Abstract
Background
Several risk factors already have been determined for dogs with degenerative mitral valve disease (DMVD). Risk factors often have been considered in isolation and have not always taken into account additional information provided by the history and physical examination (PE).
Hypothesis/Objectives
Data obtained from history and PE of dogs with DMVD provide prognostic information and can be used for risk stratification.
Animals
Client‐owned dogs (n = 244) with DMVD recruited from first opinion practice.
Methods
Prospective longitudinal follow‐up of dogs with DMVD. History and PE data were obtained at 6‐month intervals and analyzed with time‐dependent Cox models to derive relative risk of cardiac death. Independent hazard ratios were used to derive a clinical severity score (CSS), the prognostic value of which was evaluated by analyzing the median survival times for different risk groups and ROC analysis. Analysis of the progression of CSS over time also was undertaken.
Results
History of cough, exercise intolerance, decreased appetite, breathlessness (difficulty breathing) and syncope with PE findings of heart murmur intensity louder than III/VI and absence of respiratory sinus arrhythmia were independently associated with outcome and allowed development of the CSS. Clinical severity score distinguished groups of dogs with significantly different outcomes.
Conclusions and Clinical Importance
Routinely obtained clinical findings allow risk stratification of dogs with DMVD. Results of ancillary diagnostic tests may be complementary to history and PE findings and always should be interpreted in conjunction with these findings.
Keywords: Clinical diagnosis, History, Natriuretic peptide, Physical examination, Survival analysis
Abbreviations
- CHF
congestive heart failure
- CKCS
Cavalier King Charles spaniel
- CSS
clinical severity score
- DMVD
degenerative mitral valve disease
- HR
hazard ratio
- IQR
interquartile range
- MST
median survival time
- NT‐proBNP
N‐terminal pro B‐type natriuretic peptide
- PE
physical examination
- ROC
receiving operator characteristics
Degenerative mitral valve disease (DMVD) is a common condition, accounting for approximately 75% of cases of cardiac disease in dogs.1, 2, 3 The majority of DMVD cases are diagnosed and treated in first opinion practice. Clinicians detect evidence of DMVD from early stages because of the consistent presence of a characteristic heart murmur readily identified on physical examination (PE). Therefore, the challenge in managing patients with DMVD generally is not in diagnosis but rather in how to determine which patients have more advanced disease and therefore are at risk of dying of their heart disease.
Previous studies have identified prognostic risk factors.3, 4, 5, 6, 7, 8, 9, 10, 11 These studies consistently have demonstrated that increased N‐terminal pro B‐type natriuretic peptide (NT‐proBNP) concentrations and enlarged heart size indicate dogs at higher risk of developing congestive heart failure (CHF) and cardiac death. These studies simultaneously evaluated the predictive ability of numerous indices obtained from history, PE and ancillary diagnostic tests, ignoring the fact that these data usually are acquired sequentially. Therefore, results of these studies can only be applied prospectively to patients once all of the results are obtained, necessitating that all of the predictive tests are performed in all patients. A problem encountered by first opinion practitioners is that some of these indices, particularly those derived from echocardiography, can only be obtained by undertaking diagnostic tests that require substantial expertise to perform proficiently, represent considerable cost to the client and necessitate access to expensive diagnostic equipment or referral of the dog to a specialist.
Clinical investigations usually occur in a standard sequence: history taking is followed by PE after which ancillary diagnostic tests are chosen. The results of the history taking and PE therefore are known by the clinician before deciding to undertake any additional diagnostic tests. Moreover, the interpretation of the test results is made in light of prior knowledge of the nature and severity of the patient's disease. This prior knowledge informs the clinician's sense of the “pre‐test probability” of a patient having clinically relevant disease and, after evaluating the results of any tests performed, the clinician can develop a sense of the “post‐test probability”.12
The aim of this study was to examine the ability of information obtained as a part of routine history taking and PE to predict cardiac mortality in a population of dogs with DMVD. Furthermore, we aimed to use those clinical and historical factors shown to be independently predictive of a worse outcome to create a clinical severity score (CSS). The CSS could be used to categorize dogs before interpreting results of ancillary diagnostic tests. Our hypothesis was that the CSS would independently predict outcome.
