA, Rosenthal DN, et al. Right ventricular function in cardiovascular disease, part I: Anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation 2008;117:1436–1448. [DOI] [PubMed] [Google Scholar]
- 13. Ueti OM, Camargo EE, de A Ueti A, et al. Assessment of right ventricular function with doppler echocardiographic indices derived from tricuspid annular motion: Comparison with radionuclide angiography. Heart 2002;88:244–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Forfia PR, Fisher MR, Mathai SC, et al. Tricuspid annular displacement predicts survival in pulmonary hypertension. Am J Respir Crit Care Med 2006;174:1034–1041. [DOI] [PubMed] [Google Scholar]
- 15. Lobo JL, Holley A, Tapson V, et al. Prognostic significance of tricuspid annular displacement in normotensive patients with acute symptomatic pulmonary embolism. J Thromb Haemost 2014;12:1020–1027. [DOI] [PubMed] [Google Scholar]
- 16. Finocchiaro G, Knowles JW, Pavlovic A, et al. Prevalence and clinical correlates of right ventricular dysfunction in patients with hypertrophic cardiomyopathy. Am J Cardiol 2014;113:361–367. [DOI] [PubMed] [Google Scholar]
- 17. López‐Candales A, Rajagopalan N, Saxena N, et al. Right ventricular systolic function is not the sole determinant of tricuspid annular motion. Am J Cardiol 2006;98:973–977. [DOI] [PubMed] [Google Scholar]
- 18. Baumwart RD, Meurs KM, Bonagura JD. Tei index of myocardial performance applied to the right ventricle in normal dogs. J Vet Intern Med 2005;19:828–832. [DOI] [PubMed] [Google Scholar]
- 19. Pariaut R, Saelinger C, Strickland KN, et al. Tricuspid annular plane systolic excursion (TAPSE) in dogs: Reference values and impact of pulmonary hypertension. J Vet Intern Med 2012;26:1148–1154. [DOI] [PubMed] [Google Scholar]
- 20. Bussadori C, Amberger C, Le Bobinnec G, et al. Guidelines for the echocardiographic studies of suspected subaortic and pulmonic stenosis. J Vet Cardiol 2000;2:15–22. [DOI] [PubMed] [Google Scholar]
- 21. Hansson K, Häggström J, Kvart C, et al. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in Cavalier King Charles Spaniels with and without left atrial enlargement. Vet Radiol Ultrasound 2002;43:568–575. [DOI] [PubMed] [Google Scholar]
- 22. Thomas WP, Gaber CE, Jacobs GJ, et al. Recommendations for standards in transthoracic two‐dimensional echocardiography in the dog and cat. J Vet Intern Med 1993;7:247–252. [DOI] [PubMed] [Google Scholar]
- 23. Sahn DJ, DeMaria A, Kisslo J, et al. Recommendations regarding quantitation in M‐mode echocardiography: Results of a survey of echocardiographic measurements. Circulation 1978;58:1072–1083. [DOI] [PubMed] [Google Scholar]
- 24. Boon JA. Veterinary Echocardiography, 2nd ed West Sussex, UK: John Wiley & Sons; 2011:206–207. [Google Scholar]
- 25. Vizzardi E, D'Aloia A, Bordonali T, et al. Long‐term prognostic value of the right ventricular myocardial performance index compared to other indexes of right ventricular function in patients with moderate chronic heart failure. Echocardiography 2012;29:773–778. [DOI] [PubMed] [Google Scholar]
- 26. Smets P, Daminet S, Wess G. Simpson's Method of Discs for Measurement of Echocardiographic End‐Diastolic and End‐Systolic Left Ventricular Volumes: Breed‐Specific Reference Ranges in Boxer Dogs. J Vet Intern Med 2014;28:116–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Stern JA, Meurs KM, Spier AW, et al. Ambulatory electrocardiographic evaluation of clinically normal adult Boxers. J Am Vet Med Assoc 2010;236:430–433. [DOI] [PubMed] [Google Scholar]
- 28. Spier AW, Meurs KM. Assessment of heart rate variability in Boxers with arrhythmogenic right ventricular cardiomyopathy. J Am Vet Med Assoc 2004;224:534–537. [DOI] [PubMed] [Google Scholar]
- 29. Oxford EM, Danko CG, Fox PR, et al. Change in β‐catenin localization suggests involvement of the canonical Wnt pathway in Boxer dogs with arrhythmogenic right ventricular cardiomyopathy. J Vet Intern Med 2013;28:92–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Baumwart RD, Meurs KM, Raman SV. Magnetic resonance imaging of right ventricular morphology and function in Boxer dogs with arrhythmogenic right ventricular cardiomyopathy. J Vet Intern Med 2009;23:271–274. [DOI] [PubMed] [Google Scholar]
- 31. Spier AW, Meurs KM. Evaluation of spontaneous variability in the frequency of ventricular arrhythmias in Boxers with arrhythmogenic right ventricular cardiomyopathy. J Am Vet Med Assoc 2004;22:538–541. [DOI] [PubMed] [Google Scholar]


