Abstract
Background
Primary hyperaldosteronism (PHA) in cats occurs as a consequence of excessive hormone production by an adrenocortical tumor. Median survival time, association between tumor type and prognosis, and the likelihood that cats require continued medical therapy after surgery have not been systematically evaluated.
Objectives
To determine the median survival time of cats with PHA treated by unilateral adrenalectomy. To examine if tumor type, anesthesia time, or tumor location (left or right side) affect survival and if affected cats require continued postoperative treatment for persistent hypertension or hypokalemia.
Animals
Ten client‐owned cats.
Methods
Retrospective study. Cats were diagnosed with PHA based on clinical signs, increased plasma aldosterone concentration, and advanced imaging. Cats underwent unilateral adrenalectomy. Survival time (days alive after surgery) was determined for each cat. Factors affecting median survival time were investigated, including histopathology, anesthesia time, and location (side) of the tumor.
Results
Eight of 10 cats survived to discharge from the hospital post adrenalectomy. Overall median survival was 1,297 days (range 2–1,582 days). The only significant factor affecting median survival time was anesthesia time >4 hours. Tumor type and location (side) did not significantly affect median survival time. No cats required continued medical treatment for PHA.
Conclusions and Clinical Importance
Although PHA in cats is still considered an uncommon condition, it should be considered in middle to older aged cats with hypokalemic polymyopathy and systemic hypertension. Surgical correction by unilateral adrenalectomy is a viable approach to definitive treatment of PHA with no need for continued medical management.
Keywords: Adrenal tumor, Feline, Hypertension, Hypokalemia
Abbreviations
- PHA
primary hyperaldosteronism
- CRI
continuous rate infusion
- CT
computed tomography
Primary adrenal tumors account for 0.2% of all neoplasms in cats.1 Some adrenocortical tumors secrete excessive amounts of aldosterone, leading to hypokalemia and systemic arterial hypertension (Conn's syndrome). This disease was considered rare in humans and companion animals for many decades,2, 3, 4, 5 but recently has been found to be more prevalent with improved screening tests and detection in both humans and cats.6, 7 Since the first reported case of primary hyperaldosteronism in a cat in 1983,8 there have been only a few case reports of feline primary hyperaldosteronism (PHA).9, 10, 11, 12, 13, 14, 15, 16, 17, 18
Six different subtypes of PHA have been identified in humans, the most common being bilateral idiopathic hyperaldosteronism or an aldosterone‐producing adenoma.19, 20 Less common subtypes in humans include aldosterone‐producing renin‐responsive adenomas, primary adrenal hyperplasia, and genetic or familial forms of hyperaldosteronism.19, 20 In humans, the most common cause of idiopathic hyperaldosteronism is bilateral hyperplasia, which is treated medically. In human patients with evidence of a unilateral source of aldosterone excess and PHA, unilateral adrenalectomy is the treatment of choice.7 In patients with aldosterone‐secreting adenomas, surgery results in resolution of hypertension 50–60% of the time.23 Most studies in humans focus on 1 predictor of success, systemic hypertension, because most responsible tumors are benign adenomas.21 The main focus is on the duration of hypertension before surgery, because this is well documented in studies of affected humans. However, in cats, systolic blood pressure measurements are not routine. Other predictors of success were investigated in studies of affected humans, but were not significant including age, duration of hypokalemia, plasma aldosterone concentration, and other genes associated with human aldosterone biosynthesis.21 It is rare for a histopathologic diagnosis of an adrenal carcinoma to be obtained in humans.21 In cats, most cases of PHA are attributed to unilateral adrenal adenomas or carcinomas.19 There are only a few reports of unilateral adrenalectomies in cats, and perioperative complications of hemorrhage and death were reported in 8 of 18 surgical cases,11, 12, 16, 17, 19, 22, 23, 24 with 6 of those reported as fatalities.
The purpose of this study was to determine the median survival time of cats with primary hyperaldosteronism treated with by unilateral adrenalectomy. The study also evaluated the effect of tumor type, anesthesia time, and location (side) of the tumor on survival, and the requirement for postoperative treatment with antihypertensive or diuretic medication or potassium supplementation.
