Abstract
Background
The diagnostic tools for bovine respiratory disease diagnosis include clinical inspection, thoracic auscultation, and ultrasonography.
Hypothesis
Thoracic auscultation and clinical examination have limitations in the detection of lung consolidation in dairy calves.
Animals
Prospective cohort of 106 preweaned calves from 13 different dairy herds (10 with a history of active bovine respiratory disease (BRD) in calves and 3 without suspected BRD problems).
Methods
Each preweaned calf was clinically inspected using the Wisconsin calf respiratory scoring chart (CRSC) and treatment history was noted. Systematic thoracic auscultation and ultrasonography then were performed, the latter focusing on lung consolidation. Mortality was recorded over a 30‐day period.
Results
A total of 56 of 106 calves had ultrasonographic evidence of lung consolidation. The sensitivity of thoracic auscultation to detect consolidation was 5.9% (range, 0–16.7%). Only 41.1% (23/33) of calves with consolidated lungs had been treated previously by the producers. When adding CRSC and previous BRD treatment by the producer, sensitivity of detection increased to 71.4% (40/56). The area under the receiver operating characteristics curve was 0.809 (95% CI, 0.721–0.879) for the number of areas within the lungs with consolidation and 0.743 (95% CI, 0.648–0.823) for the maximal depth of consolidation as predictors of death within 1 month after examination. These were not significantly different (P = .06).
Conclusions and Clinical Importance
This study shows that thoracic auscultation is of limited value in diagnosing lung consolidation in calves. Ultrasonographic assessment of the thorax could be a useful tool to assess BRD detection efficiency on dairy farms.
Keywords: Atelectasis, Cattle, Parenchymal disease, Pneumonia, Radiology and diagnostic imaging, Respiratory tract
Abbreviations
- BRD
bovine respiratory disease
- CI
confidence interval
- COMT
comet‐tails artifact
- CRSC
calf respiratory scoring chart
- Ds
dorsal
- Md
median
- NA
not applicable
- NPV
negative predictive value
- PI
probability interval
- PPV
positive predictive value
- ROC
receiver operating characteristics
- Se
sensitivity
- Sp
specificity
- Vt
ventral
Bovine respiratory disease (BRD) is one of the main health issues in replacement dairy calves.1, 2, 3 Consequences of BRD are numerous. Relapses, mortality, propagation of infectious agents as well as retarded growth can be observed as consequences of BRD in replacement calves in addition to the associated costs of antimicrobial treatment and time for monitoring and administering treatments.2 Calves treated for BRD before 3 months of age have a 2.5 greater risk of dying after 3 months of age than untreated calves.4 One of the challenges of bovine respiratory medicine is early detection of clinical cases of BRD. This is especially important in subclinical forms of the disease, which can be easily missed and cause important economic losses.5, 6
The clinical diagnosis of BRD classically is based on clinical signs including lethargy, anorexia, abnormal breathing patterns (eg, dyspnea, tachypnea), and increased rectal temperature. Different practical tools have been developed for researchers and producers for both beef7 and dairy calves.2 These tools are of practical interest because they are based on clinical signs that can be easily and reliably assessed by producers. The limitations of these clinical signs have since been shown to lack both sensitivity and specificity to detect lung lesions.5 The lack of accuracy of clinical inspection also has been mentioned in the feedlot industry, even when performed by trained pen checkers.8
In veterinary medicine, thoracic auscultation has been mentioned as a fundamental part of the assessment of the ruminant respiratory tract.9, 10 Normal lung sounds result from the turbulence and velocity of air flow in the large airways during breathing. In cases of pneumonia, abnormal or adventitious lung sounds will be generated and are mostly characterized as crackles (or rhonchi) and wheezes. Wheezes are created by air turbulence in narrowed airways. Crackles consist of short‐duration popping sounds because of a sudden opening of obstructed airways, as is the case when mucopurulent secretions are present in the pulmonary tree during bronchopneumonia. The absence of normal lung sounds also is considered abnormal.9, 10 Clinical data concerning the efficiency of lung auscultation to detect lung lesions in cattle are lacking. In sheep, thoracic auscultation has been shown to have limitations because it can be relatively normal despite extensive lung lesions.11 To the authors' knowledge, the ability of thoracic auscultation to detect consolidated lungs has not been studied in cattle.
