Abstract
Background
An overdose of pentobarbital sodium administered IV is the most commonly used method of euthanasia in veterinary medicine. Determining death after the infusion relies on the observation of physical variables. However, it is unknown when cortical electrical activity and brainstem function are lost in a sequence of events before death.
Hypothesis/Objectives
To examine changes in the electrical activity of the cerebral cortex and brainstem during an overdose of pentobarbital sodium solution for euthanasia. Our testing hypothesis is that isoelectric pattern of the brain in support of brain death occurs before absence of electrocardiogram (ECG) activity.
Animals
Fifteen horses requiring euthanasia.
Methods
Prospective observational study. Horses with neurologic, orthopedic, and cardiac illnesses were selected and instrumented for recording of electroencephalogram, electrooculogram, brainstem auditory evoked response (BAER), and ECG. Physical and neurologic (brainstem reflexes) variables were monitored.
Results
Loss of cortical electrical activity occurred during or within 52 seconds after the infusion of euthanasia solution. Cessation of brainstem function as evidenced by a lack of brainstem reflexes and disappearance of the BAER happened subsequently. Despite undetectable heart sounds, palpable arterial pulse, and mean arterial pressure, recordable ECG was the last variable to be lost after the infusion (5.5–16 minutes after end of the infusion).
Conclusions and Clinical Importance
Overdose of pentobarbital sodium solution administered IV is an effective, fast, and humane method of euthanasia. Brain death occurs within 73–261 seconds of the infusion. Although absence of ECG activity takes longer to occur, brain death has already occurred.
Keywords: Brainstem auditory evoked response, Death, Electroencephalogram, Equine
Abbreviations
- BAER
brainstem auditory evoked response
- ECG
electrocardiogram
- EEG
electroencephalogram
- EOG
electrooculogram
- MAP
mean arterial pressure
Euthanasia is a term used to describe ending of an animal's life in a painless and minimally distressful way.1 The American Veterinary Medical Association has established recommendations to assist in the decision on when to consider euthanasia.1 Several methods of euthanasia have been approved in veterinary medicine and might vary among species.1 An overdose of barbiturates is one of the approved methods of euthanasia in horses and it is the most common method used by practicing veterinarians.1 Determining death after the infusion of an overdose of a barbiturate solution relies on the observation of physical and neurologic variables such as undetectable heart sounds, loss of an arterial pulse, and the absence of brainstem reflexes (mainly corneal and palpebral reflexes).1
Determining brain death has been a subject of debate and controversy in human medicine and a consensus on what constitutes brain death varies from country to country.2 Procedures used to confirm brain death in human medicine include computed tomography angiography, transcranial Doppler sonography, electroencephalography (EEG), somatosensory evoked potentials, and brainstem auditory evoked responses (BAER).2 However, state of disease, drugs used, and personnel expertise in performing the tests used for the confirmation of brain death could influence accurate interpretation of such diagnostic aids.2 Physiologic, behavioral, and EEG studies have been done in laboratory animals, poultry, piglets, rabbits, dogs, and frogs.3, 4, 5, 6, 7, 8, 9, 10 Brain activity during euthanasia with intravenous concentrated potassium chloride has been studied by monitoring cerebral blood flow, metabolic state, electrocorticogram, and extracellular ion concentrations in cats.11 Electrodiagnostic studies such as electroencephalography and BAERs have not been performed in equine species to examine electrical activity of the brain in support of brain death because of euthanasia procedures. Therefore, the objective of the study was to evaluate the electrical activity of the cerebral cortex and brainstem during an intravenous overdose of pentobarbital sodium solution. Our testing hypothesis is that isoelectric pattern of the brain in support of brain death occurs before absence of electrocardiogram (ECG) activity. Therefore, suggesting that this method of euthanasia is an effective and humane procedure.
Materials and Methods
Animals
This observational prospective study included 15 horses for which euthanasia was elected based on published guidelines during a study period from 2011 to 2014.1 Reasons for euthanasia included poor quality of life, intractable pain, progressive and debilitating or incapacitating disease with a poor prognosis. Horses were sourced from a research herd and patients from the William R. Pritchard Veterinary Medical Teaching Hospital.
Sedation and anesthetic protocol
All horses had an intravenous catheter placed in the jugular vein for the administration of sedatives, injectable anesthetics, and euthanasia solution. Sedative and anesthetic protocols before euthanasia were elected according to the horses’ condition or disease, temperament, apparent anxiety, clinician preference, and safety concerns for the horse, personnel, and equipment. Accordingly, 3 protocols were used: (1) intravenous sedation (IVS, n = 4 horses), (2) intravenous anesthesia (IVA, n = 4), or (3) inhalation anesthesia (IA, n = 7).