Materials and Methods
Medical records of cats that had a diagnosis of PHA caused by an adrenal tumor and were treated by adrenalectomy at 2 specialty veterinary hospitals between 2002 and 2012 were reviewed. Cats that had a diagnosis of PHA caused by an adrenal tumor and were not treated surgically were excluded. Cats that were treated by adrenalectomy but for which records were incomplete were excluded. Nine surgeries were performed at the University of Pennsylvania Veterinary Hospital by board‐certified surgeons. One surgery was performed at the Veterinary Specialty and Emergency Center – Levittown, PA by a board‐certified surgeon. PHA was diagnosed by confirmation of increased plasma aldosterone concentration and identification of a unilateral adrenal mass by ultrasonographic or computed tomographic (CT) imaging of the abdomen. Data collected included signalment, body weight, clinical signs, physical examination findings, clinicopathologic data, diagnostic imaging results, tumor size and location, surgical procedure, perioperative complications, postoperative complications, and survival times. All cats included had histopathologically confirmed adrenocortical tumors. Owners and referring veterinarians were interviewed to obtain long‐term follow‐up information. Interview questions included whether or not the cats needed continued medical treatment of PHA, date of death, known cause of death, and owner satisfaction with original surgery. Necropsy was not routinely performed.
Anesthesia and Surgery
Routine anesthesia was performed with all 10 cats as previously described.24 Routine adrenalectomy by open laparotomy was performed in 9 cats as described.25 In 1 patient, a unilateral left adrenalectomy was performed using a laparoscopic adrenalectomy procedure.24
Histopathology
Histopathology was performed by board‐certified pathologists. Tumors were classified histopathologically as adenomas or carcinomas based on the absence or presence of microscopic invasion, other features of malignancy, or both. In the carcinomas, densely packed cortical epithelial cells effaced the normal adrenal gland parenchyma with central necrosis. Invasion into the capsule was noted in carcinomas. In adrenocortical adenomas, individual neoplastic cells had round, chromatic nuclei with a central nucleolus and an extensive amount of eosinophilic granular cytoplasm.
Statistical Analysis
Median survival times were determined by the use of the Kaplan‐Meier product limit method and log rank analysis was used to compare survival curves among groups. Cox multivariable survival methods were employed to determine which factors were associated with survival time after adrenalectomy. Factors investigated included anesthesia time, histopathology (adenoma versus adenocarcinoma), and which adrenal gland was affected (right versus left). Any factors identified with a P value <.20 on univariate analysis were tested for significance in the multivariable model. Factors with P values <.05 in the multivariable model were retained. Survivor functions controlling for significant variables were plotted and the log rank test was used to determine the equality of those functions. The proportional hazards assumption was tested using Schoenfeld residuals. All analyses were performed by statistical software.1
Results
Ten cats were identified that meet the inclusion criteria between 2002 and 2012. The median age of cats was 12.3 years (range 9–15 years). The median weight was 5.0 kg (range 2.8–7.4 kg). Five were categorized as obese to overweight, 1 was of normal weight, and 4 were underweight. Eight were castrated males and 2 were spayed females. Nine were domestic short‐haired cats and 1 was a domestic long‐haired cat.
Clinical Signs and Physical Examination Findings
Initial presenting clinical signs and physical examination findings in the 10 cats included findings consistent with hypokalemic polymyopathy (generalized weakness, limb stiffness, neck ventroflexion, collapse, plantigade stance, or some combination of these; n = 10), systolic heart murmur (n = 9), hypertension (systolic blood pressure >180 mmHg26, 27; n = 8), ophthalmologic abnormalities (anisocoria, retinal hemorrhage, blindness; n = 3), polyuria (n = 3), polydipsia (n = 3), dysphagia (n = 2), and polyphagia (n = 2). Median systolic blood pressure was 189 mmHg (10 cats; range 130–230 mmHg). Two cats had hypertension documented for at least 1 month.
Diagnostic Testing
Routine serum biochemical analysis identified hypokalemia (n = 7). Serum potassium concentration was low in 7 cats and low normal (3.5 mmol/L; normal, 3.5–4.8 mmol/L) in 2 cats. Creatine kinase activity was measured in 6 cats and was increased in all cats with a median activity of 31,221 U/L (range 979–101,641 U/L; normal, 49–688 U/L). Plasma aldosterone concentrations were increased in all cats (n = 10). Aldosterone concentrations ranged from 1253 ng/dL to >3329 ng/dL, the limit of measurement (normal, 194–388 ng/dL). No changes consistent with hyperadrenocorticism were identified on the biochemical analysis. Thus, no additional adrenal function testing was performed.
