Abstract
Background
Pulmonary hypertension (PH) is common in dogs with myxomatous mitral valve disease (MMVD) but its effect on clinical outcome has not been investigated.
Hypothesis/objectives
The presence of PH worsens the outcome in dogs with MMVD. To compare survival times of dogs with MMVD and PH to those without PH.
Animals
Two hundred and twelve client‐owned dogs.
Methods
Case review study. Medical records of dogs diagnosed with ACVIM stage B2 and C MMVD between January 2010 and December 2011 were retrospectively reviewed. Long‐term outcome was determined by telephone interview or from the medical record. End of the observation period was March 2013. PH was identified if tricuspid regurgitation peak velocity was >3 m/s.
Results
Two hundred and twelve were identified. Eighty‐three dogs (39%) had PH. PH was more commonly identified in stage C compared to B2 (P < .0001). One hundred and five (49.5%) dogs died during the observation period. Median survival time for the entire study population was 567 days (95% CI 512–743). Stage C (P = .003), the presence of PH (P = .009), left atrial to aortic root ratio (LA/Ao) >1.7 (P = .0002), normalized left‐ventricular end‐diastolic diameter (LVEDn) >1.73 (P = .048), and tricuspid regurgitation pressure gradient (TRPG) >55 mmHg (P = .009) were associated with worse outcomes in the univariate analyses. The presence of TRPG >55 mmHg (HR 1.8 95% CI 1–2.9; P = .05) and LA/Ao > 1.7 (HR 2 95% CI 1.2–3.4; P = .01) remained significant predictors of worse outcome in the multivariate analysis.
Conclusions and Clinical Importance
In dogs with MMVD, moderate to severe PH worsens outcome.
Keywords: Canine, Doppler echocardiography, Echocardiography, Heart failure, Heart valve
Abbreviations
- CHF
congestive heart failure
- CI
confidence interval
- E max
peak velocity of E wave of transmitral flow
- HR
hazard ratio
- Edt
E‐wave deceleration time of transmitral flow
- HF
heart failure
- LA/Ao
left atrial to aortic root ratio
- LVEDDn
normalized left‐ventricular end‐diastolic diameter
- LVESDn
normalized left‐ventricular end‐systolic diameter
- MMVD
myxomatous mitral valve disease
- TR
velocity of tricuspid regurgitation
- PH
pulmonary hypertension
- RVenl
right‐ventricular enlargement
- TRPG
peak tricuspid regurgitation pressure gradient
Pulmonary hypertension (PH) is a common complication in dogs affected by myxomatous mitral valve disease (MMVD).1 PH caused by left‐sided heart failure (HF) is initially the consequence of passive back transmission of increased left‐ventricular filling pressure to the pulmonary capillaries. This stage is generally considered reversible. However, if pulmonary venous pressure remains increased or continues to increase, pulmonary artery vasoconstriction and then pulmonary artery and vein remodeling might occur, at which time PH can become irreversible.2 Right‐heart catheterization is considered the “gold standard” for diagnosis of PH.3 In veterinary medicine, right‐heart catheterization is rarely performed in routine practice, and echocardiography is the standard noninvasive technique for the diagnosis of PH.4 PH is associated with a worse prognosis in human patients with left‐sided HF, including those with MMVD.5, 6 In veterinary medicine, there are currently no data regarding the prognostic importance of PH in dogs with MMVD. Accordingly, we hypothesized that dogs with stage B2 and C MMVD and echocardiographically identified PH have shorter survival time than dogs with MMVD that do not have PH. The objective of this study was to compare survival times of dogs with PH to those without PH in a population of dogs with ACVIM stages B2 or C MMVD.7
Materials and Methods
The medical records of dogs examined between January 2010 and December 2011 at seven referral cardiology centers in United States , Italy and Sweden, were reviewed. Search criteria included dogs with ACVIM stage B2 or C MMVD with or without an echocardiographic diagnosis of PH.
