Abstract
Background
Itraconazole is commonly used to treat systemic fungal infections in dogs, but problems exist with absorption and cost.
Objective
To determine oral bioequivalence of generic and compounded itraconazole compared to original innovator (brand name) itraconazole in healthy dogs.
Animals
Nine healthy, adult research Beagle dogs.
Methods
A randomized, 3‐way, 3‐period, crossover design with an 8‐day washout period. After a 12‐hour fast, each dog received 100 mg (average: 10.5 mg/kg) of either innovator itraconazole, an approved human generic capsule, or compounded itraconazole (compounded using a commercially available compounding vehicle) with a small meal. Plasma was collected at predetermined intervals for high pressure liquid chromatography analysis. Concentration data were analyzed using noncompartmental pharmacokinetics to determine area under the curve (AUC), peak concentration (CMAX), and terminal half‐life. Bioequivalence tests compared generic and compounded itraconazole to the reference formulation.
Results
Average ratios of compounded and generic formulations to the reference formulation of itraconazole for AUC were 5.52% and 104.2%, respectively, and for CMAX were 4.14% and 86.34%, respectively. A test of bioequivalence using 2 one‐sided tests and 90% confidence intervals did not meet bioequivalence criteria for either formulation.
Conclusion and Clinical Importance
Neither generic nor compounded itraconazole is bioequivalent to the reference formulation in dogs. However, pharmacokinetic data for generic formulation were similar enough that therapeutic concentrations could be achieved. Compounded itraconazole produced such low plasma concentrations, it is unlikely to be effective; therefore, compounded itraconazole should not be used in dogs.
Keywords: Bioavailability, Blastomyces, Fungal infection, Histoplasma, Pneumonia
Abbreviations
- AMDUCA
Animal Medical Drug Clarification Act
- AUC
area under the curve
- CL
systemic clearance
- CMAX
peak concentration
- Cn
last measured concentration point
- FDA
US Food and Drug Administration
- HPLC
high pressure liquid chromatography
- LOQ
limit of quantification
- MRT
mean residence time
- QC
quality control
- VD
volume of distribution
- λZ
terminal slope of the curve
Fungal infections of dogs are common in the southeastern United States, with blastomycosis and histoplasmosis being the most commonly recognized systemic diseases. Itraconazole is one of the drugs used for their treatment. The original innovator itraconazole1 was first introduced onto the market in 1992 as an oral capsule; it is also now available in an oral liquid formulation. Itraconazole is highly lipophilic and practically insoluble in water.1 Absorption of oral itraconazole capsules is variable because the drug requires an acidic environment for dissolution; administration with a meal is suggested to further aid in bioavailability. To verify adequate dosage or overdose, plasma itraconazole concentrations can be measured; however, this is not usually carried out.
In dogs infected with blastomycosis, a 74% response rate occurs when treating with 5 mg/kg/d of the original innovator formulation of itraconazole.2 Blastomycosis requires a minimum of 60 days' treatment; and treatment is usually continued for 30 days beyond resolution of clinical signs. Unfortunately, the original formulation can be cost prohibitive for owners, especially those with large‐breed dogs that need extended treatment. Recently, approved human generic oral formulations of itraconazole became available. Itraconazole has also been compounded for veterinary patients by pharmacists using bulk itraconazole powder, but use of compounded formulations is not recommended2, 3 because their pharmacokinetics are unknown. Treatment failures can occur, in which case the innovator formulation might need to be used for successful treatment (authors' anecdotal observations).
According to the US Food and Drug Administration (FDA), compounding of a drug is acceptable when there is a need for a different concentration or a more palatable oral formulation, such as for small animals. Under the Animal Medical Drug Clarification Act (AMDUCA), compounding is legal if conditions listed in the AMDUCA extralabel drug use regulations4 are followed. The US Pharmacopeial Convention lists precise requirements for compounding nonsterile dosage formulations,5 and compounded products must be made from commercial sources of drug rather than bulk drugs, which are defined as active ingredients used in the manufacture of finished dosage forms.4
The purpose of this study was to determine the bioequivalence of 3 formulations of itraconazole in healthy dogs. For our comparison, we used a descriptive analysis of the oral pharmacokinetic parameters derived from each formulation: original innovator capsules, generic capsules,2 and compounded capsules.3 In addition, we performed bioequivalence analysis using the tests accepted by the FDA.6 Bioequivalence tests examine the average ratios of each test formulation to the reference formulation for 2 critical pharmacokinetic parameters: area under the plasma concentration versus time curve (AUC) and peak concentration (CMAX).