Imaging
Radiographic interpretation was made by board‐certified radiologists. Echocardiography was performed in 1 cat and showed left ventricular hypertrophy. Left ventricular hypertrophy was observed to be improved in a subsequent echocardiogram. All cats had either a full abdominal ultrasound examination (n = 8) or CT scan (n = 2) before surgery and a unilateral adrenal mass was found in all cases. The size of the adrenal tumors measured by imaging ranged from 1 to 4 cm. Tumor invasion of the vena cava was not seen in any cat evaluated by ultrasound examination. Tumor invasion of the caudal vena cava was seen by CT scan in 1 cat and invasion was verified in surgery.
Anesthesia
Anesthetic times were defined as the period from induction to extubation. An anesthetic protocol was determined for each cat based on information obtained by physical examination, diagnostic testing, and patient history. All anesthesia was performed by a board‐certified anesthesiologist or criticalist.
All cats survived the intraoperative period. Intraoperative complications included hypotension (n = 10), minimal hemorrhage not necessitating transfusion (n = 5), hemorrhage necessitating a packed red blood cell transfusion (n = 2), and ventricular arrhythmias (n = 1). The median anesthetic time was 211 minutes (range 70–310 minutes). Hypotension and arrhythmias were responsive to treatment.
Surgical Procedures
All surgeries were performed by a board‐certified surgeon. All cats underwent unilateral adrenalectomy. Six of the adrenal tumors were on the right side and 4 were on the left side. Vascular invasion was seen in 7 cats during surgery. Five adrenal tumors were visualized invading the phrenicoabdominal vein, 2 tumors invaded the caudal vena cava, and 1 of these with caudal vena cava invasion also had invasion near the renal vein. Both the tumors that invaded the caudal vena cava were right‐sided tumors. Tumor size ranged from 1 to 6 cm (long axis). Complete resection of the mass was achieved in 9 patients. In 1 patient, the adrenal tumor had extensive caudal vena cava and local abdominal invasion and was not deemed completely resectable by the surgeon. In that case, a partial adrenal resection and biopsy with a guillotine method was performed and packed red blood cell transfusion was given intraoperatively. In the 1 case in which a pancreatic mass was observed, a partial pancreatectomy of the distal right limb was performed for an excisional biopsy. In another case, an abnormally mottled spleen was seen on ultrasound examination and a concurrent splenectomy was performed for biopsy.
Postoperative Management and Complications
The average perioperative hospital stay (admission to discharge) was 4.8 days (range 3–10 days). Two cats were euthanized 2 days and 10 days postoperatively for complications associated with the surgery. These included lethargy, anorexia with hepatic lipidosis, anemia, metabolic acidosis, and continued hypotension. One cat had a nonresectable tumor and the other had a concurrent pancreatic mass. Both cats were not improving postoperatively, and the owners elected euthanasia without necropsy. Other complications in the remaining cats included lethargy (n = 5), anemia (n = 5), anorexia (n = 2), vomiting (n = 1), dysphagia (n = 2), hyperthermia (n = 1), upper respiratory infection (n = 1), and constipation (n = 1). Only 1 patient received 1 transfusion with packed red blood cells postoperatively. The remaining anemic patients did not receive blood transfusions. One patient had an esophagostomy tube placed before discharge. Systolic blood pressures were measured at different intervals after surgery and all results were within normal limits (normal, 100–180 mmHg).26, 27 Serum potassium concentrations were within normal limits in all patients before being discharged (normal, 3.5–4.8 mmol/L).
Histopathologic Diagnosis
Six tumors were adrenocortical carcinomas and 4 were adrenocortical adenomas. One cat also had splenic intermediate cell lymphoma and 1 cat had pancreatic carcinoma.
Long‐Term Outcome
Two of the 8 cats discharged from the hospital were readmitted for lethargy and anorexia 6 and 7 days after discharge, respectively. Both cats responded to supportive treatment (including fluid therapy, pain management, gastroprotectant medication, and 1 received an appetite stimulant) and were discharged. At this second hospitalization, these 2 cats did not need medical treatment for PHA (antihypertensive medication, potassium supplementation, or spironolactone). None of the 8 surviving cats needed continued medical treatment after unilateral adrenalectomy. Data from re‐evaluation at 6–15 days were available for 6 cats. Systolic blood pressures were measured, and all results were within normal limits (normal, 100–180 mmHg).26, 27 Serum potassium concentration levels also were measured and were within normal limits (normal, 3.5–4.8 mmol/L).