Inclusion Criteria
Dogs with ACVIM Stage B2 or Stage C MMVD that had been subject to physical examination, thoracic radiography, and echocardiography were considered for inclusion in the study population. Echocardiographic inclusion criteria were: 2‐D detection of mitral valve prolapse, any degree of mitral valve leaflet thickening or both; color Doppler identification of any degree of mitral valve regurgitation; and M‐mode left‐ventricular fractional shortening >20%; left aortic root ratio (LA/Ao) > 1.5,8 normalized end‐diastolic left‐ventricular diameter >1.73, or both.9 The presence of tricuspid valve regurgitation (TR) was an absolute inclusion criterion; all included dogs had color Doppler evidence of TR. Dogs were classified as ACVIM stage B2 MMVD if they did not have present or past clinical signs of congestive heart failure (CHF) or radiographic evidence of pulmonary edema or pulmonary venous congestion. Dogs were classified as ACVIM stage C MMVD if they had presented with past or current clinical signs of CHF in conjunction with past or current evidence of pulmonary edema, and pulmonary venous congestion on thoracic radiographs.
Exclusion Criteria
Dogs were excluded if they had congenital heart disease or other acquired cardiovascular disorders such as bacterial endocarditis or dilated cardiomyopathy. Dogs with MMVD without cardiac remodeling (stage B1) and those with MMVD and refractory CHF (stage D) were also excluded. Dogs with stage D have often right‐sided HF and decreased right‐ventricular function. In these circumstances, the Doppler‐derived estimated pulmonary artery pressure does not adequately reflect pulmonary vascular resistance and might underestimate the severity of PH.6 Dogs were also excluded if they had a history of clinically symptomatic systemic diseases or identifiable causes of PH other than MMVD such as clinically evidence of respiratory disease. Clinically evidence of respiratory disease was defined as the presence of moderate to marked bronchial, interstitial or alveolar pulmonary pattern in dogs that had diffuse pulmonary crackles on physical examination. Dogs with tracheal collapse were also excluded, as were dogs with a history of a positive heartworm test.
Baseline Data
Data obtained from the case records were: breed, sex, age, body weight, and treatment at the time of examination. Echocardiographic data retrieved were: normalized left‐ventricular end‐diastolic (LVEDDn) and end‐systolic diameter (LVESDn),9 LA/Ao, peak E‐wave velocity (E max) and E‐wave deceleration time (Edt) of the transmitral flow, peak velocity of tricuspid regurgitation (peak TR) from whatever echocardiographic view provided the highest velocity and the presence of right‐ventricular enlargement (RVenl). Right‐ventricular enlargement was subjectively assessed based on 2‐D echocardiographic examination. The clinical dataset was reviewed by a single investigator (MB).
Echocardiographic Measurements
The LA/Ao was obtained from the 2‐D short‐axis view.8, 10 LVDDn and LVESDn were calculated according to Cornell's method of allometric scaling: LVEDDn = LVEDD/BW0.294 and LVESDn = LVESD/BW0.315.9 Right‐ventricular enlargement was subjectively assessed and classified as yes/no. Assessment of TR by color Doppler was obtained with the aliasing velocity set between 60 and 90 cm/s. When Doppler spectrograms of TR were not recorded or were not measurable, dogs were considered not affected by PH if they had trivial TR and there was no evidence of RVenl. Peak velocity of TR was obtained under color Doppler guidance. Systolic pulmonary pressure was estimated calculating the peak tricuspid regurgitation gradient (TRPG) using the simplified Bernouilli equation: TRPG = 4 × TR2. PH was diagnosed if the peak TR velocity was >3 m/s corresponding to a TRPG <36 mmHg.
Clinical Progress and Survival
Investigators, trained senior veterinary students or veterinary technician conducted telephone interviews with dog owners or reviewed medical records to determine the clinical outcome of each dog. Clinical progression of dogs for which it was not possible to contact the owner was assessed by review of medical records. Survival time was counted from the day of diagnosis of stage B2 or C MMVD to either the day of death or closing time of the study (March 31, 2013). End‐point of the study was death (all causes).