Materials and Methods
Study Population
The research protocol was approved by the University of Tennessee Institutional Animal Care and Use Committee. Nine healthy, adult Beagle research dogs with body weights between 7.4 and 13.7 kg (mean 9.8 kg) were studied.
Experimental Protocol
A 3‐way, 3‐period, randomized crossover experimental design was used with an 8‐day washout period. The 3 itraconazole formulations were original innovator, generic, and compounded capsules. Compounded itraconazole was made using a bulk powder. The powder obtained was authenticated with a certificate of analysis,3 weighed using a gram scale, compounded in a commercially available compounding vehicle,4 and then placed into gelatin capsules. All formulations were administered as a 100 mg capsule to each dog (average dose 10.5 mg/kg). Twelve hours before administration of each formulation of itraconazole, intravenous jugular catheters were aseptically placed and secured with neck bandages and food was withheld. Time zero blood samples were collected, and itraconazole was PO administered followed by a small meal (approximately 60 mL of gruel) approximately 2 minutes after the drug was administered. All dogs received an identical meal of commercially available maintenance canine food. If a dog did not immediately voluntarily eat, syringe feeding was performed to ensure that food was ingested within the first 10 minutes. Blood samples (3 mL) were collected at 20, 40, and 60 minutes and then at 2, 4, 6, 8, 10, and 12 hours. Catheters were removed after the last sample was collected.
Blood samples were immediately placed on ice and then centrifuged in batches. The plasma was separated and stored in plastic cryovials at −70 to −80°C. Plasma samples were shipped frozen on ice to the North Carolina State University Clinical Pharmacology Laboratory for itraconazole concentration analysis.
Itraconazole Analysis
The quantitative determination of itraconazole in plasma samples was performed by high pressure liquid chromatography (HPLC) as previously described.7, 8 All experimental plasma samples, quality control (QC) samples, calibration samples, and blank (control) plasma samples were prepared identically.
The analytical reference standard of itraconazole was obtained as a pure substance.5 Itraconazole was dissolved in HPLC‐grade acetonitrile to make a 1 mg/mL stock solution. From this stock solution, further dilutions were made to use as fortifying solutions for plasma in order to generate calibration curves and QC standards in canine plasma. The stock solution was kept at 4°C in a tightly sealed, dark vial, which we determined to be stable throughout the duration of the study. Itraconazole spiking solutions were added to blank canine (control) plasma, to prepare 9 calibration standards (range 0.01–10 μg/mL). Blank (control) plasma samples were also analyzed with each day's run to check for interfering peaks and estimate background noise. All calibration curves were linear with an R 2 value of 0.99 or greater. Limit of quantification (LOQ) for itraconazole in canine plasma was 0.01 μg/mL, which was determined from the lowest point on a linear calibration curve that produced an acceptable signal‐to‐noise ratio with a range of 0.01–20 μg/mL. Quality control samples were analyzed each day and compared against the calibration curve. The laboratory used guidelines published by the US Pharmacopeial Convention.9
Pharmacokinetic Analysis
Concentrations for itraconazole after the oral dose of each formulation in each dog were analyzed using noncompartmental analysis and a pharmacokinetic program.6 The AUC from time 0 to the last measured concentration, defined by the LOQ, was calculated using the log‐linear trapezoidal method. The AUC from time 0 to infinity was calculated by adding the terminal portion of the curve to AUC0‐cn. The terminal portion of the curve was estimated from the relationship Cn/λZ,, where λZ is the terminal slope of the curve and Cn is the last measured concentration point. Mean residence time (MRT), systemic clearance (CL), and apparent volume of distribution (VD) were calculated using statistical moment theory according to methods described by Gibaldi and Perrier.10 Because an accompanying intravenous dose of the drug was not administered, the values for CL and VD were expressed as per the fraction absorbed (CL/F and VD/F).