Materials and Methods
This prospective longitudinal study had permission from the Royal Veterinary College Welfare and Ethics committee and informed consent was obtained from all of the owners before enrolment in the study. The veterinarians from 2 first‐opinion practices in London referred client‐owned dogs to a research clinic for evaluation and collection of data. The first‐opinion clinicians remained in charge of the management of the cases at all times. Data were collected from December 2004 to the 15th of January 2014. Inclusion criteria were the presence of a left apical systolic murmur suggestive of DMVD with echocardiographic confirmation of mitral regurgitation caused by degenerative valvular changes (eg, valvular thickening, prolapse or both). Exclusion criteria were the presence of other cardiac or noncardiac illnesses at presentation. Animals that developed comorbidities during the study period were not excluded.
Owners were invited to participate in the study after detection of the cardiac murmur and dogs were followed‐up approximately every 6 months until they died, were euthanized or were withdrawn from the study. History, PE and ancillary diagnostic tests always were obtained in the same order and according to the same protocol as described previously.10, 13, 14 Thoracic radiographs were not obtained as part of the study but were recommended to the primary clinician when deemed necessary; the presence of CHF was suspected on the basis of the combination of clinical signs, echocardiographic measurements, increased NT‐proBNP concentrations and response to treatment.
Owners were questioned about the presence of cough, exercise intolerance, breathlessness (difficulty breathing), changes in appetite and body weight over the previous 6 months and whether or not the dog had experienced syncope. All answers were recorded as yes/no but the owners’ comments and descriptions also were noted. Physical examination included body condition score, murmur description, heart rate and rhythm (as regular or subjectively consistent with respiratory sinus arrhythmia), respiratory rate, presence of dyspnea or cough on examination, presence of ascites, and other comments about cardiovascular signs and signs from other systems. All data were collected by the same investigator (AB) and archived in paper files and then transferred by a qualified veterinary nurse to a customized electronic database.1
Statistical Analysis
Statistical analyses were performed using commercially available software2 , 3 and freeware.4 For the construction of the different models, the assumptions were verified as required. The level of significance was set at 5% for all analyses.
The cause of death was divided into cardiac‐related and noncardiac causes, and the latter right‐censored (as nonevents) at the time of death to account for cardiac mortality only. Patients were considered to have experienced cardiac‐related death when no other cause of death was known or suspected and death occurred after a variable period of progressive cardiac remodeling in conjunction with increased NT‐proBNP concentration. Clinical evidence of CHF including the presence of body cavity effusions (ascites, pleural effusion or pericardial effusion), lung crackles on auscultation or radiographic evidence of CHF was considered to support the assumption that death was cardiac‐related. Response to appropriate treatment for CHF also was considered supportive evidence of a cardiac cause for the patient's death. Sudden death was defined as any unexpected natural death, and was classified as cardiac‐related death unless another reason was known. Dogs also were right‐censored if they were lost to follow‐up or alive at the end of the study. Dogs were considered lost to follow‐up if they had not been presented at the research clinic or the first‐opinion practice for >9 months, and the last date of presentation was assigned as their right‐censoring date.
For ease of clinical use, the continuous variables age, body weight and heart rate at presentation were dichotomized: the cut‐off value was determined from receiver operating characteristics (ROC) curves as the point that provided the highest mean of sensitivity and specificity to identify cardiac mortality. Body condition score was categorized as suboptimal (<3/5) or adequate (≥3/5). The breed of the dogs also was categorized as Cavalier King Charles spaniel (CKCS): yes or no.
Effects of Historical and PE Data on Time to Cardiac Death
A time‐dependent Cox model was used to assess the association between risk of cardiac death and the explanatory variables. Survival interval was defined as the time between consultations or between last consultation and right‐censoring date or date of death caused by DMVD. The risk of each time interval was calculated using the command: “coxph (surv (start, end, status))” from the freeware survival analysis package, where start, end and status referred to the starting and ending time of each time interval and status was coded as either event (cardiac‐related death) or censored. Variables with P < .10 in the univariable analysis were included in a manual backward stepwise manner to build the multivariable regression model for survival (named herein as risk score multivariable model). Two‐way interactions were assessed for all of the significant covariates present in the final risk score multivariable model by checking the effect these interactions had 1 by 1 on the final model. Risk was quantified as hazard ratio (HR) with 95% confidence interval.
Derivation of Clinical Severity Score
The CSS for each dog on each visit was generated using the HR derived from all factors shown to be significant independent predictors of outcome in the multivariable survival analysis. When a risk factor was present, the dog was assigned the value of the HR; when a factor was not present the dog was assigned a value of 1. The product of the resulting scores for all independently predictive historical and clinical covariates was generated and counted as the CSS for that dog on that visit. For ease of handling and interpretation, base 2 log transformation was applied before further analysis.