Statistical Analysis
Baseline descriptive statistics were presented as mean and standard deviation for normally distributed continuous variables, whereas non‐normally distributed variables were presented as median and range. Categorical variables were analyzed with Chi‐Square analysis. Between‐group analyses of baseline variables were performed using analysis of variance or test for median as appropriate for the error residuals distribution. Effects on survival of the following variables at baseline were evaluated: ACVIM stage, presence of PH, LA/Ao > 1.7, LVEDDn > 1.73, LVESDn > 1.4, TRPG > 55 mmHg. The 55 mmHg TRPG was chosen based on visual inspection of penalized spline hazard plots of pressure gradient modeling mortality on a logarithmic scale relative to the absolute value on a linear scale for TRPG suggesting a linear increase in hazard for values >50 mmHg (Fig 1). Time to event analyses were carried out in univariate analysis by way of Kaplan–Meier product limit estimates. Dogs that were lost to follow‐up before closing time of the study were censored after last visit. Cox semiparametric regression models were used to generate multivariate models. When developing multivariate models continuous variables were categorized by visual inspection of log hazards plotted on a continuum by way of penalized spline with four degrees of freedom and based on clinical rationale and previous literature. Model relative goodness of fits was analyzed by Akaike information criterion and compared using a Chi‐Square one degree of freedom test. Tests for proportionality were carried out by visual inspection of Schoenfeld residuals, negative log estimated SDF, and formal hypothesis testing of covariate by log (time) interactions followed by Wald Chi‐Square statistics and deemed proportional. Additional sensitivity analyses were carried out using parametric accelerated time failure models affirmatively validating the Cox proportionality assumptions. All analyses were deemed significant at P ≤ .05 and carried out using a commercial software.1
Figure 1.
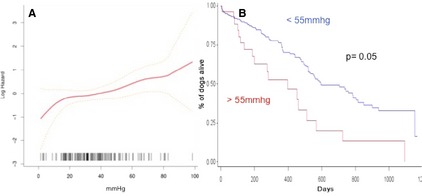
(A) Penalized spline (df = 4) hazard plots of pressure gradient modeling death on a logarithmic scale relative to the absolute value on a linear scale for trans–tricuspidal pressure gradient (TRPG). Visual inspection suggest a linear increase in the hazard for values of TRPG > 50 mmHg. Yellow graph lines represent 95% Confidence interval (95% CI). (B) Graph depicting survival times as Kaplan–Meier curves for TRPG greater and less than 55 mmHg.
Results
Baseline Characteristics
Baseline characteristics for the general population and for dogs with and without PH are summarized in Table 1. Two hundred and twelve dogs, 121 (57%) males (41 neutered) and 91 (43%) females (67 spayed) from 41 breeds were included in the study. In decreasing magnitude of representation, breeds included: mixed (n = 48; 23%), Cavalier King Charles spaniel (n = 30; 14%), Dachshunds (n = 16; 7%) and Miniature Poodle (n = 12; 6%). One hundred dogs were in stage B2 (47%) and 112 (53%) were in stage C of MMVD. PH was diagnosed in 83 (39%) dogs. Age, weight, LVESDn, E max and presence/absence of RVenl were not different between dogs with and without PH. PH was significantly more common in dogs with stage C MMVD (24; 29% stage B2, 59; 71% stage C; P < .001). Dogs with PH had significantly larger left atria, LVEDDn and TRPG compared to dogs without PH (Table 1). At baseline, 131 (62%) dogs were receiving medical treatment for heart disease (41 stage B2 and 90 stage C). Twenty seven dogs received furosemide in combination with an ACE‐I, 48 dogs furosemide in combination with an ACE‐I and pimobendan, 5 dogs furosemide in combination with an ACE—I, pimobendan and spironolactone. Of dogs that received only a single drug, one received furosemide, 18 dogs received an ACE‐I, 2 dogs, pimobendan and 3 dogs spironolactone. All dogs received at least an ACE‐I, pimobendan and furosemide after diagnosed with ACVIM stage C.
Table 1.