Intravenous sedation consisted of administration of xylazine hydrochloride at a dosage of 0.25 mg/kg to relieve anxiety and facilitate electrode placement. Four horses (#1–4) were included in this group (orthopedic = 2, neurologic = 1, cardiac disease = 1). BAER was not performed in these horses because of equipment safety concerns.
Intravenous anesthesia consisted of administration of xylazine hydrochloride at a dosage of 1 mg/kg IV followed 5 minutes later by administration of ketamine hydrochloride at 2.2 mg/kg IV. Four horses (#5–8) received IA (neurologic = 3, orthopedic disease = 1). The electrodes for the recording of the study were placed once the horses were anesthetized. A BAER was performed in 3 of 4 horses.
In the group of horses euthanized while under inhalation anesthesia, horses were first sedated with xylazine and induced with ketamine as in the IVA group. Seven horses (#9–15) received inhalation anesthesia (neurologic = 6, orthopedic disease = 1). Reasons for undergoing anesthesia included myelography, computed tomography, and magnetic resonance imaging. Inhalation anesthesia was maintained with isoflurane (except one horse [#13] that received desflurane) delivered in 100% oxygen via a large animal anesthesia machine and breathing circuit. In addition, this one horse (#13) received IV propofol at a dosage of 2 mg/kg. Before euthanasia, the anesthetic level was maintained such that the EEG recorded continuous activity (no burst suppression). BAER was performed in 5 of 7 horses because of equipment availability.
Physical and neurologic variables
Physical variables included audible heart rate (beats per minute [bpm]) and rhythm, and the presence and quality of the arterial pulse. The neurologic variables consisted of presence or absence of brainstem reflexes such as direct pupillary light, corneal, and palpebral reflexes. The subcortical dazzle reflex was also monitored. These variables were monitored as follows: before receiving any medication (sedation), after instrumentation (EEG, EOG, ECG, and BAER), within 1 minute immediately before euthanasia solution infusion, within 20 seconds after the initiation of the infusion, immediately after the end of the infusion, and every 30 seconds thereafter until these variables were undetectable. Monitoring at these specific time points were not always possible in horses from the sedation group because of safety concerns. However, once horses from this group collapsed, variables were recorded immediately after collapse and every 30 seconds thereafter. Personnel assistance was used for monitoring physical (1st assistant) and neurologic (2nd assistant) variables. Mean arterial blood pressure (MAP) was continuously recorded in the inhalation anesthesia group.
Electrophysiologic examination
The examination consisted of EEG, EOG, electrocardiography (ECG), and BAER as described elsewhere.12, 13 The equipment used for EEG, EOG, and ECG was a digital EEG system (stationary or wireless),1, 2 with integrated video monitoring. Stationary or wireless (telemetry) digital EEG systems were used based upon equipment availability or safety concerns (eg, standing sedation versus anesthesia). Instrumentation for these procedures has been described elsewhere.12 Needle electrodes were placed SC in the scalp of the horse for the recording of EEG.12 Baseline recordings were performed before euthanasia in all horses. When possible, recordings were continuous throughout the procedure.
An evoked potential system3 was used for the recording of BAER. However, BAER was not evaluated in nonanesthetized horses for equipment safety reasons. One set of baseline tracings (an average of 200 responses using both derivations [vertex to mastoid, and vertex to C2]13 run simultaneously) with a single duplicate recorded for each ear were done before euthanasia. Immediately after this recording, infusion of euthanasia began and recordings were made continuously. Each complete recording took 90 seconds total. These were repeated continuously until BAER was absent (no peaks could be detected). The noise applied to the ear under evaluation was 90 dB normal hearing level (nHL) with a masking noise for the contralateral ear of 60 dB nHL.13 Identification of visible peaks were labeled from I to V; these were consistent with auditory function.13
Euthanasia protocol
Euthanasia consisted of intravenous injection of a combination of both pentobarbital sodium4 (390 mg/mL) and phenytoin sodium (50 mg/mL) at a dosage of 77–109 mg/kg for a total volume of 100 mL for horses above 400 kg of body weight. This dosage protocol is routinely used by most practicing veterinarians. The study was approved by an institutional animal care and use committee and owner consent was obtained.
Statistical analysis
Mean, standard deviation (SD), median, and range values are presented. No attempts were made to compare the results from the 3 groups of horses because of the low numbers of horses with different disorders, different euthanasia protocols, and variation in euthanasia solution dosages.