Assessment of the CSS
The number of independently predictive clinical signs present at a dog's first visit was used to classify all dogs into 4 groups: very low risk (no risk factors), low risk (1–2 risk factors), intermediate risk (3–4 risk factors) and high risk (≥5 risk factors). Considering only cardiac‐related mortality, median survival times for these 4 groups were estimated using the Kaplan–Meier method and compared using the log rank test.
For the assessment of the prognostic capability of 1‐year cardiac mortality from first consultation, CSS at first presentation was assessed using ROC curve analysis. The point that provided the highest mean of sensitivity and specificity to identify cardiac mortality was used as cut‐off and the likelihood ratios were calculated.
Progression of CSS Over Time
For the assessment of the progression of individual CSS over time, 3 groups of dogs were assessed: those that experienced cardiac‐related death, those that died of other causes and those that remained alive, as described previously14 and explained in Data S1.
Results
From December 2004 to January 2014, 1,217 visits of 374 dogs were recorded. From these, 63 dogs were presented only at the first evaluation and no further information was available from them, 5 dogs were diagnosed with causes other than DMVD for their murmurs, and 62 were age‐ and weight‐matched dogs followed‐up as normal controls but not affected by DMVD, resulting in a total of 130 dogs excluded from the study. The remaining 244 dogs (138 males and 106 females) had a mean age of 10.4 ± 2.9 years and median body weight of 10.0 kg (IQR, 7.6–12.9) at presentation. These included: 97 CKCS, 51 cross breeds, 22 Jack Russell terriers, 16 Yorkshire terriers, 9 each Chihuahuas and Shih‐Tzus, 5 Pomeranians, 4 Poodles, 3 Bichon Frises, 2 each Chinese crested, Lurchers, Malteses, Norfolk terriers, Staffordshire Bullterriers, Tibetan terriers and Whippets and 1 each of 13 other pure breeds. These dogs were examined a median number of 4 times (range, 1–14).
Thirty‐five dogs were presented in CHF as defined by the study criteria. At the end of the study, 66 dogs had died of their cardiac disease, 78 dogs had died of noncardiac causes, 81 dogs were still alive and 19 were lost to follow‐up after a variable period of time. Of the 66 dogs that died of DMVD, 33 were euthanized because of worsening of clinical signs consistent with CHF and 33 dogs died suddenly. Forty‐nine of these 66 dogs (75%) had radiographic confirmation of heart disease as the cause of their clinical signs, 12 presented with cavity effusions attributed to advanced DMVD, 4 died suddenly due to acute decompensation of their CHF signs and 1 was euthanized because of progressive deterioration of its quality of life over a period of several months during which it was refractory to diuresis; all of the 66 dogs had cardiac enlargement and progressive increases in NT‐proBNP with increased concentration on the last consultation before death. The estimated MST for cardiac mortality of the 244 dogs was 1,977 days (95% CI, 1,751–2,203 days).
Results of the univariable analysis of individual factors recorded from history and PE are shown in Table 1. The risk score multivariable model then was constructed with the variables: CKCS (yes/no), history of cough, of exercise intolerance, of decreased appetite, of breathlessness (difficulty breathing) and of syncope; and PE assessment of body condition score, murmur grade, heart rate, heart rhythm (sinus arrhythmia/sinus rhythm) and presence of dyspnea. No interaction was detected between any pair of variables remaining in the final multivariable model. The resulting multivariable model is presented in Table 2.
Table 1.