Descriptive statistics for all 212 dogs, dogs without pulmonary hypertension (PH), and dogs with PH
| All (n = 212) | No PH (129) | PH (n = 83) | P‐Value | |
|---|---|---|---|---|
| Age (years) (n = 212) | 10.6 ± 2.6 | 10.6 ± 2.6 | 10.7 ± 2.7 | .94 |
| Sex (F/M) (n = 212) | 91/121 (43%/57%) | 55/74 (43%/57%) | 36/57 (43%/57%) | .91 |
| Weight (kg) (n = 212) | 8.6 (1.2–80.7) | 9.1 (1.2–80.7) | 7.7 (1.6–67) | .19 |
| ACVIM stage (n = 212) | 100 B2 (47%) | 76 B2 (59%) | 24 B2 (29%) | .0022 |
| 112 C (53%) | 53 C (41%) | 59 C (71%) | .0023 | |
| LA/Ao (n = 211) | 2 (1.3–3.9) | 1.9 (1.3–3.2) | 2.3 (1.4–3.9) | <.0001 |
| LVEDDn (n = 211) | 2.02 (0.8–2.9) | 1.9 (0.9–2.9) | 2.1 (0.8–2.9) | .006 |
| LVESDn (n = 211) | 1.05 (0.2–1.9) | 1.04 (0.4–1.9) | 1.04 (0.2–1.8) | .877 |
| E peak (m/s) (n = 117) | 1.28 ± 0.40 | 1.3 ± 0.3 | 1.42 ± 0.37 | .08 |
| TRPG (mmHg) (n = 191) | 33.2 (1.4–98.4) | 24.1 (1.4–35.5) | 46.2 (36.0–98.4) | <.0001 |
| RV enlargement (yes/no) (n = 123) | 15 (12%) | 7(47%) | 8 (53%) | .067 |
P‐value is referring to differences between dogs with and without PH. No PH, no pulmonary hypertension, ACVIM stage, class of heart failure according to ACVIM classification; LA/Ao, left‐atrial to aortic root ratio; LVEDDn, normalized left‐ventricle end‐diastolic diameter indexed; LVESDn, normalized left‐ventricle end‐systolic diameter indexed; E peak, peak velocity of E wave of transmitral flow; TRPG, tricuspid regurgitation pressure gradient; RV, right ventricle.
Survival Analysis and Progression
During the observation period, 105 dogs (49.5%) died or were euthanized because of refractory CHF. Of these dogs 26 were ACVIM stage B2 and 79 ACVIM stage C MMVD. The median survival time was 567 days (95% CI 512–743). Median survival times for stage B2 and C dogs were 784 days (95% lower CI 576, upper CI nonestimable) and 491 days (95% CI 440–561), respectively. The median survival time for dogs without PH was 758 days (95% CI 527–848) and for those with PH, 456 days (95% CI 360–567). Of the six variables used as predictors in the univariate analysis, ACVIM stage C (HR 1.8, 95% CI 1.2–2.7; P = .003), the presence of PH (HR 1.6 95% CI 1.1–2.4; P = .009), LA/Ao > 1.7 (HR 2.4, 95% CI 1.5–4; P = .0002), LVEDDn > 1.73 (HR 1.7, 95% CI 1–1.7; P = .048), and TRPG > 55 mmHg (HR 2.3 95% CI 1.4–3.8; P = .002) were associated with worse outcomes (Figs 1, 2). The presence of TRPG > 55 mmHg (HR 1.8 95% CI 1–2.9; P = .05) and LA/Ao > 1.7 (HR 2 95% CI 1.2–3.4; P = .01) remained significant predictors of poor outcome in the multivariate analysis.
Figure 2.
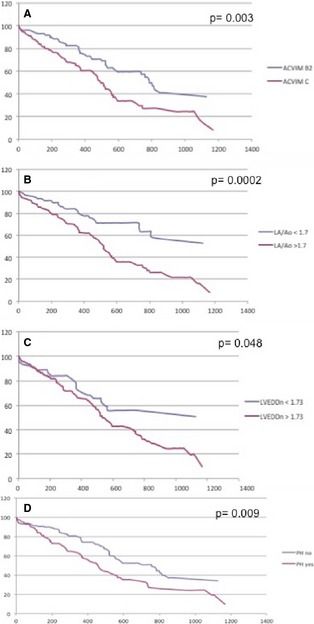
Graphs depicting survival times as Kaplan–Meier curves for variable affecting survival in dogs with myxomatous mitral valve degeneration. (A) myxomatous mitral valve disease (MMVD) dogs in ACVIM stage B2 and C. (B) Dogs with left‐atrial to aortic root ratio (LA/Ao) > 1.7. (C) Dogs with normalized left‐ventricular end‐diastolic dimension (LVEDDn)> 1.73. (D) Dogs with and without pulmonary hypertension (PH).