Results
Fifteen horses of Thoroughbred (n = 5), Quarter horse (n = 4), Arabian (n = 2), Morgan (n = 2), Warmblood (n = 1), and Tennessee Walking horse (n = 1) breeds were included in the study. There were 8 males (castrated = 7, intact = 1), and 7 females. The mean age was 10.8 years (median 14, range 20 days to 17 years). Ten horses had neurologic disease as follows: cervical compressive myelopathy (n = 4), progressive multifocal spinal cord disease (n = 3: undetermined etiology, n = 2/3; scoliosis, n = 1/3), occipitoatlantoaxial malformation with compression of the cervical spinal cord (n = 1), equine protozoal myeloencephalitis (n = 1), and meningoencephalomyelitis because of Halicephalobus gingivalis (n = 1). Four horses had orthopedic disease: chronic multiple osteoarthritis (n = 2), bilateral femoral osteochondrosis (n = 1), and bilateral pelvic fracture (n = 1). One horse had atrial fibrillation with severe atrioventricular heart block.
The mean infusion time was 46.8 seconds (SD 23.1, median 38, range 28–115 seconds) in adult horses. Two foals received 20 and 30 mL of euthanasia solution infused over 21 and 32 seconds, respectively. The mean infusion time for all horses was 44.1 seconds (SD 22.7, median 37, range 21 to 115 seconds). Heart rate increased during and immediately after the administration of euthanasia solution (before infusion: mean 40.4 bpm, SD 15.4, median 32, range 30–80; immediately after the infusion: mean 54.3, SD 12, median 52, range 36–80 bpm). Visible and audible breaths were not evident by the end of the infusion. Within 1 minute after euthanasia, heart sounds (mean 43.2, SD 12.1, median 38, range 25–60 seconds) were not audible and arterial pulse was undetectable. The MAP decreased from a mean of 83 mmHg (SD 5.6, median 80, range 75–89 mmHg) before euthanasia to 56.7 mmHg (SD 9.9, median 60, range 58–66 mmHg) after the infusion. Mean arterial pressure (MAP) was undetectable at a mean time of 52.6 seconds (SD 9.3, median 59, range 40–60 seconds) after the end of the infusion. All horses had intact brainstem reflexes before euthanasia.
A 10‐minute baseline EEG was recorded in all horses before euthanasia. Interpretable EEG was obtained in standing horses under sedation before infusion of euthanasia solution. However, interpretation was difficult during the infusion because of movement artifact. Based on unpublished isoflurane data from another EEG study in horses (DCW, MA), a minimal alveolar concentration of less than 1.2 was maintained to obtain continuous EEG activity without suppression. Burst suppression,14 defined as an isoelectric pattern alternating with bursts of high voltage activity, was noted in 2 horses anesthetized with isoflurane after infusion of 20–40 mL of euthanasia solution (Fig 1). Lack of detection of EEG (a continuous isoelectric pattern) occurred at a mean time of 52.6 seconds (SD 26.6, median 41, range 25–111 seconds) from time 0 (defined as the start of the infusion). Undetectable EEG occurred before (Fig 2A) and after (Fig 2B) termination of the infusion in 4 and 9 horses, respectively. In 2 horses (#2 and 3) from the sedation group, electrodes were lost as the horses collapsed. A reduced number of electrodes (9 plus ground) were placed promptly (<15 seconds) after collapse and an isoelectric pattern was noted; making it difficult to determine at what time point the EEG became isoelectric. In the group of 9 horses, loss of EEG activity occurred from 2 to 52 seconds (mean 23.7, SD 21.3, median 18 seconds) after termination of the infusion. The horse with the longest time to loss of EEG activity had atrial fibrillation and the longest time of infusion (Fig 3). This horse collapsed 17 second after the termination of the infusion, and lost EEG activity 29 seconds later. A different horse from the sedation group collapsed 5 seconds after the termination of the infusion and lost EEG activity 13 seconds later. Lack of brainstem reflexes occurred at a mean time of 81.1 seconds (SD 39, median 80, range 36–169 seconds) after the end of the infusion. A breath‐like movement (perceived as an agonal breath) concurrent with undetectable brainstem reflexes was observed in 3 horses (Fig 4). A baseline BAER was recorded in 8 of 8 horses before euthanasia (Fig 5A). Decreased amplitudes of all waves were noted seconds after the termination of the infusion (Figs 5B,C). Loss of detectable BAER was seen at a mean time of 122.6 seconds (SD 69.6, median 88, range 73–261 seconds) after completion of the infusion (Fig 5D). In one horse, a second breath‐like movement was observed and recorded on EEG at approximate 8 seconds after BAER became absent (not shown).