Since the 1990s, noninvasive assessment of lung parenchyma has been reported as a valuable tool to monitor thoracic lesions associated with pneumonia and pleuritis.12, 13, 14, 15, 16, 17 Thoracic ultrasonography is correlated with both radiographic15, 16 and macroscopic findings.14, 16, 18 It can be done quickly calf‐side, and therefore has the potential to be used by bovine practitioners and researchers in a field setting. We recently showed that this tool can be used even if the examiner is not familiar with nonreproductive ultrasound examination.19
We hypothesized that pneumonia in replacement heifer calves associated with lung consolidation may be under diagnosed by dairy producers using treatment records and clinical score assessments as well as by veterinarians using thoracic auscultation findings.
The objective of this study was to compare thoracic ultrasonographic findings with clinical score, auscultation, and treatment history in preweaned dairy calves from herds with and without known problems of enzootic pneumonia in replacement calves.
Materials and Methods
The experimental protocol was approved by the Comité d'éthique et d'utilisation des animaux of the Université de Montréal. We used a convenience sample of 13 dairy farms regularly visited by the bovine ambulatory clinic (Faculté de médecine vétérinaire, St‐Hyacinthe). The study was performed during the fall of 2012. The choice of most of the participating farms was based on their recent history in relation to BRD in preweaned calves. The producers on whose farms BRD was known as a recurrent or active problem were contacted and asked to participate in the study. A maximum of 10 calves were examined per farm. The 10 oldest preweaned calves were recruited if >10 were present. We also included 3 dairy farms without active BRD problems as reported by the producer and the herd veterinarian. The medical record of each calf was assessed on the day of examination, specifically for previous treatments for BRD by the producer or herd veterinarian using approved parenteral antibiotic administration.
Clinical Score Assessment
Each calf was identified and assessed using a widely used clinical score sheet from the University of Wisconsin (Calf Respiratory Scoring Chart, CRSC).1 , 21 Briefly, this 12‐point score is based on 4 different criteria including rectal temperature, nasal discharge, and eye and ear scores. Each criterion is noted on a 0–3 scale, with 0 associated with the lowest risk of being sick and 3 with the highest risk of BRD. This clinical score sheet has been widely as a practical tool for calf ranchers when screening calves for acute BRD. The recommendations have been to treat calves because of high BRD presumption if the score is ≥5, and to monitor calves with scores of 4. Calves with scores of ≤3 are considered healthy. The score of each calf was noted and stored for further analysis, serving as a tool that theoretically would have allowed the producer to detect BRD calves on the day of the examination.
Thoracic Auscultation
The thorax was divided into 3 equal longitudinal regions (Fig 1): dorsal, middle, and ventral. Because our objective was to focus on enzootic bronchopneumonia, we concentrated our examination on the middle and ventral parts of the thorax. These are the most common locations of bovine bacterial bronchopneumonia.20, 21 The medium and ventral parts of the right (r) and left (l) thorax then were divided into 4 quadrants (Ar/Br/Cr/Dr and Al/Bl/Cl/Dl) that were auscultated using a stethoscope2 (Fig 1). The presence of abnormal lung sounds including crackles, wheezes, and pleural friction rubs and the absence of respiratory noises were recorded as abnormal. Otherwise auscultation was considered normal. The procedure was repeated on both sides. The veterinarian who performed the auscultations was blind to the ultrasonographic lesions and was not aware of the treatment history of the calf.
Figure 1.
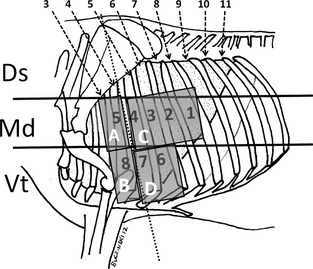
Thoracic ultrasonographic and auscultation sites used in preweaned calves. Sites 1–8: Ultrasonographic sites at which thoracic examination was performed systematically. The median (Md) to ventral (Vt) parts of the thorax were divided into 4 auscultation areas (A–D). The auscultation findings were compared with ultrasonographic findings. The dorsal (Ds) third of the thorax was not examined.