Overall median survival was 1,297 days (range 2–1,582 days). Anesthesia time was the only variable with a P value <.20 on univariate analysis (P = .035) and was retained in the model. As anesthesia time increased, the hazard for death increased. For every 60 minute increase in anesthesia time, the hazard increased by 8.7 (95% CI, 1.2–65.2; P = .035). Figure 1 shows the survival curves for cats stratified by > or <4 hours of anesthesia time. Cats with <4 hours of anesthesia time had a median survival of 1,329 days, whereas cats with >4 hours of anesthesia time had a median survival time of 10 days. These survival curves were significantly different (P = .049). There was no significant difference in survival between cats with adenoma (median, 1,329 days) compared with cats with adenocarcinoma (248 days; P = .20). There also was no significant difference in survival between right‐ (median, 1,297 days) and left‐ (248 days) sided tumors (P = .80).
Figure 1.
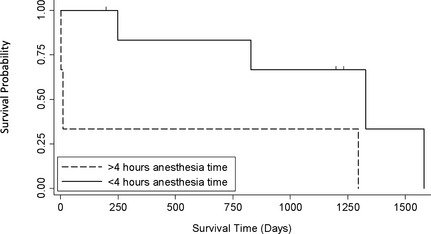
Survival curves for cats stratified by more or <4 hours of anesthesia time. Cats with <4 hours of anesthesia time had a median survival of 1,329 days, whereas cats with >4 hours of anesthesia time had a median survival time of 10 days (P = 0.049). The vertical tick marks represent the 3 patients that are still alive at the time these data were collected and their days still alive since discharge.
No deaths were known to be directly related to the adrenal tumor in any of the cats discharged from the hospital. Two cats were euthanized for chronic kidney disease (1,582 and 1,297 days), 1 was euthanized for cardiomyopathy and subsequent aortic thrombus (1,329 days), 1 for oral squamous cell carcinoma (823 days), and 1 for unknown cause (248 days).
Discussion
With appropriate case selection, this study suggests that unilateral adrenalectomy can be an efficacious treatment for PHA in cats. Overall, median survival was 1,297 days (range 2–1,582 days). Three cats were still alive at last follow‐up (194, 1,195, 1,229 days after surgery). In this study, the only significant factor affecting median survival time was anesthesia time >4 hours. The tumor type and location (side) did not significantly affect median survival time. None of the cats in the study needed additional medical treatment because their hypokalemia and clinical signs resolved with tumor removal.
For cats that underwent surgical adrenalectomy in other studies, survival times were between 240 and 1,803 days.8, 9, 17 Unfortunately, perioperative complications resulted in a high frequency of euthanasia before discharge. These major complications such as hemorrhage and sepsis were reported in 8 of 18 surgical cases11, 12, 16, 17, 25 and 6 of those were fatalities. In our study, 2 of 10 cats did not survive to discharge because of postoperative complications associated with hypotension, metabolic acidosis, lethargy, anorexia, and hepatic lipidosis.
One cat that was euthanized because of postoperative complications had a nonresectable tumor with both caudal vena cava invasion and extensive local invasion into the renal vein and surrounding tissue. This cat most likely was not an optimal surgical candidate, but the owner chose to pursue surgery. A more complicated invasive adrenal tumor such as one invading into the vasculature may lengthen the anesthesia time, which significantly affected median survival in this study. Adrenalectomy and venotomy for thrombus removal are more technically demanding than adrenalectomy alone. A recent report of adrenal tumors in dogs showed that there was no significant difference in morbidity or mortality between those undergoing adrenalectomy with or without vena cava tumor thrombi.28 The reported mortality rate was 22% overall. The authors concluded that correct case selection and surgeon experience might have influenced their results.28 It is not clear what factors might have contributed to the higher survival rate in our study compared with the previous studies with mortality of 6/18 cats,11, 12, 16, 17, 25 but the majority of cases were performed at a tertiary referral center with anesthesia and intensive care specialists similar to the study of dogs described above.28 Proper selection of surgical cases such as patients without known metastatic disease and extensive local invasion of the adrenal tumor are most likely important factors for overall survival and may contribute to the higher survival rate in this study. However, our median survival time may not accurately reflect a true overall survival rate of all cats undergoing adrenalectomy for PHA if these same selective criteria were not used.