Univariable time‐dependent Cox regression analysis of factors associated with cardiac mortality derived from the history and physical examination
| nDogs (nVisits) | HR (95% CI) | P‐Value | |
|---|---|---|---|
| Signalment | |||
| Age (>13.3 years old/≤13.3 years olda) | 68 (164)/212 (821) | 0.92 (0.47–1.79) | .806 |
| Body weight (>15 kg/<15 kga) | 42 (148)/209 (836) | 0.60 (0.26–1.40) | .240 |
| Sex (female /malea) | 106 (418)/138 (567) | 0.91 (0.54–1.49) | .718 |
| CKCS (yes/noa) | 97 (425)/147 (560) | 2.09 (1.27–3.43) | .004 |
| History | |||
| Cough (yes/noa) | 123 (306)/184 (679) | 7.69 (4.32–13.73) | <.001 |
| Exercise intolerance (yes/noa) | 54 (122)/232 (863) | 5.12 (3.07–8.55) | <.001 |
| Appetite (decreased/normala) | 49 (71)/238 (914) | 3.30 (1.79–6.10) | <.001 |
| Weight loss (yes/noa) | 143 (260)/219 (725) | 1.30 (0.77–2.20) | .320 |
| Breathlessness (yes/noa) | 141 (311)/196 (674) | 4.10 (2.45–6.86) | <.001 |
| Syncope (yes/noa) | 37 (58)/228 (927) | 2.73 (1.39–5.34) | .003 |
| Physical examination | |||
| Body Cond. score (<3/≥3a) | 111 (232)/222 (749) | 1.93 (1.16–3.19) | .011 |
| Murmur grade PE (>III/≤IIIa) | 156 (461)/169 (524) | 7.22 (3.68–14.18) | <.001 |
| Heart rate PE (>135/≤135a) | 156 (397)/178 (588) | 3.64 (2.15–6.16) | <.001 |
| Rhythm PE (SR/SAa) | 160 (376)/196 (609) | 3.81 (2.27–6.41) | <.001 |
| Dyspnea PE (yes/noa) | 31 (41)/232 (941) | 15.66 (8.72–28.14) | <.001 |
nDogs, total number of dogs with the factor at a particular visit; nVisits, total number of visits a factor was detected; HR, hazard ratio; CI, confidence interval; CKCS, Cavalier King Charles spaniel; PE, physical examination; SR, assumed sinus rhythm; SA, assumed sinus arrhythmia.
Shows the reference category for the dichotomous variables.
Variables with the p‐value in marked bold were considered significant at the univariable level.
Table 2.
Multivariable time‐dependent Cox regression analysis of factors independently associated with cardiac mortality derived from the history and physical examination
| Factors | HR (95% CI) | P‐Value |
|---|---|---|
| History of Cough (yes/noa) | 3.85 (2.11–7.03) | <.001 |
| History of exercise intolerance (yes/noa) | 2.50 (1.43–4.36) | .001 |
| History of appetite (decreased/normala) | 2.36 (1.26–4.43) | .007 |
| History of breathlessness (yes/noa) | 1.93 (1.09–3.41) | .024 |
| History of syncope (yes/noa) | 2.28 (1.15–4.52) | .018 |
| Murmur grade (>III/≤IIIa) | 3.08 (1.51–6.28) | .002 |
| Rhythm on PE (SR/SAa) | 2.33 (1.35–4.03) | .002 |
HR, hazard ratio; CI, confidence interval; PE, physical examination; SR, assumed sinus rhythm; SA, assumed sinus arrhythmia.
Shows the reference category for the dichotomous variables.
According to the risk score multivariable model HRs, the CSS was calculated for each dog at every consultation. Dogs with no negative prognostic factors from the risk score multivariable model had a log2 (CSS) score = 0; dogs with 1 factor had a score between 0.9 and 1.9; 2 factors = 2.1–3.6; 3 factors = 3.4–4.9; 4 factors = 5.0–6.1; 5 factors = 6.4–7.3; and 6 factors = 8.2–8.3. None of the dogs had all 7 factors at any of the visits. Taking only the observations from the first visit for each dog, and grouping them as very low risk, low risk, intermediate risk and high risk, there was a significant difference among groups over time in the proportion that had not experienced cardiac mortality (P < .001; Table 3 and Fig 1).
Table 3.
Median survival time for the four severity groups according to the number of risk factors shown at presentation
| Risk Factors | n | Median Survival Time (95% CI) | IQR | Statistical Comparison | |
|---|---|---|---|---|---|
| Very low | None | 63 | nd | nd | A |
| Low | 1 or 2 | 114 | 1,977 (nd) | nd | A |
| Intermediate | 3 or 4 | 57 | 1,013 (17–2,009) | 312–1,954 | B |
| High | 5 or more | 10 | 167 (19–314) | 96–980 | C |
| Overall | 244 | 1,977 (1,751–2,202) |
A significant difference between the groups is indicated by different letters.
CI, confidence interval; IQR, interquartile range; nd, not determined.
Figure 1.
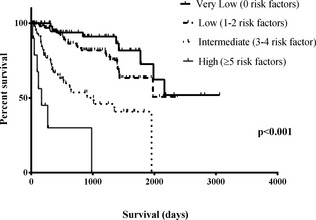
Kaplan–Meier survival curves of dogs categorized according to the number of risk factors manifested at first presentation.