Figure 1.
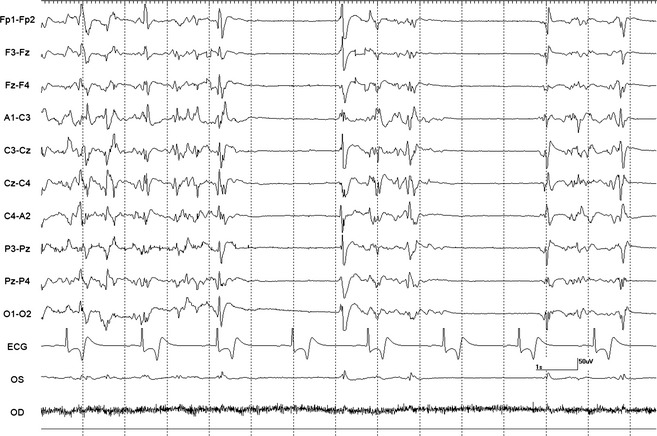
Burst suppression. This EEG is showing burst suppression activity in horse 13. Note the high voltage activity with intermittent electrical suppression. Note: Electroencephalogram: Even numbers = right side, odd numbers = left side, z = midline. Fp = frontopolar, F = frontal, C = central, P = parietal, O = occipital, A = aural, EOG: OD = right eye or OS = left eye, ECG. Calibration bar shown is for EEG and EOG = 1 second (second), 50 μV (microvolts). Calibration bar for ECG is not shown.
Figure 2.
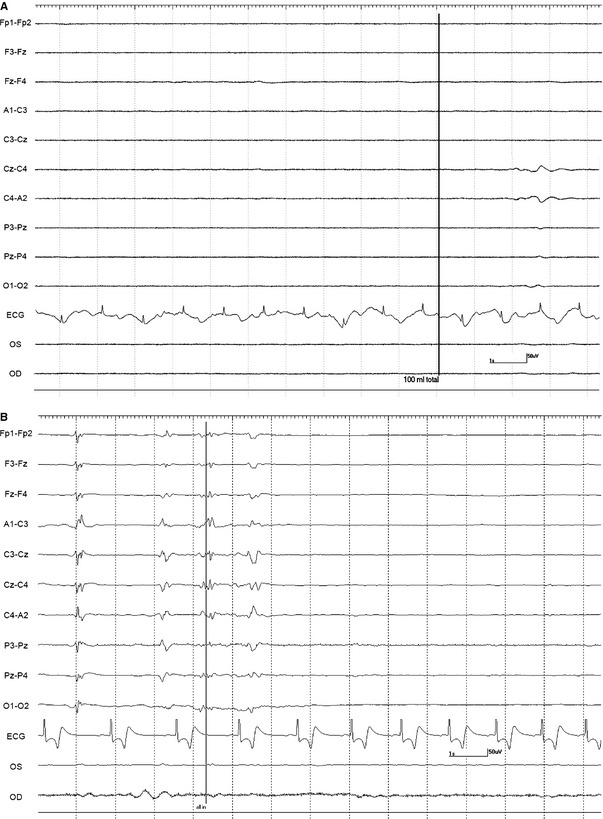
Isoelectric recording. (2A) This EEG is isoelectric in horse 10 before the end of the infusion marked with black vertical line and labeled 100 mL total (volume of pentobarbital sodium solution). (2B) This EEG is showing isoelectric pattern 1.5 seconds after the end of the infusion marked with a black vertical line (labeled: all in) in horse 13 (see Fig 1. for Electroencephalogram details).
Figure 3.
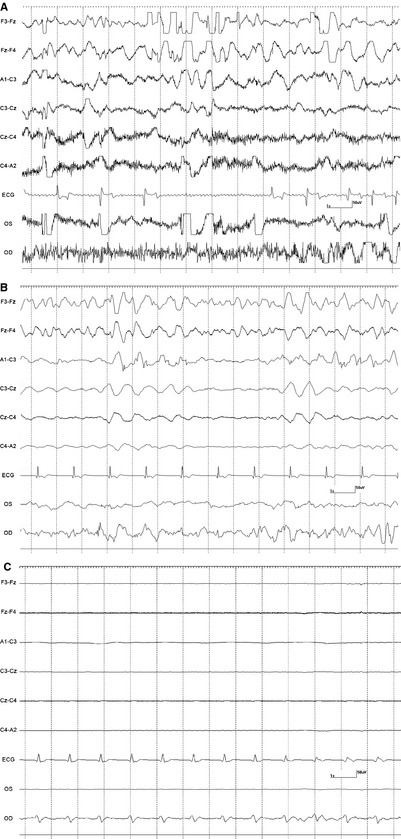
Electroencephalogram, electrooculogram, and electrocardiogram from horse 4. (3A) Baseline EEG and ECG. Movement artifact (large potentials often exceeding amplifier range [as evidenced by flattening of signal]) and muscle artifact (fast activity prominent in lower channels) obscure the EEG and EOG. Montage modified (fewer channels) than those of previous figures. Note atrial fibrillation. (3B) Decreased artifact in EEG. The ECG became regular after the infusion of euthanasia solution. (3C) ECG remained regular as EEG activity became undetectable. OD electrodes are picking ECG activity in this figure (see Fig 1. for Electroencephalogram details).