One of the included cases in this study did undergo laparscopic adrenalectomy instead of a laparotomy procedure.24 Studies in humans have shown that in appropriately selected cases, laparscopic adrenalectomy is an effective tool for controlling PHA.29 It is unknown whether this procedure affected the median survival rate in our patients, but this patient did undergo complete resection of the left adrenal gland and median survival time was approximately 44 months.24 The use of laparoscopic adrenalectomy is based on surgeon experience and appropriate selection of cases such as cats without intravascular invasion and metastatic disease.24
In all cases, imaging identified a mass associated with the adrenal gland. It was important for surgical planning to address invasion of tumor into the surrounding vasculature.18 Two tumors invaded the caudal vena cava at surgery and both were right‐sided adrenal tumors. Because right‐sided tumors were reported more likely to invade the vena cava,25, 30 the location (side) of the tumor was investigated as a potential factor for survival, but location was not a factor affecting median survival time. Only 1 adrenal tumor was reported before surgery as invasive, and this tumor was imaged by CT. The second was imaged by ultrasound examination and vascular invasion was not reported. No data are available in cats addressing which imaging modality is most sensitive and specific for caval tumor invasion. In a recent study of dogs, abdominal ultrasound examination was 100% sensitive and 96% specific in identifying the presence of a tumor in the caudal vena cava.31 CT has been shown to have a sensitivity of 92% and a specificity of 100% for detection of tumor thrombi in dogs.32 Although either imaging modality may be used, the results may be patient, machine, and radiologist dependent. The CT scan may hold an advantage attributable to the use of contrast enhancement as well as potential concurrent imaging of the chest cavity for metastasis.
Anesthesia time has been included in analyses of survival risk factors for adrenalectomy in dogs, but has been variable with its effect on median survival times.33, 34 A recent study showed that anesthetic deaths are more common in cats than dogs and that most of these deaths occurred postoperatively.35 In a study of dogs undergoing unilateral adrenalectomy for pheochromocytoma, it was seen that decreased anesthetic time significantly improved survival.36 In our study population, anesthesia time >4 hours significantly affected the median survival time and affected cats were more likely to die before discharge. Although this outcome may be multifactorial, the most common intraoperative complication was hypotension, which occurred in every cat. Marked or prolonged intraoperative hypotension is associated with increased anesthetic‐related morbidity and mortality in veterinary patients.36, 37 Fifty‐seven percent of deaths intra‐ and postoperatively were associated with cardiovascular or respiratory causes in cats.36 Increasing the intended duration of procedure and fluid therapy associated with hypotension also were associated with increased odds of anesthetic‐related death.35, 37 As mentioned previously, the complexity of the adrenalectomy procedure also may contribute to an anesthetic time >4 hours. In these adrenal surgeries in cats, decreasing anesthesia time and careful intraoperative monitoring and treatment may improve survival.
Medical management alone for PHA has been described in 3 cats17 and normally entails the use of spironolactone treatment, potassium supplementation, and antihypertensive drugs. Correction of hypokalemia was possible in 1 cat, but the others either required increasing doses of spironolactone to maintain normokalemia or normokalemia could not be achieved. In this study, 7 cats were hypokalemic on presentation (<3.5 mmol/L). Two cats had serum potassium concentrations of 3.5 mmol/L (normal, 3.5–4.8 mmol/L) and displayed polymyopathy consistent with hypokalemia. All 9 of these cats achieved normokalemia and resolution polymyopathy post adrenalectomy (normal, 3.5–4.8 mmol/L) without continued potassium supplementation. Hypertension also was refractory to medical management.17 Reported survival times for cats treated medically range from 50 to 984 days.8, 9, 17 However, medical management only addresses clinical signs and does not treat the underlying adrenal mass, acute tumor hemorrhage,32 distant metastasis,1, 6, 30 or tumor invasion into the caudal vena cava or renal vessels.1
Hypertension was resolved in all cats in which blood pressure was measured postoperatively. This outcome is consistent with previous studies in which up to 82% of cats had complete resolution of hypertension.19 In humans, resolution of hypertension occurs in 50–60% of patients after unilateral adrenalectomy.21 A lesser degree of mild hypertension persists in as many as 40% of human patients, but the criteria for hypertension in humans are more stringent.26, 27 In humans, predictors for resolution of hypertension after adrenalectomy include shorter duration of hypertension, number of hypertensive medications, preoperative response to spironolactone, and benign adenoma rather than hyperplasia or carcinoma.20 Nine cats had systolic heart murmurs at presentation, which may have been caused by concurrent disease, but may be exacerbated by the underlying hyperaldosteronism. Systemic hypertension may contribute to myocardial hypertrophy,19 and resolution of systemic hypertension actually may improve left ventricular function in some patients as demonstrated in 1 patient in this study in which repeated echocardiography was performed. Cardiac abnormalities also may develop because hypokalemia increases automaticity and delays ventricular repolarization.38, 39
Chronic renal disease can be the primary cause of arterial hypertension and hypokalemia in cats; it can also be a consequence of PHA. Arterial hypertension and hypokalemia are commonly treated symptomatically without a thorough search for the underlying cause in cats.6 Blood pressure measurement is not always considered part of the minimum database for routine evaluation of cats. This can lead to a lack of recognition of PHA as seen in the human population. This failure to recognize the disorder may exclude a number of cats from receiving appropriate treatment and potential cure for their underlying disease.