A total of 31 dogs died of cardiac‐related causes within the first 365 days after initial presentation (median, 171 days; IQR, 106–304 days). The area under the ROC curve for the individual CSS at presentation to detect 1‐year cardiac mortality was 0.83 (95% CI, 0.74–0.91), with sensitivity = 0.81 and specificity = 0.77 for the log2 transformed CSS = 3.30. Using this cut‐off value, the positive likelihood ratio was 3.51 and the negative likelihood ratio 0.25.
For the assessment of the progression of the CSS over time, 125 dogs met the inclusion criteria: 33 dogs in Group 1, 44 dogs in Group 2 and 48 dogs in Group 3 (42 alive and 6 lost to follow‐up). After graphical assessment of the progression of the log2 (CSS) over time (Fig 2), a linear repeated measures model showed that score was overall different among groups (P < .001) and among visits (P < .001). Post hoc analysis showed that the log2 (CSS) was higher at each visit for group 1 compared with the other 2 groups, and that there was no significant difference between groups 2 and 3 at any visit (Table 4).
Figure 2.
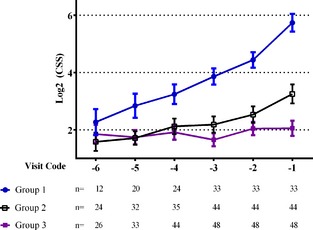
Graphic assessment of the progression of the mean individual clinical severity score (CSS) (standard error of the mean) over time in the 3 study groups. Group 1: dogs suffering cardiac death; Group 2: dogs dying of non‐cardiac causes; Group 3: dogs alive or lost to follow‐up.
Table 4.
Estimated adjusted mean ± SE for the individual log2 (CSS) for each group and visit
| Visit‐3 | Visit‐2 | Visit‐1 | Overall | |
|---|---|---|---|---|
| Group 1 | 3.86 ± 0.31 | 4.44 ± 0.31 | 5.74 ± 0.31 |
4.68 ± 0.27 A |
| Group 2 | 2.18 ± 0.27 | 2.53 ± 0.27 | 3.25 ± 0.27 |
2.65 ± 0.23 B |
| Group 3 | 1.65 ± 0.26 | 2.04 ± 0.26 | 2.05 ± 0.26 |
1.91 ± 0.22 B |
| Overall |
2.60 ± 0.16 A |
3.02 ± 0.16 B |
3.63 ± 0.16 C |
A significant difference was found between the groups with different letters.
Group 1: dogs suffering cardiac death; Group 2: dogs dying of non‐cardiac causes; Group 3: dogs alive or lost to follow‐up.
SE, standard error; CSS, clinical severity score.
Discussion
The findings support our original hypothesis. Several commonly observed historical and clinical findings in dogs with DMVD are independently predictive of a worse outcome. Affected dogs that die of their disease show a progressively higher number of these signs as their disease progresses, and finding several of these signs in combination is much more strongly predictive of a worse outcome than any individual sign in isolation.
Degenerative mitral valve disease is characterized by mitral valve insufficiency that compromises forward stroke volume and causes left‐sided volume overload eventually resulting in the activation of compensatory mechanisms. The presence of an apical systolic heart murmur usually is the first detectable clinical finding in patients with DMVD.15, 16 Decreased cardiac output may cause some of the initial clinical signs, which may only be present during circumstances that necessitate increased cardiac demand (ie, exercise intolerance). In small breed dogs, DMVD progresses slowly over months to years.1 The rate of progression is variable among dogs and many do not die of their DMVD.11 This was apparent in our population, in which from a total of 144 dogs that died, only 66 did so because of their cardiac disease, representing 46% of all deaths.
Our initial observation (subsequently supported by this study) was that small breed dogs with DMVD tend to develop several, sometimes subtle, clinical signs before they develop CHF as defined in our study. We therefore aimed to analyze factors that can be obtained in general veterinary practice before performing any ancillary diagnostic tests and that, under normal circumstances, consciously or sub‐consciously, are already guiding and dictating the clinician's subsequent decisions regarding the patient.