Bioequivalence Analysis
In addition to descriptive pharmacokinetic analysis of the 3 itraconazole formulations in dogs, we performed bioequivalence analysis using the tests accepted by the FDA.6 Bioequivalence, sometimes referred to as relative bioavailability, indicates that the test (generic and compounded itraconazole) and reference (innovator) formulations are pharmacokinetically equivalent and are predicted to produce the same therapeutic effect.
The procedure accepted by the FDA6 uses a statistical analysis for the pharmacokinetic parameters AUC and CMAX based on 2 one‐sided test procedures of a comparison of the test and reference formulation.11 This approach is termed average bioequivalence6 and involves the calculation of a 90% confidence interval for the ratio of the average of the test and reference formulation parameter. To claim bioequivalence, the calculated 90% confidence interval should fall within a bioequivalence limit from 80% to 125% for the ratio of the product averages. The FDA guidance6 on this test recommends that it is performed as a crossover study with random assignment of the possible sequences of drug administration. It also recommends that the pharmacokinetic parameters be evaluated using a log‐transformed scale because logarithmic transformations are more likely to follow a normal distribution. A power calculation was also performed for this analysis: Power = 1 − probability of a type II statistical error.
Results
All dogs tolerated each drug administration. No vomiting or regurgitation occurred. Some dogs did not readily eat the small meal after drug administration and had to be syringe fed. Samples were successfully collected from all dogs in the study at each predetermined time point and processed for analysis.
Pharmacokinetic Analysis
The pharmacokinetic analysis and plasma drug concentrations versus time curves for each itraconazole formulation are presented in Table 1 and Figure 1. The AUC and CMAX attained from the compounded formulation were small in comparison to the generic and reference formulations. In contrast, the AUC, half‐life, MRT, and CMAX were similar for the generic and reference formulations, but high variability was observed as shown by Geometric mean CV%.
Table 1.
Pharmacokinetic parameters for itraconazole determined from noncompartmental analysis in healthy Beagle dogs (n = 9) administered each of 3 formulations (compounded, generic, and reference standard)
| Value | Unit | Compounded | Generic | Reference | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Mean | CV% | n | Mean | CV% | n | Mean | CV% | ||
| AUC%ex | % | 7 | 41.31 | 75.40 | 9 | 31.37 | 45.40 | 9 | 32.55 | 38.80 |
| AUC0‐cn | μg h/mL | 9 | ND | ND | 9 | 8.45 | 31.76 | 9 | 8.10 | 56.25 |
| AUC0‐∞ | μg h/mL | 7 | 1.10 | 179.9 | 9 | 13.68 | 50.19 | 9 | 12.58 | 72.95 |
| CL/F | mL/h/kg | 7 | 9.04 | 200.4 | 9 | 0.76 | 40.72 | 9 | 0.82 | 78.08 |
| Cmax | μg/mL | 9 | 0.06 | 62.00 | 9 | 1.19 | 35.59 | 9 | 1.37 | 58.30 |
| T1/2 | hour | 7 | 10.84 | 144.7 | 9 | 6.79 | 79.26 | 9 | 7.14 | 38.37 |
| Elim. rate | hour | 7 | 0.06 | 144.7 | 9 | 0.10 | 79.26 | 9 | 0.10 | 38.37 |
| MRT | hour | 7 | 18.96 | 112.9 | 9 | 12.16 | 60.46 | 9 | 11.43 | 31.22 |
| Tmax | hour | 8 | 4.2 | 51.85 | 9 | 3.4 | 48.79 | 9 | 3.0 | 60.84 |
| VD/F | L/kg | 7 | 141.3 | 60.0 | 9 | 7.410 | 60.0 | 9 | 8.46 | 64.7 |
Mean, geometric mean; CV%, geometric mean coefficient of variation; AUC0‐cn, area under the curve from time zero to the last measured time point; AUC0‐∞, area under the curve from time zero to infinity; AUC%ex, percent of area under the curve extrapolated from time Cn to infinity; CL/F, clearance per fraction absorbed; CMAX, peak plasma concentration; T1/2, terminal half‐life; Elim. rate, terminal elimination rate; MRT, mean residence time; TMAX, time to peak concentration; VD/F, apparent volume of distribution per fraction absorbed; ND, could not be determined because of excessive low plasma concentrations.