Investigation of suspected PHA includes measuring plasma aldosterone concentrations, as performed in this study. Additional diagnostic tests include aldosterone:renin ratio testing.6, 22 Although considered a gold standard in human medicine,7, 20 measurement of plasma renin activity is not as easily performed in veterinary medicine. Results of plasma renin activity should be interpreted in comparison with a control population that may not be readily available and leads to increased cost. For handling, approximately 4 mL of blood is needed from a cat with instant freezing and sending of serum samples. Repeated sampling also may be necessary as 1 normal aldosterone:renin ratio result does not exclude PHA in cats.6 Because of these difficulties with obtaining and measuring plasma renin activity, it is not a practical test at this time. In humans and cats, a fludrocortisone suppression test also has been suggested to determine if an aldosterone:renin ratio is increased attributable to PHA. However, this test has not been validated in the feline population at this time and may have false negatives.7 At this time, clinical signs coupled with a thorough physical examination, laboratory abnormalities, and abdominal imaging are the gold standard for diagnosis of PHA. Furthermore, none of these cats was diagnosed with hyperadrenocorticism concurrently, and postoperative monitoring and treatment for Addison's disease was not necessary.
Eight of the cats were hypertensive on presentation, but clinical signs associated with hypertension such as blindness caused by retinal detachment were not as common as previously reported.19 Five cats presented with ocular changes such as anisocoria or ocular hemorrhage on ophthalmologic examination, but only 2 cats had associated blindness with retinal detachment. Only 1 blind cat with retinal detachment survived to discharge, and it was reported by the owner that the blindness resolved gradually over a few weeks after adrenalectomy. Half of these cats presented with ocular changes associated with hypertension and a careful ophthalmologic examination is warranted when these patients present for evaluation.
For all cats in which the reason for death was available from referral veterinarians or owners, no deaths appeared to be directly related to the adrenal tumor. However, 2 cats were euthanized because of chronic renal disease that could have been exacerbated by the PHA.
Limitations of this study were consistent with those of other retrospective studies. There were a very small number of cases over 10 years, because PHA is a rare disease. There was no available control group for comparison in this study. Because of the small data group, the Kaplan‐Meier survival curves included all patients, even the two that did not survive to discharge. It is possible with a larger cohort of cats that the survival curve for those that survive to discharge may be similar for cats with > and <4 hours anesthesia time. However, exclusion of those cats would necessitate a larger group of patients with >4 hours of anesthesia time. Complete data and follow‐up were not always available and cause of death was not always definitively known. Data to address other predictors of success for survival time such as duration of hypertension and response to specific medications such as spironolactone alone were not available. Data regarding complete staging and postoperative monitoring were not consistently available in this population of cats and thus reliable conclusions about disease‐free interval could not be made. To obtain more complete follow‐up data, all referring veterinarians and owners were contacted and questioned, which can introduce bias. There may have been a case selection bias influenced by owner and surgeon decisions regarding a patient's suitability for surgery.
Conclusion
Unilateral adrenalectomy for cats with PHA may be a viable treatment modality with appropriate case selection. The tumor type (adenoma versus adenocarcinoma) or the location (side) of the tumor was not a significant factor for long‐term survival if the tumor was completely resected during surgery. However, overall median survival time was affected by an anesthesia time of >4 hours. With complete resection of the adrenal tumor, the clinical signs resolve and cats do not need any additional medical treatment related to PHA.
Increased screening for systemic hypertension and recognition of common clinical signs may lead to improvement in earlier detection and treatment of feline PHA in the future.
Acknowledgment
Conflict of Interest Declaration: Authors disclose no conflict of interest.