Figure 4.
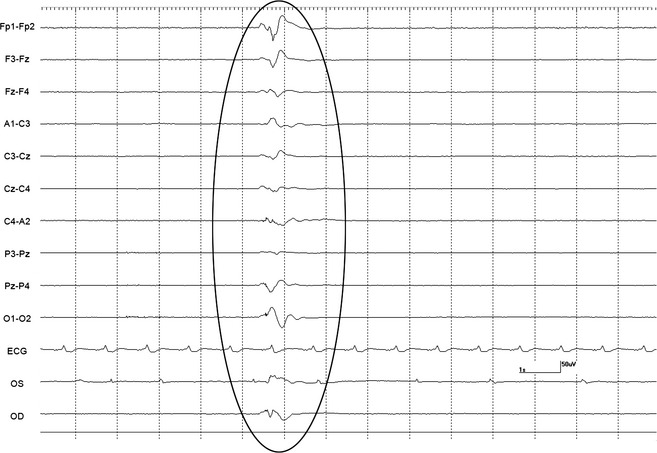
Agonal breath. This movement artifact (not cerebral electrical activity) was because of agonal breath (oval) at the time of absent brainstem reflexes in horse 3. Note isoelectric pattern and ongoing ECG (see Fig 1. for Electroencephalogram details).
Figure 5.
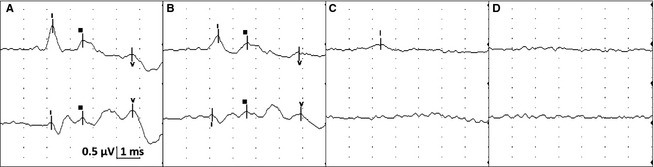
BAER. For all figures: Top tracing is vertex to mastoid (V‐M) and bottom tracing is vertex to C2 (V‐C2) recorded simultaneously. This figure represents stimulation of the right ear only. Calibration indicated for all figures (0.5 μV = microvolts, 1 ms = 1 millisecond per division). (5A) Baseline BAER in horse 15 (before euthanasia solution infusion). (5B) BAER done at the time of absent brainstem reflexes in horse 15. (5C) BAER done just before becoming absent. No clearly identifiable peaks, with the exception of wave I on V‐M. (5D) Absent BAER.
Despite undetectable heart sounds and the absence of a palpable arterial pulse, ECG monitoring showed ongoing ECG activity until a mean time of 559.1 seconds (SD 217.9, median 501, range 330–979 seconds) from termination of the infusion in all horses of all groups. During this time, brainstem reflexes and brain electrical activity did not return, and MAP was not recordable. In the horse with atrial fibrillation (Fig 3A), the heart rhythm became regular based on ECG (Fig 3B) after euthanasia solution administration. Before the occurrence of undetectable ECG in all horses, the ECG waves became irregular in shape, size, and rhythm (Fig 6).
Figure 6.
Electrocardiogram. Note the abnormal morphology of the ECG with isoelectric EEG recorded 10 seconds before undetectable ECG in horse 10 (see Fig 1. for Electroencephalogram details).
Discussion
This study showed that euthanasia with an overdose of pentobarbital sodium administered IV is an effective, fast, and humane method to terminate life in horses. Absence of detectable cortical electrical activity can occur during the administration of an overdose of pentobarbital (4 horses) or within 52 seconds after completion of the infusion (9 horses). The exact time at what 2 horses lost cortical electrical activity was not determined, but thought to be either during or shortly after (<15 seconds) the end of the infusion. This lack of EEG activity appeared to be irreversible based on continuous recording for several minutes with no recovery of EEG activity. Brainstem function was lost second based on absent brainstem reflexes and BAER. Brainstem reflexes were undetectable before loss of the BAER. Agonal breaths were observed concurrently with the loss of brainstem reflexes. Although heart sounds and a palpable arterial pulse were undetectable, ECG activity was the last variable to be lost. Absence and lack of recovery of any detectable brain electrical activity, based on EEG and BAER, supported the diagnosis of brain death in these horses.