Ultrasonographic Examination Procedure
The same area that was auscultated was systematically scanned from the 8th to the 4th intercostal space (Fig 1). A total of 8 sites for each side of the thorax were screened for the presence of abnormal ultrasonographic findings.15 Ultrasonography was performed using a 8.5 MHz linear probe3 that was directly applied on the thorax after 70% isopropyl alcohol had been sprayed on the area of interest to improve image quality without clipping.4 , 19 The different anomalies noted were the presence of comet‐tail artifacts (COMT), the number of sites with COMT (ΣsCOMT), pleural fluid accumulation, pleural irregularity, and consolidated lung. If present, depth of consolidation (cm, DEPTH) was directly counted on the grid on the screen of the ultrasound unit [Fig 2]).17, 19 The COMT were identified when ≥1 reverberation artifacts (with comet‐tail shape) were observed from the pleura to the deeper part of the ultrasonogram during the scanning of the site.17 Pleural fluid accumulation was diagnosed if disruptions between the parietal and the visceral pleura were observed during examination. Pleural irregularity was noted if, in contrast to a smooth hyperechoic line, the pleural line was serrated with an irregular shape. Pleural thickening was a subjective assessment of the pleural line which is a thin line (<1 mm) in healthy animals. Any suspicion of thickening during the examination was noted by the operator, but no measurements were taken to assess pleural thickness. Lung consolidation was defined as the ability to observe the abnormal lung parenchyma as a heterogenous hypoechoic to echoic area.
Figure 2.
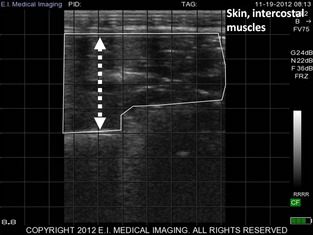
Ultrasonographic evidence of consolidated lung in a preweaned dairy calf with bronchopneumonia. Consolidated lung in a Holstein calf (delimited by the white line), the DEPTH of consolidated lung parenchyma is indicated by the double arrowline (4 cm, each square is 1 cm2), 8.5 MHz linear probe, maximal depth 8.8 cm.
Consolidation was considered clinically relevant when DEPTH was ≥1 cm. This threshold of 1 cm was chosen as previously recommended4 and to avoid any misclassification with pleural thickening, pleural irregularity or both. It can also be easily measured using the 1 cm grid screen of the ultrasound unit.3 The number of sites in which consolidation was observed was also noted (ΣsDEPTH). The maximal depth of the screen was set at 8.8 cm for the entire study.
Follow‐up
Calves that died within 30 days after the ultrasonographic examination were recorded by the producers.
Statistical Analysis
The data obtained were analyzed using commercial statistical software.5 , 6 The descriptive statistics were summarized as median, minimum, and maximum values for the calves' ages, CRSC, DEPTH, ΣsDEPTH, COMT, and ΣsCOMT because most of the data were not normally distributed. To assess the impact of performing unilateral ultrasonography (as a less time‐consuming technique for future field studies), concordance between the presence of consolidated lesions between the left and right sides of the thorax was assessed using the Kappa test for inter‐rater agreement. The Kappa agreement was judged as slight when 0 ≤ κ ≤ 0.20, fair when 0.21 ≤ κ≤ 0.40, moderate when 0.41 ≤ κ ≤ 0.60, substantial when 0.61 ≤ κ ≤ 0.80, and almost perfect when 0.81 ≤ κ ≤ 1.
The sensitivity, specificity as well as positive and negative predictive values were obtained for each auscultation area. The results of left and right findings (normal or abnormal lung sounds) were pooled because no significant difference was observed between left and right sides of the thorax (chi‐squared test). Four different areas therefore were created as follows: A(Ar+Al), B(Br+Bl), C(Cr+Cl), and D(Dr+Dl). They then were compared with the presence of lung consolidation which was considered the gold standard for this study. A theoretical “producer diagnosis accuracy” to assess the ability to detect lung consolidation was built using the theoretical ability of the producer to assess animal with pneumonia (either by having previously treated the animal for BRD or if on the day of the study the CRSC was ≥5). The sensitivity, specificity as well as positive and negative predictive values were calculated for the previous treatment history, clinical scores, and producer diagnostic accuracy using ultrasonographic lesions of lung consolidation as a reference method for lung infection detection.
A Wilcoxon rank sum analysis was performed to compare the age of calves with and without ultrasonographic evidence of lung consolidation because age was not normally distributed.
A Pearson chi‐squared test was used to assess the difference in calves with or without ultrasonographic evidence of lung consolidation with regard to the probability of having been previously treated for bronchopneumonia. The same test was used for other categorical data assessments.
The relationship between clinical score and DEPTH, ΣsDEPTH or ΣsCOMT was assessed using the nonparametric Spearman correlation statistics. The relation between DEPTH and ΣsDEPTH was assessed using a Pearson correlation statistic.
The diagnostic accuracy of the ultrasonographic parameters DEPTH and ΣsDEPTH for predicting death within 30 days after the initial examination was assessed by comparing the area under the receiver operating characteristics (ROC) curve using the DeLong comparison test.22 Statistical significance was set at P < .05.