Discussion
Results of this study demonstrate that echocardiographic evidence of PH is common in dogs with ACVIM stage B2 and C MMVD and that it is associated with a poorer prognosis. Prevalence of detectable and quantifiable PH in these population of dogs was 39%. Previous studies have reported a prevalence of PH in dogs with MMVD between 14 and 53%.11, 12, 13, 14 One possible reason for the difference observed between studies is the use of a different TR velocity cut off to establish the presence of PH. In this study PH was diagnosed if TR velocity was more than 3 m/s. Other studies have used a lower cut off.12, 13 Prevalence of the disease is also related to the population studied. One large study, reported a lower prevalence of PH in a population of 617 dogs with different stages of MMVD.12 However, in that study, more than half of the included dogs had a mild form of MMVD not associated with cardiac remodeling. Two studies have reported that the prevalence of PH was associated with severity of MMVD.12, 15 Similarly, in this study, dogs with stage C MMVD had a significantly greater prevalence of PH compared to dogs in stage B2. This is not surprising as PH with left‐sided heart disease is initially the consequence of passive back transmission of increased left‐ventricular filling pressure to the pulmonary capillaries.2 Accordingly, humans and animals with more severe mitral regurgitation, and therefore higher left‐atrial pressure, have an increased risk of developing PH. In our study about one third of dogs with PH were in stage B2; this finding suggests that a certain number of dogs considered pre‐clinical for left‐sided HF have high left‐atrial pressure. Dogs with stage B2 MMVD represents a population that is heterogeneous with respect to cardiac remodeling; in some dogs, cardiac enlargement is mild and in others, severe, despite the fact, that clinical signs are, by definition, absent. Therefore, it is possible that dogs with more severe remodeling have higher left‐atrial pressure and are actually in left‐sided HF but clinical signs were missed by the owner. More objective measures of clinical signs might help identify this subset of dogs. Recently two studies have suggested that resting respiratory rate represent a useful objective clinical variable to identify dogs with left‐sided HF.14, 16
Univariate analysis indicated that stage of the disease, left‐atrial enlargement, left‐ventricular enlargement, presence of PH and a TRPG > 55 mmHg were predictors of survival. However, only left‐atrial enlargement and a TRPG > 55 mmHg were predictors of death in the multivariate analysis. In dogs with MMVD, left‐atrial size is related to severity of MR and is an independent predictor of progression and death.17, 18, 19 Degree of left‐atrial enlargement has also been associated with severity of PH in dogs with MMVD.12, 15 The fact that PH was not an independent predictor of death in multivariate analysis is likely because of the fact it is correlated with left‐atrial enlargement. However, TRPG > 55 mmHg remained an independent negative predictor of survival. If pulmonary venous pressure remains increased or continues to increase in humans and animals with left‐sided HF, local and systemic release of angiotensin II, tumor necrosis factor, endothelin‐1 together with impaired nitric‐oxide mediated pulmonary vasodilation lead to pulmonary artery and vein remodeling and the process becomes irreversible.2 In these humans and animals, pulmonary vascular resistance is increased and they develop PH that is out of proportion to left‐atrial pressure or, “reactive PH.”3 A systolic pulmonary arterial pressure >50 mmHg has been reported to be associated with poor outcome in human beings with MMVD.5 In dogs with MMVD, a TRPG at or above 48 mmHg suggests the presence of irreversible PH.15 Another study in humans with cardiomyopathies demonstrated that each 5‐mmHg increase in baseline of mean pulmonary arterial pressure, increased hazard of death by 25%.20 Similarly, in this study, visual inspection of spline curve for TRPG shows that there is a linear increase for the risk of death with TRPG more than 50 mmHg (Fig 2). Concomitant chronic respiratory diseases, such as tracheal collapse, chronic bronchitis or interstitial lung disease, also commonly affect small breed dogs with MMVD.21 These diseases might contribute to the development of PH in dogs with MMVD. Therefore, it is possible that PH in some of the dogs included in this study could be induced by concomitant respiratory disease. However, none of the included dogs had a history, clinical signs, and radiographic evidence of severe respiratory disease and this study did not include dogs of breeds known to be predisposed to interstitial lung disease. Moreover, our results show that dogs with more advanced MMVD were more likely to have PH. These data indirectly support the contention that MMVD is most likely the cause of PH in this sample population.