Figure 1.
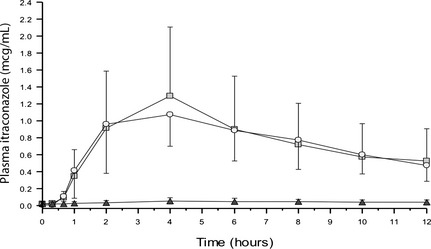
Plasma itraconazole concentrations over time for 9 healthy Beagle dogs after oral administration of 100 mg innovator‐formulated (□), generic (∘), and compounded (▴) itraconazole. The error bars represent standard deviation.
Bioequivalence Analysis
Bioequivalence results for compounded and generic itraconazole are presented in Tables 2 and 3. The average ratios of AUC and CMAX for compounded itraconazole versus the reference formulation did not meet the acceptance criteria for bioequivalence. Although the generic formulation of itraconazole appeared to perform similar to the reference formulation, it also did not meet acceptance criteria for bioequivalence. As seen in Table 4, the AUC and CMAX average ratios were 104.2% and 86.34%, respectively, of the reference formulation. However, the upper 90% confidence interval for AUC for the generic formulation was 152.6%, exceeding the acceptable range of 80–125%. The lower 90% confidence intervals were 71.25% and 60.08% for AUC and CMAX, respectively, which were both below the acceptable range. Bioequivalence data for individual dogs are presented online as supplemental materials.
Table 2.
Bioequivalence data for reference and compounded itraconazole administered to 9 healthy Beagle dogs
| Subject | AUC (μg h/mL) | CMAX (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Compounded | Difference | Ratio (%) | Reference | Compounded | Difference | Ratio (%) | |
| Mean | 9.10 | 0.46 | −8.64 | 5.64 | 1.54 | 0.06 | −1.49 | 4.39 |
| SD | 4.53 | 0.34 | 4.48 | 3.55 | 1.02 | 0.08 | −0.94 | 7.91 |
| Range | 3.58–17.61 | 0–1.04 | −17.15 to −3.33 | 0–9.64 | 0.46–2.98 | 0–0.1 | −2.95 to −0.43 | 0–7.91 |
Difference, difference between the compounded and reference formulations; Ratio (%), ratio of the value for the compounded formulation to reference formulation expressed as a percent; SD, standard deviation.
Values were log‐transformed for comparisons.
Table 3.
Bioequivalence data for reference and generic itraconazole administered to 9 healthy Beagle dogs
| Subject | AUC (μg h/mL) | CMAX (μg/mL) | ||||||
|---|---|---|---|---|---|---|---|---|
| Reference | Generic | Difference | Ratio (%) | Reference | Generic | Difference | Ratio (%) | |
| Mean | 9.10 | 8.82 | −0.28 | 124.5 | 1.54 | 1.25 | −0.30 | 101.7 |
| SD | 4.53 | 2.78 | 5.18 | 83.35 | 0.74 | 0.39 | 0.88 | 56.56 |
| Range | 3.58–17.61 | 5.74–12.84 | −4.77 to 8.53 | 41.27–303.15 | 0.46–2.98 | 0.71–1.76 | −2.28 to 0.69 | 23.65–194.83 |
Difference, difference between the generic and reference formulations; Ratio (%), ratio of the value for the generic formulation to reference formulation expressed as a percent; SD, standard deviation.
Values were log‐transformed for comparisons.
Table 4.