The work was performed at the University of Pennsylvania Veterinary Hospital, School of Veterinary Medicine, Department of Clinical Studies ‐ Philadelphia, PA and at the Veterinary Specialty and Emergency Center û Levittown, PA. The study was not supported by a grant. The study was not presented at any meetings. There are no special acknowledgments.
Footnote
Stata version 11, StataCorp, College Station, TX
References
- 1. Lunn KF, Page RL. Tumors of the endocrine system In: Withrow SJ, Vail DM, eds. Withrow and McEwen's Small Animal Clinical Oncology, 5th ed St. Louis, MO: Saunders Elsevier; 2013:504–531. [Google Scholar]
- 2. Conn JW. Primary aldosteronism, a new clinical syndrome. J Lab Clin Med 1955;45:3–17. [PubMed] [Google Scholar]
- 3. Gunn‐Moore D. Feline endocrinopathies. Vet Clin North Am Small Anim Pract 2005;35:171–210. vii. Review. [DOI] [PubMed] [Google Scholar]
- 4. Ahn A. Hyperaldosteronism in cats. Semin Vet Med Surg (Small Anim) 1994;9:153–157. [PubMed] [Google Scholar]
- 5. Chiaramonte D, Greco DS. Feline adrenal disorders. Clin Tech Small Anim Pract 2007;22:26–31. [DOI] [PubMed] [Google Scholar]
- 6. Djajadiningrat‐Laanen S, Galac S, Kooistra H. Primary hyperaldosteronism: Expanding the diagnostic net. J Feline Med Surg 2011;13:641–650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. McKenzie TJ, Lillegard JB, Young WF Jr, et al. Aldosteronomas–state of the art. Surg Clin North Am 2009;89:1241–1253. Review. [DOI] [PubMed] [Google Scholar]
- 8. Eger CE, Robinson WF, Huxtable CRR. Primary aldosteronism (Conn's syndrome) in a cat; a case report and review of comparative aspects. J Small Anim Pract 1983;24:293–307. [Google Scholar]
- 9. Flood SM, Randolph JF, Gelzer AR. Primary hyperaldosteroinism in two cats. J Am Anim Hosp Assoc 1999;35:411–416. [DOI] [PubMed] [Google Scholar]
- 10. Briscoe K, Barrs VR, Foster DF. Hyperaldosteronism and hyperprogesteronism in a cat. J Feline Med Surg 2009;11:758–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. MacKay AD, Holt PE, Sparkes AH. Successful surgical treatment of a cat with primary aldosteronism. J Feline Med Surg 1999;1:117–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. DeClue AE, Breshears LA, Pardo ID, et al. Hyperaldosteronism and hyperprogesteronism in a cat with an adrenal cortical carcinoma. J Vet Intern Med 2005;19:355–358. [DOI] [PubMed] [Google Scholar]
- 13. Calsyn JD, Green RA, Davis GJ, et al. Adrenal pheochromocytoma with contralateral adrenocortical adenoma in a cat. J Am Anim Hosp Assoc 2010;46:36–42. [DOI] [PubMed] [Google Scholar]
- 14. Renschler JS, Dean GA. What is your diagnosis? Abdominal mass aspirate in a cat with an increased Na:K ratio. Vet Clin Pathol 2009;38:69–72. [DOI] [PubMed] [Google Scholar]
- 15. Rose SA, Kyles AE, Labelle P, et al. Adrenalectomy and caval thrombectomy in a cat with primary hyperaldosteronism. J Am Anim Hosp Assoc 2007;43:209–214. [DOI] [PubMed] [Google Scholar]
- 16. Rijnberk A, Voorhout G, Kooistra HS, et al. Hyperaldosteronism in a cat with metastasised adrenocortical tumour. Vet Q 2001;23:38–43. [DOI] [PubMed] [Google Scholar]
- 17. Ash RA, Harvey AM, Tasker S. Primary hyperaldosteronism in the cat: A series of 13 cases. J Feline Med Surg 2005;7:173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Moore LE, Biller DS, Smith TA. Use of abdominal ultrasonography in the diagnosis of primary hyperaldosteronism in a cat. J Am Vet Med Assoc 2000;217:213–215. 197. [DOI] [PubMed] [Google Scholar]
- 19. Schulman RL. Feline primary hyperaldosteronism. Vet Clin North Am Small Anim Pract 2010;40:353–359. [DOI] [PubMed] [Google Scholar]