This study has some limitations. First, diagnosis of PH was only based on peak velocity of TR. Right‐heart catheterization is considered the “gold standard” for the definitive diagnosis of PH.3 However, in veterinary medicine right‐ventricular catheterization is rarely performed in routine practice and PH hypertension is generally diagnosed based on the presence of clinical and echocardiographic findings. Moreover, peak velocity of TR is affected by right‐ventricular contractility.3, 4 In this study, we reported if the right‐ventricle was subjectively enlarged but right‐ventricular function was not assessed. Second, TRPG might be underestimated in some dogs because of technical difficulties in obtaining an ideal alignment with eccentric tricuspid regurgitant jets. In this study assessment of TR was obtained from the echocardiographic view providing the highest peak velocity and best alignment. Third, TR might not be present in all dogs with PH. Fourth, this study has a retrospective design. Retrospective studies increase the risk of uncontrolled systematic errors. Furthermore, because of the retrospective design, all variables were not available at the baseline. Finally, because of the many combinations of drugs administered, and the retrospective nature of the study, it was not possible to analyze the effects of treatment in the studied population. However, all stage C dogs were treated with standard treatment including an ACE‐I, pimobendan and furosemide after their inclusion in the study.
Clinical Relevance
This study demonstrated that PH is a commonly associated with stage B and C MMVD in dogs and that the presence of PH is associated with an increased risk of death. Furthermore, the finding that about one third of dogs with PH in this study were classified as ACVIM stage B2 suggests that this stage of the disease includes a heterogeneous group of dogs.
Acknowledgments
This study was not supported by a grant. The Authors acknowledge Dr Giulio Menciotti, and Stephanie Apple for helping with the organization of the data for this study and Dr Sunshine Lahmers for her revision and suggestions.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Off‐label Antimicrobial Declaration: Authors declare no off‐label use of antimicrobials.
Cases for this study were referred to the institutions and clinics listed above. Analysis of the data was performed at the institution of the corresponding author.
Preliminary results of this study have been presented at the 23rd ECVIM‐CA Congress 12–14th September 2013, Liverpool, United Kingdom.
Footnote
SAS 9.3 (Cary, NC 2013).