Bioequivalence calculations for log‐transformed values of AUC and CMAX for comparison of compounded and generic itraconazole to the reference formulation administered PO to 9 healthy Beagle dogs
| Value | AUC | CMAX | ||
|---|---|---|---|---|
| Compounded | Generic | Compounded | Generic | |
| 90% CIlower (%) | 3.72 | 71.25 | 2.84 | 60.08 |
| 90% CIupper (%) | 8.18 | 152.6 | 6.02 | 124.1a |
| Ratio (%) | 5.52 | 104.2 | 4.13 | 86.34 |
| Difference | −2.90 | 0.04 | −3.19 | −0.15 |
| SE (+/−) | 0.22 | 0.22 | 0.21 | 0.21 |
| Power | 0.24 | 0.25 | 0.25 | 0.26 |
CI, confidence interval; Difference, difference between the test (compounded or generic) and reference formulations; Ratio (%), ratio of the value for the test (compounded or generic) to reference formulation expressed as a percent; SE, standard error between formulations.
Statistically bioequivalent.
Also shown in Table 4 is the power calculation for this analysis. The power of the test was in the range of 0.24–0.26 for all calculations. This is much less than the desired power level of >0.80 to prove bioequivalence.
Discussion
Lack of bioequivalence was found for generic and compounded itraconazole in comparison with the reference formulation in this study. The average AUC for the generic formulation was higher, the half‐life similar, and CMAX only slightly lower than for the reference standard. Although the generic formulation did not meet the statistical criteria to claim bioequivalence to the brand name product, the absolute ratio of generic formulation to brand name formulation was higher for AUC with a value of 104.2%. In contrast, the compounded formulation of itraconazole had very low absorption and bioavailability.
The most likely reason for the lack of statistical bioequivalence between the generic and brand name products is low statistical power. Posthoc calculations revealed the power of the test was only 0.25 and 0.26 for AUC and CMAX, respectively (Table 4). The 2 most common reasons for underpowered tests are high interindividual variability and low numbers of subjects in a study. High interindividual variability in this study, as evidenced by the high coefficients of variation for measured parameters, is the likely cause of poor power. It is likely that bioequivalence could have been demonstrated for the generic product if a larger number of subjects had been used.
High interindividual variability, because of drug insolubility and variable metabolism among dogs, is an inherent problem with itraconazole administration. Itraconazole is a highly lipophilic but poorly soluble (Biopharmaceutics Classification System Class II) drug.12 It must dissolve in the gastrointestinal tract to be absorbed, which is difficult to accomplish. Once dissolved, however, it is highly permeable and will easily diffuse across the intestinal epithelium. The original innovator, itraconazole is formulated with specially designed sugar spheres coated with the drug in the capsule to increase surface area and solubility of the drug. The absorption of this formulation of itraconazole is also improved with a low gastric pH and the presence of food, though feeding induces high variability in gastric emptying time in dogs.13 For these reasons, the absorption of itraconazole capsules in dogs is variable, as was observed in this study.
Generic itraconazole, available as capsules, has been approved for use in people and has been commercially available since 2004. There are 3 companies that supply generic capsules. The product we selected for this study was manufactured by the parent company of the original innovator formulation of itraconazole. It is not known whether results of this study would be applicable to the other 2 formulations of generic itraconazole. Based on the results of this study, use of this generic form of itraconazole in dogs with systemic fungal infections could produce equivalent therapeutic results while substantially decreasing treatment costs. Clinicians should be wary that the generic and innovator itraconazole are not interchangeable, and treatment failure remains possible. Clinicians can have blood samples tested to ensure that adequate plasma concentrations are attained,14 but therapeutic plasma itraconazole concentrations have not been established for dogs. In people, trough serum itraconazole concentrations of at least 0.5–1.0 μg/mL, measured by HPLC, have been associated with therapeutic success.14 If a bioassay method is used to measure itraconazole concentrations, the values in people are 3.3 times higher than those obtained by HPLC because of presence of the bioactive metabolite hydroxyitraconazole.