Electroencephalography has been used for decades to aid in the determination of brain death in human medicine.15 Electroencephalography reflects cerebral cortical activity modulated by diencephalic and brainstem influences. An isoelectric pattern on EEG supports the absence of cerebral electrical activity. However, barbiturate administration and hypothermia can preclude proper diagnosis of brain death.16 Barbiturates can cause burst suppression and even an isoelectric pattern.17 Therefore, determining brain death in patients treated with barbiturates can be challenging. Halogenated inhalation anesthetics, such as isoflurane, can also cause burst suppression and isoelectric patterns.17 Propofol can also cause burst suppression in humans; however, the single horse that received propofol did not demonstrate this pattern.17 In the present study, only 2 horses displayed burst suppression and both horses were anesthetized with isoflurane. However, burst suppression was not observed until the infusion of pentobarbital sodium. The sedative (xylazine hydrochloride) administered to the horses in this study is not associated with burst suppression or isoelectric patterns.12 Ketamine hydrochloride, the induction agent, does not induce these EEG patterns.
Brainstem evoked response is used to assess the auditory pathway which includes the cochlear nerve, caudal, and cranial brainstem.18 Therefore, BAER could be used as a diagnostic aid to evaluate the presence or absence of brainstem function.18 However, BAER is considered to have a moderate prognostic value and low to moderate validity to confirm brain death depending upon the disease process (eg, severe brainstem injury).2, 19 To fulfill the criteria of brain death in people with sufficient brainstem damage, BAER waves are absent after wave I or occasionally after wave II.19 Complete absence of BAER could indicate deafness because of peripheral auditory dysfunction and not brain death exclusively.19 To avoid misinterpretation of BAER in our study, a baseline BAER was recorded in 8 horses. All BAER waves were present in these 8 horses and considered to be within published reference ranges.20, 21 The amplitude of all waves decreased and interpeak intervals increased within seconds after termination of pentobarbital infusion. Loss of waves II to V (brainstem, Fig 5C) occurred first, and wave I was the last wave to become undetectable (Fig 5D). Complete absence of BAER is in support of brain death in the absence of severe brainstem disease in these horses (n = 8 of 8). BAER can persist despite high doses of barbiturates in people and animals.22, 23, 24, 25
Factors that influence EEG and BAER recordings and interpretation such as disease and artifacts were considered. In this study, 3 horses had diseases that could have altered EEG and BAER findings. Two horses had multifocal brain disease with brainstem involvement (altered state of consciousness [stupor], multiple cranial nerve abnormalities). BAER was not performed in these 2 horses. The horse with atrial fibrillation took longer to lose ECG activity but its baseline EEG did not show obvious abnormalities. Artifacts such as those generated by movement, electrical interference, or hospital equipment (eg, ventilator) could interfere with proper EEG interpretation and determination of brain death. Movement artifacts were observed in standing horses resulting in difficulty in interpreting EEG as euthanasia solution was administered. Body temperature should be noted when using BAER as an aid to determine brain death because hypothermia can alter BAER (increased interpeak latencies) in people.26 This finding has not been investigated in horses. Body temperature in these horses did not decrease below 36.7°C (98°F), and BAER baseline was within reference ranges at this temperature.