Risk stratification is the statistical process that enables detection and categorization of characteristics associated with increased probability of experiencing an unwanted outcome.17 Several risk factors for CHF and cardiac death for dogs with DMVD already have been reported in the veterinary literature, including age, sex, breed, murmur intensity, cardiac silhouette size on thoracic radiographs, echocardiographically detected severity of valvular changes, degree of mitral regurgitation, left atrial size, left ventricular size in diastole, transmitral flow pattern and circulating concentrations of cardiac biomarkers.5, 7, 8, 10, 11, 13, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27 Some studies have shown that a higher heart rate, exercise intolerance, increased respiratory effort, dyspnea, cough, syncope and arrhythmias are significant predictors of worse outcome at the univariable level but these factors do not consistently appear in final multivariable models of different studies.6, 11, 28
Analyzing all of the data routinely collected from history and PE, we found that all of those we examined, except for weight loss, were associated with cardiac mortality at the univariable level (Table 1). Our multivariable model identified 7 independent risk factors that allowed us to develop a clinical scoring system of the patients’ disease severity. The CSS had an accuracy determined by ROC analysis of 83% (95% CI, 74–91%) for the prediction of cardiac‐related death in the following year in our population. Moreover, the likelihood ratio confirms that the CSS system is helpful to predict which dogs are more likely to die of their DMVD, and also which are unlikely to do so. This implies that the information collected by a clinician before choosing to perform any ancillary diagnostic test is already informative about the prognosis and illustrates the importance of performing a thorough clinical evaluation of cases before undertaking additional tests.
The subsequent analysis of the change over time in the CSS in dogs that ultimately die of their DMVD indicates a gradual increase, implying an increase in the number of clinical signs that manifest over a period of a few years. Dogs with DMVD that die of noncardiac diseases tend to develop fewer clinical signs. Although some clinical signs of advanced heart disease are shared by other potentially life‐threatening conditions (eg, cough, loss of appetite, exercise intolerance), dogs that die of other disease develop a few, but not many, of the clinical signs associated with progressive DMVD. Their CSS therefore may increase before death but not to the same extent that it does in dogs dying of their heart disease.
Using a single aspect of history or PE to guide decision making is fraught with potential problems. The advantage of the CSS system is that it allows the integration of several aspects of the history and PE and therefore will have increased relevance in the clinical setting. We found that the development of cough during the progression of DMVD was strongly and independently associated with higher hazard of cardiac‐related death, having the highest HR of any single factor in the final multivariable model. A recent report found that cough in dogs with DMVD is not likely to be due to CHF.29 Most of the dogs that developed a cough during the clinical progress of their disease in our population did so at a much earlier stage than they developed overt signs of CHF. Therefore, the explanation that cough is caused by left atrial enlargement and concomitant airway abnormalities rather than overt CHF appears to be supported by these results. The development of cough in a patient with DMVD however still should be regarded as possible evidence of progression of that patient's disease.
As shown previously by our group, heart rate and heart rate variability change gradually with progression of disease.14 However, these factors also change in dogs with DMVD that die of noncardiac causes. The resulting conclusion is that these are insensitive prognostic markers for cardiac death because of DMVD, but possess excellent negative predictive value. This is reflected in the multivariable model in this study showing that dogs with a regular sinus rhythm have a higher risk of dying of DMVD than dogs that maintain their respiratory sinus arrhythmia on PE.
The severity of mitral regurgitation has been shown to be associated with the intensity of the resultant murmur.19, 30 In this study, we observed that a heart murmur intensity higher than III/VI is associated with a higher risk of cardiac mortality than a murmur of grade III/VI or lower, demonstrating that the intensity of the murmur is associated with outcome.
Certain weaknesses of this study should be acknowledged. One limitation is the subjective nature of the owners’ perceptions of a patient's clinical signs. This however reflects the reality of relying on observations made by clients in practice. Another limitation is that we did not ask owners to record resting respiratory rate at home. Published evidence suggests that the onset of CHF may be heralded by an increased respiratory rate.31 Dogs with subclinical heart disease have normal respiratory rates.32 This would therefore be a useful clinical sign to monitor in patients but would not be information available to a clinician on the initial presentation of a patient with DMVD, because clients would need to be instructed to obtain this information. Another limitation is that the period of time between visits was not the same for all dogs and that the exact date of appearance of clinical signs was not known. This may have influenced the results. The cumulative nature of the data analyzed however makes it unlikely that once 1 of these signs appeared the dog would not present with that sign on subsequent visits. A last limitation is that euthanasia represents a unique situation in veterinary medicine and a common factor influencing any veterinary study assessing survival. Euthanasia generally is requested by an owner who fears the pet no long has an adequate quality of life, but this does not necessarily reflect the severity of DMVD. As with any novel clinical prediction tool, the first development phase needs to be followed by a prospective validation before it can be adopted in clinical practice. Despite the above limitations, we consider that this model provides a basis for a validation study in a larger population in which the assessment of the impact of the implementation of this risk stratification system in practice can be evaluated.