There are no reported bioequivalence studies for compounded itraconazole, to the authors' knowledge. Regardless, compounding pharmacies promote their use for animals, and veterinarians and pet owners trust that they will be therapeutically equivalent to the brand name product. Veterinarians must be cognizant of the limitations of compounding medications. Compounding from bulk chemicals can produce a product that is much inferior to an original or generic formulation.4 In addition, compounding veterinary itraconazole from a bulk powder is prohibited by federal regulations.4 Very low absorption and bioinequivalence of compounded itraconazole were demonstrated in this study. The most likely reason is that the compounded formulation does not facilitate dissolution and thus absorption of the drug. The bulk form of itraconazole is a powder and does not contain any excipients or other formulation properties to improve oral absorption. Without modifications, this powder will not dissolve in water‐based liquids, such as those found in the stomach. Also, itraconazole can adsorb to glassware or plastics used during compounding, thus decreasing the potency of the product.15 The compounded product used in this study produced AUC and CMAX concentrations in dogs that were approximately 5% of the innovator product. Because of the poor performance of the compounded formulation, it should not be used as a substitute for brand name or generic itraconazole formulations.
The mean terminal half‐life of itraconazole in the dog is 28.0 ± 2.9 hours.16 Ordinarily, a minimum washout period between crossover studies is 7 half‐lives, which allows for >99% of the drug to be eliminated. The reported half‐life corresponds to a washout period of 7.6 days; therefore, this study used a washout of 8 days. Because very low, but detectable, itraconazole concentrations were observed in some dogs in samples collected at the zero time point, a longer washout period would have been desirable. Another limitation of the study is that samples were not collected long enough for a complete delineation of the AUC. The AUC left to be extrapolated beyond the last measured time point was very high (approximately 30% for generic and 40% for compounded itraconazole). This limitation could have been avoided if samples had been collected for a longer period of time and if there were more samples.
In conclusion, this study validates the recommendation against the use of compounded itraconazole for treatment of systemic fungal infections in dogs. The generic itraconazole used in this study was not bioequivalent to the original innovator itraconazole, but it could be considered for use in fungal infection because of the favorable oral pharmacokinetics observed with the added benefit of a lower cost.
Supporting information
Table S1. Individual bioequivalence data for reference and compounded itraconazole administered to 9 healthy Beagle dogs.
Table S2. Individual bioequivalence data for reference and generic itraconazole administered to 9 healthy Beagle dogs.
Acknowledgments
This project was supported by a grant from the Companion Animal Fund, University of Tennessee College of Veterinary Medicine. The authors thank Ms Delta Dise, North Carolina State University Clinical Pharmacology Laboratory, for her expert technical assistance and HPLC analysis, and Ms Missy Smith LMVT, University of Tennessee, for her technical assistance.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Footnotes
Sporanox (Itraconazole) Capsules 100 mg. Manufactured by: PriCara, Division of Ortho‐McNeil‐Janssen Pharmaceuticals, Inc. Pharmaceuticals, Inc, Raritan, NJ. Capsule contents manufactured by: Janssen Pharmaceutica N.V., Olen, Belgium
Itraconazole Capsules (100 mg); Patriot Pharmaceuticals, LLC, Horsham, PA. Capsule contents manufactured by: Janssen Pharmaceutica N.V., Olen, Belgium