The 2 horses with the longest infusion times (65 and 115 seconds) were the ones who took the longest to lose all ECG activity (962 and 979 seconds) after end of the infusion. The infusion of a smaller volume per time likely prolonged the time for full effect of pentobarbital solution. However, one of these horses had atrial fibrillation with periods of no ventricular activity (based on recorded ECG) for over 8–9 seconds, which likely impacted the distribution time and effects of euthanasia solution. The mean infusion time of pentobarbital solution for the remaining 13 horses was 37.1 seconds, and the mean time to absent ECG activity was 495.8 seconds postinfusion. As this variable (loss of ECG) is frequently used to determine time of death, administration of an overdose of pentobarbital sodium should be performed quickly. The distribution of an overdose of pentobarbital might be delayed with prolonged infusion, therefore possibly prolonging the effect on the brain (perception). In another euthanasia study using different premedication protocols (detomidine versus no detomidine administration) and variable dosages of pentobarbital sodium (high versus low), the mean time to asystole varied according to the protocol used.27 In that study, asystole occurred almost 4 minutes earlier in horses that received sedation compared to unsedated horses.27 Although sedated horses took approximately 8 seconds longer to collapse than unsedated horses, the documentation that asystole occurred earlier, led the authors to conclude that the combination of sedation with high doses of pentobarbital resulted in faster cardiac death.27 The overall mean infusion time in that study was 17 seconds (range 6–45 seconds).27
Pain, anxiety, and distress by a conscious horse could be minimized by administering IV sedation before euthanasia. In horses with standing sedation, 2 horses had isoelectric EEG patterns at the time of electrode replacement (<15 seconds after collapse) and 2 other horses took 18 and 46 seconds postinfusion to reach cerebral silence. The horse that took the longest time had atrial fibrillation, which likely played a role in the prolongation to effect. A larger number of horses are needed to validate these findings. However, the results of this study are encouraging because an isoelectric pattern on EEG supports a lack of conscious perception of pain and distress as euthanasia is occurring and while brain death and eventually asystole take place. A study by Chalifoux and Dallaire demonstrated that EEG was lost 4 minutes after euthanasia with carbon monoxide in dogs and that cessation of ECG occurred at 19 minutes.7 The study by Buhl27 showed that asystole in horses occurred up to 15 minutes later which is similar to our study (up to 16 minutes later in the 2 horses with the longest infusion times which one had atrial fibrillation). Removing these 2 horses, absence of ECG activity occurred up to 12 minutes (mean time 8.3 minutes) postinfusion of euthanasia solution. Respiratory arrest was noted earlier with no observable or auscultable breaths by the end of the infusion. A few breath‐like movements occurred at a time where EEG activity and brainstem reflexes were absent; and therefore considered reflexive (agonal breath: not a true breath).
In conclusion, an intravenous overdose of pentobarbital sodium solution is an effective, fast, and humane method of euthanasia. Rapid administration of an intravenous overdose of pentobarbital sodium solution might decrease the time to asystole after the infusion. Respiratory arrest occurs during or around the end of the infusion. Further, cerebral cortical activity becomes undetectable before the end or shortly after (less than 1 minute) the end of the infusion. This might support lack of conscious perception while brain death is happening. Brainstem function is absent next as evidenced by lack of brainstem reflexes and BAER. Lastly, absence of ECG activity occurs at a time on which brain death has already occurred and there is no cardiac output as evidenced by undetectable heart sounds, arterial pulse, and MAP. It is possible that cardiac death occurs earlier and that the ongoing ECG activity represents ineffective contraction with no cardiac output (electrical mechanical dissociation) as the remaining cardiac muscle ATP is being utilized. Future studies should be directed at assessing brain and cardiac death in horses with severe illnesses on which cardiovascular or metabolic derangements, hypovolemia, and hypotension might compromise and extend the distribution time of euthanasia solution to reach the brain and heart.
Acknowledgments
The authors thank Mr. John Doval from the UCD Media Lab for the illustrations, and Ms. Jennifer P. Vergara for technical assistance. The study was performed at the University of California at Davis. The study was supported by gifts from anonymous donors toward the Comparative Neurology Research Group at UCD.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Footnotes
Neurofax 9100, Nihon Kohden America, Inc., Foothill Ranch, CA
Neurofax Wireless Input 1000A, Nihon Kohden America Inc., Foothill Ranch, CA