In summary, this study shows that data obtained from history and PE, specifically history of cough, exercise intolerance, decreased appetite, breathlessness (difficulty breathing) and syncope as well as murmur grades louder than III/VI and loss of respiratory sinus arrhythmia, are independent indicators of disease progression for DMVD and are predictive of cardiac death. This knowledge can be developed into a scoring system to assist clinical decision making and may improve the interpretation of subsequent diagnostic test results for improved management of cases both in first opinion and referral practice.
Supporting information
Data S1. Progression of CSS over time.
Acknowledgments
This study was performed at the Royal Veterinary College, London, UK. This manuscript complies with the Royal Veterinary College's Good Research Practice Policy on Publications (manuscript number CSS_00762).
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: The authors declare no off‐label use of antimicrobials.
Footnotes
FileMaker Pro 11.0v3, Santa Clara, CA
IBM SPSS Statistics 20, Armonk, NY
GraphPad Prism 6, San Diego, CA
R version 3.0.2, R Core Team (2013). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. http://www.R-project.org/
References
- 1. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 2. Haggstrom J, Hoglund K, Borgarelli M. An update on treatment and prognostic indicators in canine myxomatous mitral valve disease. J Small Anim Pract 2009;50(Suppl 1):25–33. [DOI] [PubMed] [Google Scholar]
- 3. Borgarelli M, Haggstrom J. Canine degenerative myxomatous mitral valve disease: Natural history, clinical presentation and therapy. Vet Clin North Am Small Anim Pract 2010;40:651–663. [DOI] [PubMed] [Google Scholar]
- 4. Chetboul V, Serres F, Tissier R, et al. Association of plasma N‐terminal pro‐B‐type natriuretic peptide concentration with mitral regurgitation severity and outcome in dogs with asymptomatic degenerative mitral valve disease. J Vet Intern Med 2009;23:984–994. [DOI] [PubMed] [Google Scholar]
- 5. Lord P, Hansson K, Kvart C, Haggstrom J. Rate of change of heart size before congestive heart failure in dogs with mitral regurgitation. J Small Anim Pract 2010;51:210–218. [DOI] [PubMed] [Google Scholar]
- 6. Borgarelli M, Crosara S, Lamb K, et al. Survival characteristics and prognostic variables of dogs with preclinical chronic degenerative mitral valve disease attributable to myxomatous degeneration. J Vet Intern Med 2012;26:69–75. [DOI] [PubMed] [Google Scholar]
- 7. Pedersen HD, Lorentzen KA, Kristensen BO. Echocardiographic mitral valve prolapse in cavalier King Charles spaniels: Epidemiology and prognostic significance for regurgitation. Vet Rec 1999;144:315–320. [DOI] [PubMed] [Google Scholar]
- 8. Boswood A, Murphy A. The effect of heart disease, heart failure and diuresis on selected laboratory and electrocardiographic parameters in dogs. J Vet Cardiol 2006;8:1–9. [DOI] [PubMed] [Google Scholar]
- 9. Reynolds CA, Brown DC, Rush JE, et al. Prediction of first onset of congestive heart failure in dogs with degenerative mitral valve disease: The PREDICT cohort study. J Vet Cardiol 2012;14:193–202. [DOI] [PubMed] [Google Scholar]
- 10. Moonarmart W, Boswood A, Luis Fuentes V, et al. N‐terminal pro B‐type natriuretic peptide and left ventricular diameter independently predict mortality in dogs with mitral valve disease. J Small Anim Pract 2010;51:84–96. [DOI] [PubMed] [Google Scholar]
- 11. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 12. Gill CJ, Sabin L, Schmid CH. Why clinicians are natural bayesians. BMJ 2005;330:1080–1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hezzell MJ, Boswood A, Chang YM, et al. The combined prognostic potential of serum high‐sensitivity cardiac troponin I and N‐terminal pro‐B‐type natriuretic peptide concentrations in dogs with degenerative mitral valve disease. J Vet Intern Med 2012;26:302–311. [DOI] [PubMed] [Google Scholar]