References
- 1. Kellihan HB, Stepien RL. Pulmonary hypertension in dogs: Diagnosis and therapy. Vet Clin North Am Small Anim Pract 2010;40:623–641. [DOI] [PubMed] [Google Scholar]
- 2. Guazzi M, Arena R. Pulmonary Hypertension with left sided heart disease. Nat Rev Cardiol 2010;7:648–659. [DOI] [PubMed] [Google Scholar]
- 3. Galie N, Hoeper MM, Humbert M, et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. Eur Respir J 2009;34:1219–1263. [DOI] [PubMed] [Google Scholar]
- 4. Kellihan HB, Stepien RL. Pulmonary hypertension in canine degenerative mitral valve disease. J Vet Cardiol 2012;14:149–164. [DOI] [PubMed] [Google Scholar]
- 5. Barbieri A, Bursi F, Grigioni F, et al. Prognostic and therapeutic implications of pulmonary hypertension complicating degenerative mitral regurgitation due to flail leaflet: A multicenter long‐term international study. Eur Heart J 2011;32:751–759. [DOI] [PubMed] [Google Scholar]
- 6. Ghio S, Gavazzi A, Campana C, et al. Independent and additive prognostic value of right ventricular systolic function and pulmonary artery pressure in patients with chronic heart failure. J Am Coll Cardiol 2001;37:183–188. [DOI] [PubMed] [Google Scholar]
- 7. Atkins C, Bonagura J, Ettinger S, et al. Guidelines for the diagnosis and treatment of canine chronic valvular heart disease. J Vet Intern Med 2009;23:1142–1150. [DOI] [PubMed] [Google Scholar]
- 8. Hansson K, Haggstrom J, Kvart C, et al. Left atrial to aortic root indices using two‐dimensional and M‐mode echocardiography in Cavalier King Charles Spaniels with and without left atrial enlargement. Vet Radiol Ultrasound 2002;43:568–575. [DOI] [PubMed] [Google Scholar]
- 9. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 10. Rinshiw M, Hollis NE. Evaluation of four 2‐D dimensional echocardiographic methods of assessing left atrial size in dogs. J Vet Intern Med 2000;14:429–435. [DOI] [PubMed] [Google Scholar]
- 11. Borgarelli M, Zini E, D'Agnolo G, et al. Comparison of primary mitral valve disease in German Shepherd dogs and in small breeds. J Vet Cardiol 2004;6:27–34. [DOI] [PubMed] [Google Scholar]
- 12. Serres FJ, Chetbul V, Tissier R, et al. Doppler echocardiography‐derived evidence of pulmonary arterial hypertension in dogs with degenerative mitral valve disease: 86 cases (2001–2005). J Am Vet Med Assoc 2006;229:1772–1778. [DOI] [PubMed] [Google Scholar]
- 13. Guglielmini C, Civitella C, Diana A, et al. Serum cardiac Tn I concentration in dogs with with precapillary and post capilllary pulmonary hypertension. J Vet Intern Med 2010;24:145–152. [DOI] [PubMed] [Google Scholar]
- 14. Schober KE, Hart TM, Stern JA, et al. Detection of congestive heart failure in dogs by Doppler echocardiography. J Vet Intern Med 2010;24:1358–1368. [DOI] [PubMed] [Google Scholar]
- 15. Chiavegato D, Borgarelli M, D'Agnolo G, et al. Pulmonary hypertension in dogs with mitral regurgitation attributable to myxomatous valve disease. Vet Radiol Ultrasound 2009;50:253–258. [DOI] [PubMed] [Google Scholar]
- 16. Ohad DG, Rishniw M, Ljungvall I, et al. Sleeping and resting respiratory rates in dogs with subclinical heart disease. J Am Vet Med Assoc 2013;243:839–843. [DOI] [PubMed] [Google Scholar]
- 17. Borgarelli M, Savarino P, Crosara S, et al. Survival characteristics and prognostic variables of dogs with mitral regurgitation attributable to myxomatous valve disease. J Vet Intern Med 2008;22:120–128. [DOI] [PubMed] [Google Scholar]
- 18. Haggstrom J, Boswood A, O'Grady M, et al. Effect of pimobendan or benazepril hydrochloride on survival times in dogs with congestive heart failure caused by naturally occurring myxomatous mitral valve disease: The QUEST study. J Vet Intern Med 2008;22:1124–1135. [DOI] [PubMed] [Google Scholar]
- 19. Borgarelli M, Crosara S, Lamb K, et al. Survival characteristics and prognostic variables of dogs with preclinical chronic degenerative mitral valve disease attributable to myxomatous degeneration. J Vet Intern Med 2012;26:69–75. [DOI] [PubMed] [Google Scholar]
- 20. Cappola TP, Felker GM, Kao WH, et al. Pulmonary hypertension and risk of death in cardiomyopathy: Patients with myocarditis are at higher risk. Circulation 2002;105:1663–1668. [DOI] [PubMed] [Google Scholar]
- 21. Ferasin L, Crews L, Biller DS, et al. Risk factors for coughing in dogs with naturally acquired myxomatous mitral valve disease. J Vet Intern Med 2013;27:286–292. [DOI] [PubMed] [Google Scholar]