Itraconazole Powder (Certificate of Analysis: item/Lots:691367/Lot:11290921, 685429/Lot:11290922, 685430/Lot: 11290923, 685431/Lot;11290924); Letco Medical, Decatur, AL
Ora‐Blend, Paddock Laboratories, Minneapolis, MN
Research Diagnostics Inc, Flanders, NJ
Phoenix Pharmacokinetic Software; Phoenix WinNonlin, Pharsight Inc, Mountain View, CA
References
- 1. Davis JL, Papich MG, Heit MC. Antifungal and antiviral drugs In: Riviere JE, Papich MG, eds. Veterinary Pharmacology and Therapeutics, 9th ed Ames, IA: Wiley‐Blackwell; 2009:1013–1049. [Google Scholar]
- 2. Legendre AM. Blastomycosis In: Greene CE, ed. Infectious Disease of the Dog and Cat, 4th ed St. Louis, MO: Elsevier Saunders; 2012:606–614. [Google Scholar]
- 3. Legendre AM, Rohrbach BW, Toal RL, et al. Treatment of blastomycosis with itraconazole in 112 dogs. J Vet Intern Med 1996;10:365–371. [DOI] [PubMed] [Google Scholar]
- 4. US Food and Drug Administration . Guidance for FDA Staff and Industry: Compliance Policy Guides Manual, Sec. 608.400, Compounding of Drugs for Use in Animals. Rockville, MD: FDA; 2003. Available at: http://www.fda.gov/ICECI/ComplianceManuals/CompliancePolicyGuidanceManual/ucm074656.htm. Accessed April 26, 2013. [Google Scholar]
- 5. United States Pharmacopeial Convention . General Chapter <795> Pharmaceutical compounding – Nonsterile preparations (34th Revision). Rockville, MD: United States Pharmacopeial Convention; 2010. Available at: http://forums.pharmacyonesource.com/phos/attachments/phos/pharmacy_ops/883/1/USP%2034_795.pdf. Accessed May 2, 2013. [Google Scholar]
- 6. US Food and Drug Administration . Guidance for industry: Bioavailability and bioequivalence studies for orally administered drug products — General considerations (Revision 1). Rockville, MD: FDA; 2003. Available at: http://www.fda.gov/downloads/Drugs/…/Guidances/ucm070124.pdf. Accessed April 26, 2013. [Google Scholar]
- 7. Smith JA, Papich MG, Russell G, Mitchell MA. Effects of compounding on pharmacokinetics of itraconazole in black‐footed penguins (Spheniscus demersus). J Zoo Wildl Med 2010;41:487–495. [DOI] [PubMed] [Google Scholar]
- 8. Davis JL, Salmon JH, Papich MG. Pharmacokinetics and tissue distribution of itraconazole after oral and intravenous administration to horses. Am J Vet Res 2005;66:1694–1701. [DOI] [PubMed] [Google Scholar]
- 9. United States Pharmacopeial Convention . General Chapter 1225 Validation of Compendial Procedures. Rockville, MD: United States Pharmacopeial Convention; 2010. [Google Scholar]
- 10. Gibaldi M, Perrier D. Pharmacokinetics, 2nd ed New York, NY: Marcel Dekker; 1982. [Google Scholar]
- 11. Schuirmann DJ. A comparison of the two‐one‐sided tests procedure and the power approach for assessing the equivalence of average bioavailability. J Pharmacokinet Biopharm 1987;15:657–680. [DOI] [PubMed] [Google Scholar]
- 12. Kasim NA, Whitehouse M, Ramachandran C, et al. Molecular properties of WHO essential drugs and provisional biopharmaceutical classification. Mol Pharm 2004;1:85–96. [DOI] [PubMed] [Google Scholar]
- 13. Martinez MN, Papich MG. Factors influencing the gastric residence of dosage forms in dogs. J Pharm Sci 2009;98:844–860. [DOI] [PubMed] [Google Scholar]
- 14. Goodwin ML, Drew RH. Antifungal serum concentration monitoring: an update. J Antimicrob Chemother 2008;61:17–25. [DOI] [PubMed] [Google Scholar]
- 15. Wong JW, Nisar U‐R, Yuen KH. Liquid chromatographic method for the determination of plasma itraconazole and its hydroxyl metabolite in pharmacokinetic/bioavailability studies. J Chromatogr B 2003;798:355–360. [DOI] [PubMed] [Google Scholar]
- 16. Yoo SD, Kang E, Shin S, et al. Interspecies comparison of the oral absorption of itraconazole in laboratory animals. Arch Pharm Res 2002;25:387–391. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Individual bioequivalence data for reference and compounded itraconazole administered to 9 healthy Beagle dogs.
Table S2. Individual bioequivalence data for reference and generic itraconazole administered to 9 healthy Beagle dogs.