- 20. Young WF. Primary aldosteronism: Renaissance of a syndrome. Clin Endcrinol (Oxford) 2007;66:607–618. [DOI] [PubMed] [Google Scholar]
- 21. Wang W, Hu W, Zhang X, et al. Predictors of successful outcome after adrenalectomy for primary aldosteronism. Int Surg 2012;97:104–111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Galac S, Reusch CE, Kooistra HS, et al. Clinical Endocrinology of Dogs and Cats, 2nd ed Hannover: Schlütersche; 2010:93–154. [Google Scholar]
- 23. Duesberg C, Peterson ME. Adrenal disorders in cats. Vet Clin North Am Small Anim Pract 1997;27:321–347. Review. [DOI] [PubMed] [Google Scholar]
- 24. Smith RR, Mayhew PD, Berent AC. Laparoscopic adrenalectomy for management of a functional adrenal tumor in a cat. J Am Vet Med Assoc 2012;241:368–372. [DOI] [PubMed] [Google Scholar]
- 25. Adin CA, Nelson RW. Endocrine system In: Tobias KM, Johnston SA. Small Animal Veterinary Surgery. 1st ed St. Louis, MO: Saunders Elsevier; 2012: 2033–2041. [Google Scholar]
- 26. Stepien RL. Blood pressure assessment In: Ettinger SJ, Feldman EC, eds. Veterinary Internal Medicine, 6th ed Philadelphia, PA: Saunders; 2004:470–472. [Google Scholar]
- 27. Brown S, Atkins C, Bagley R, et al. Guidelines for the identification, evaluation and management of systemic hypertension in dogs and cats. J Vet Intern Med 2007;21:542–558. [DOI] [PubMed] [Google Scholar]
- 28. Kyles AE, Feldman EC, De Cock HE, et al. Surgical management of adrenal gland tumors with and without associated tumor thrombi in dogs: 40 cases (1994–2001). J Am Vet Med Assoc 2003;223:654–662. [DOI] [PubMed] [Google Scholar]
- 29. Pang TC, Bambach C, Monaghan JC, et al. Outcomes of laparoscopic adrenalectomy for hyperaldosteronism. ANZ J Surg 2007;77:768–773. [DOI] [PubMed] [Google Scholar]
- 30. Massari F, Nicoli S, Romanelli G, et al. Adrenalectomy in dogs with adrenal gland tumors: 52 cases (2002‐2008). J Am Anim Hosp Assoc 2011;239:216–221. [DOI] [PubMed] [Google Scholar]
- 31. Davis MK, Schochet RA, Wrigley R. Ultrasonographic identification of vascular invasion by adrenal tumors in dogs. Vet Radiol Ultrasound 2012;53:442–445. [DOI] [PubMed] [Google Scholar]
- 32. Schultz RM, Wisner ER, Johnson EG. Contrast‐enhanced computed tomography as a pre‐operative indicator of vascular invasion from adrenal masses in dogs. Vet Radiol Ultrasound 2009;38:738–746. [DOI] [PubMed] [Google Scholar]
- 33. Lang JM, Schertel E, Kennedy S, et al. Elective and emergency surgical management of adrenal gland tumors: 60 cases (1999‐2006). J Am Anim Hosp Assoc 2011;47:428–435. [DOI] [PubMed] [Google Scholar]
- 34. Herrera MA, Mehl ML, Kass PH, et al. Predictive factors and the effect of phenoxybenzamine on outcome in dogs undergoing adrenalectomy for pheochromocytoma. J Vet Intern Med 2008;22:1333–1339. [DOI] [PubMed] [Google Scholar]
- 35. Brodbelt DC. Feline anesthetic deaths in veterinary practice. Top Companion Anim Med 2010;25:189–194. [DOI] [PubMed] [Google Scholar]
- 36. Brodbelt DC. Perioperative mortality in small animal anesthesia. Vet J 2009;182:152–161. [DOI] [PubMed] [Google Scholar]
- 37. Brodbelt DC, Pfeifer DU, Young JL. Risk factors for anaesthetic‐related death in cats: Results from the confidential enquiry into perioperative small animal fatalities (CEPSAF). Br J Anaesth 2007;99:617–623. [DOI] [PubMed] [Google Scholar]
- 38. Rose BD, Post TW. Clinical Physiology of Acid‐Base and Electrolyte Disorders, 5th ed New York, NY: McGraw‐Hill; 2001:836–887. [Google Scholar]
- 39. DiBartola SP. Management of hypokalaemia and hyperkalaemia. J Feline Med Surg 2001;3:181–183. [DOI] [PMC free article] [PubMed] [Google Scholar]