VikingQuest, Nicolet Biomedical Inc., Madison, WI
Euthasol®, Virbac AH, Inc., Fort Worth, TX
References
- 1. AVMA Guidelines for the euthanasia of animals: 2013 Edition. J Am Vet Med Assoc 2013;1–102. [Google Scholar]
- 2. Welschehold S, Boor S, Reuland K, et al. Technical aids in the diagnosis of brain death. Dtsch Arztebl Int 2012;109:624–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. McKeegan DEF, Reimert HGM, Hindle VA, et al. Physiological and behavioral responses of poultry exposed to gas‐filled high expansion foam. Poult Sci 2013;92:1145–1154. [DOI] [PubMed] [Google Scholar]
- 4. Kongara K, Mcllhone AE, Kells NJ, et al. Electroencephalographic evaluation of decapitation of the anesthetized rat. Lab Anim 2014;48:15–19. [DOI] [PubMed] [Google Scholar]
- 5. Baumans V, Meijer JC, Haberham ZL, et al. Euthanasia of piglets: gas or injection? Tijdschr Diergeneeskd 1998;123:738–742. [PubMed] [Google Scholar]
- 6. Hellebrekers LJ, Baumans V, Bertens APMG, et al. On the use of T61 for euthanasia of domestic and laboratory animals; an ethical evaluation. Lab Anim 1990;24:200–204. [DOI] [PubMed] [Google Scholar]
- 7. Chalifoux A, Dallaire A. Physiologic and behavioral evaluation of CO euthanasia of adult dogs. Am J Vet Res 1983;44:2412–2417. [PubMed] [Google Scholar]
- 8. Lalonde‐Robert V, Desgent S, Duss S, et al. Electroencephalographic and physiologic changes after tricaine methanesulfonate immersion of African clawed frogs. J Am Assoc Lab Anim Sci 2012;51:622–627. [PMC free article] [PubMed] [Google Scholar]
- 9. Mikeska JA, Klemm WR. EEG evaluation of humaneness of asphyxia and decapitation euthanasia of the laboratory rat. Lab Anim Sci 1975;25:175–179. [PubMed] [Google Scholar]
- 10. Holson RR. Euthanasia by decapitation: evidence that this technique produces prompt painless unconsciousness in laboratory rodents. Neurotoxicol Teratol 1992;14:253–257. [DOI] [PubMed] [Google Scholar]
- 11. Mayevsky A, Barbiro‐Michaely E, Ligeti L, et al. Effects of euthanasia on brain physiological activities monitored in real‐time. Neurol Res 2002;24:647–651. [DOI] [PubMed] [Google Scholar]
- 12. Williams DC, Aleman M, Holliday TA, et al. Quantitative and qualitative characteristics of the electroencephalogram in normal horses following sedative administration. J Vet Intern Med 2012;26:645–653. [DOI] [PubMed] [Google Scholar]
- 13. Aleman M, Puchalski SM, Williams DC, et al. Brainstem auditory evoked responses in horses with temporohyoid osteoarthropathy. J Vet Intern Med 2008;22:1196–1202. [DOI] [PubMed] [Google Scholar]
- 14. Short TG, Leslie K. ‘Known unknows and unknown unknows’: electroencephalographic burst suppression and mortality. Br J Anaesth 2014;113:897–899. [DOI] [PubMed] [Google Scholar]
- 15. Yingying S, Qinglin Y, Gang L, et al. Diagnosis of brain death: confirmatory tests after clinical test. Chin Med 2014;127:1272–1277. [PubMed] [Google Scholar]
- 16. Monteiro LM, Bollen CW, van Huffelen AC, et al. Transcranial doppler ultrasonography to confirm brain death: a meta‐analysis. Intensive Care Med 2006;32:1937–1944. [DOI] [PubMed] [Google Scholar]
- 17. Sloan TB. Anesthetic effects on electrophysiologic recordings. J Clin Neurophysiol 1998;15:217–226. [DOI] [PubMed] [Google Scholar]
- 18. Spehlmann R. The normal BAEP In: Spehlmann R, ed. Evoked Potential Primer. Stoneham, MA: Butterworth Publishers; 1985:204–216. [Google Scholar]
- 19. Spehlmann R. Coma In: Spehlmann R, ed. Evoked Potential Primer. Stoneham, MA: Butterworth Publishers, 1985; 217–235. [Google Scholar]
- 20. Aleman M, Holliday TA, Nieto JE, et al. Brainstem auditory evoked responses in an equine patient population. Part I: adult horses. J Vet Intern Med 2014;28:1310–1317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Aleman M, Madigan JE, Williams DC, et al. Brainstem auditory evoked responses in an equine patient population. Part II: Foals. J Vet Inter Med 2014;28:1318–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stockard JJ, Stockard JE, Sharbrough FW. Nonpathological factors influencing brainstem auditory evoked potentials. Am J EEG Technol 1978;18:177. [Google Scholar]
- 23. Bobbin RP, May JG, Lemoine RL. Effects of pentobarbital and ketamine on brain stem auditory potentials: latency and amplitude intensity functions after intraperitoneal administration. Arch Otolaryngol 1979;105:467–470. [DOI] [PubMed] [Google Scholar]
- 24. Sutton LN, Frewen T, Marsh R, et al. The effects of deep barbiturate coma on multimodality evoked potentials. J Neurosurg 1982;57:178–185. [DOI] [PubMed] [Google Scholar]
- 25. Marsh RR, Frewen TC, Sutton LN, et al. Resistane of the auditory brain stem response to high barbiturate levels. Otolaryngol Head Neck Surg 1984;92:685–688. [DOI] [PubMed] [Google Scholar]
- 26. Stockard JJ, Sharbrough FW, Tinker JA. Effects of hypothermia on the human brainstem auditory response. Ann Neurol 1978;3:368–370. [DOI] [PubMed] [Google Scholar]
- 27. Buhl R, Andersen LO, Karlshoj M, et al. Evaluation of clinical and electrocardiographic changes during the euthanasia of horses. Vet J 2013;196:483–491. [DOI] [PubMed] [Google Scholar]