- 14. López‐Alvarez J, Boswood A, Moonarmart W, et al. Longitudinal electrocardiographic evaluation of dogs with degenerative mitral valve disease. J Vet Intern Med 2014;28:393–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Haggstrom J, Kvart C, Hansson K. Heart sounds and murmurs: Changes related to severity of chronic valvular disease in the Cavalier King Charles spaniel. J Vet Intern Med 1995;9:75–85. [DOI] [PubMed] [Google Scholar]
- 16. Pedersen HD, Haggstrom J, Falk T, et al. Auscultation in mild mitral regurgitation in dogs: Observer variation, effects of physical maneuvers, and agreement with color Doppler echocardiography and phonocardiography. J Vet Intern Med 1999;13:56–64. [PubMed] [Google Scholar]
- 17. Miller CC, Reardon MJ, Safi HJ. Risk Stratification: A Practical Guide for Clinicians. Cambridge: Cambridge University Press; 2001. [Google Scholar]
- 18. Haggstrom J, Hansson K, Kvart C, Swenson L. Chronic valvular disease in the cavalier King Charles spaniel in Sweden. Vet Rec 1992;131:549–553. [PubMed] [Google Scholar]
- 19. Olsen LH, Martinussen T, Pedersen HD. Early echocardiographic predictors of myxomatous mitral valve disease in dachshunds. Vet Rec 2003;152:293–297. [DOI] [PubMed] [Google Scholar]
- 20. Haggstrom J, Hamlin RL, Hansson K, Kvart C. Heart rate variability in relation to severity of mitral regurgitation in Cavalier King Charles spaniels. J Small Anim Pract 1996;37:69–75. [DOI] [PubMed] [Google Scholar]
- 21. Haggstrom J, Hansson K, Kvart C, et al. Relationship between different natriuretic peptides and severity of naturally acquired mitral regurgitation in dogs with chronic myxomatous valve disease. J Vet Cardiol 2000;2:7–16. [DOI] [PubMed] [Google Scholar]
- 22. Fujii Y, Wakao Y. Spectral analysis of heart rate variability in dogs with mild mitral regurgitation. Am J Vet Res 2003;64:145–148. [DOI] [PubMed] [Google Scholar]
- 23. Kittleson MD, Brown WA. Regurgitant fraction measured by using the proximal isovelocity surface area method in dogs with chronic myxomatous mitral valve disease. J Vet Intern Med 2003;17:84–88. [DOI] [PubMed] [Google Scholar]
- 24. Doxey S, Boswood A. Differences between breeds of dog in a measure of heart rate variability. Vet Rec 2004;154:713–717. [DOI] [PubMed] [Google Scholar]
- 25. DeFrancesco TC, Rush JE, Rozanski EA, et al. Prospective clinical evaluation of an ELISA B‐type natriuretic peptide assay in the diagnosis of congestive heart failure in dogs presenting with cough or dyspnea. J Vet Intern Med 2007;21:243–250. [DOI] [PubMed] [Google Scholar]
- 26. Boswood A, Dukes‐McEwan J, Loureiro J, et al. The diagnostic accuracy of different natriuretic peptides in the investigation of canine cardiac disease. J Small Anim Pract 2008;49:26–32. [DOI] [PubMed] [Google Scholar]
- 27. Tarnow I, Olsen LH, Kvart C, et al. Predictive value of natriuretic peptides in dogs with mitral valve disease. Vet J 2009;180:195–201. [DOI] [PubMed] [Google Scholar]
- 28. Haggstrom J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: The QUEST study. J Vet Intern Med 2008;22:1124–1135. [DOI] [PubMed] [Google Scholar]
- 29. Ferasin L, Crews L, Biller DS, et al. Risk factors for coughing in dogs with naturally acquired myxomatous mitral valve disease. J Vet Intern Med 2013;27:286–292. [DOI] [PubMed] [Google Scholar]
- 30. Ljungvall I, Ahlstrom C, Hoglund K, et al. Use of signal analysis of heart sounds and murmurs to assess severity of mitral valve regurgitation attributable to myxomatous mitral valve disease in dogs. Am J Vet Res 2009;70:604–613. [DOI] [PubMed] [Google Scholar]
- 31. Schober KE, Hart TM, Stern JA, et al. Detection of congestive heart failure in dogs by Doppler echocardiography. J Vet Intern Med 2010;24:1358–1368. [DOI] [PubMed] [Google Scholar]
- 32. Ohad DG, Rishniw M, Ljungvall I, et al. Sleeping and resting respiratory rates in dogs with subclinical heart disease. J Am Vet Med Assoc 2013;243:839–843. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1. Progression of CSS over time.