Results
One hundred and six calves were recruited in this study from the 13 participating farms. Two to 10 calves were chosen from each farm (median, 10). The median age of the calves was 36 days (range, 2–116 days). The median rectal temperature was 39.0°C (range, 37.5–40.7°C) and the median clinical score was 4 (range, from 1 to 11).
Fifty‐six calves (53%) had ultrasonographic evidence of consolidation (DEPTH ≥ 1 cm; Table 1). The median number of sites with observed consolidation was 1 (range, 0–12 of the 16 sites observed). The age of calves with (median, 36.5 days; range, 5–116 days) or without (34.5 days; range, 2–108 days) ultrasonographic evidence of lung consolidation did not differ (P = .41).
Table 1.
Median, minimum, and maximum age distribution of calves with (n = 56) or without (n = 50) ultrasonographic evidence of lung consolidation in 13 Holstein dairy farms
| Farm | Consolidated Calvesa | Non Consolidated Calvesa | Total | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Median Age (d) | Min | Max | n | Median Age (d) | Min | Max | n | ||
| 1 | 21 | 24 | 38 | 2 | 10 | 6 | 14 | 2 | 4 |
| 2 | 25 | 21 | 55 | 5 | 59 | 26 | 71 | 5 | 10 |
| 3 | 44 | 5 | 59 | 4 | 24 | 2 | 33 | 3 | 7 |
| 4 | 33 | 15 | 44 | 7 | 16 | 10 | 36 | 3 | 10 |
| 5 | 31 | 15 | 38 | 7 | 39 | 37 | 41 | 2 | 9 |
| 6 | 31 | 22 | 37 | 3 | 44 | – | – | 1 | 4 |
| 7 | – | – | – | 0 | 21 | 18 | 23 | 2 | 2 |
| 8 | 39 | 35 | 54 | 3 | 30 | 23 | 44 | 7 | 10 |
| 9 | 38 | 24 | 51 | 5 | 38 | 14 | 64 | 5 | 10 |
| 10 | 30 | 16 | 37 | 6 | 19 | 7 | 22 | 4 | 10 |
| 11 | 77 | 31 | 95 | 3 | 49 | 11 | 76 | 7 | 10 |
| 12 | 64 | 35 | 85 | 6 | 42 | 39 | 44 | 4 | 10 |
| 13 | 85 | 11 | 116 | 5 | 97 | 50 | 108 | 5 | 10 |
Consolidation was defined based as the presence of at least 1 site with lung consolidation depth ≥1 cm; en dash, no data required (n of 0 or 1).
Interestingly, of the 3 herds without known problems of calf pneumonia, ultrasonographically consolidated lungs were found in 2 (in 3 of 10 and in 6 of 10 calves, respectively). In these herds, the CRSC was compatible with treatment requirement (CRSC ≥5) in 2 of 3 and in 2 of 6 consolidated calves. In the remaining herd without anticipated BRD problems, only 2 calves were available for the study and neither had lung lesions.
Significant pleural effusion was not observed in any calves. Pleural irregularity was noted in 17 of 106 calves, and in 14 calves, the calves also had DEPTH ≥1. COMT were observed in all but 2 calves. The median ΣsCOMT was 6 (range, 0 to 14 out of the 16 sites observed) and did not differ in calves with (median, 6; range, 1–14 sites) or without (median, 5; range, 0–14 sites) ultrasonographic evidence of lung consolidation (P = .43).The localization of lung consolidation is summarized in Table 2. Lesions of consolidation were found on the right side of the thorax in 40 cases and on the left side of the thorax in 39 cases. Overall, the kappa value on agreement of the presence of consolidation between the right and left thorax was fair at 0.33 (95% confidence interval (CI) from slight to moderate, 0.14–0.52). No significant correlation was noted between ΣsCOMT and the clinical score (Spearman correlation, r s = −0.05; P = .60). A significant correlation was found between DEPTH and ΣsDEPTH (Pearson correlation, r = 0.75; P < .0001).
Table 2.
Localization of consolidation lesions using systematic thoracic ultrasonography in 106 dairy calves from farms with enzootic pneumonia
| Right Side of the Thorax | |||
|---|---|---|---|
| Left Side of the Thorax | Consolidateda | Normal | Total |
| Consolidateda | 23 | 16 | 39 |
| Normal | 17 | 50 | 67 |
| Total | 40 | 66 | 106 |
Consolidation was defined as 1 or more site in which the depth of consolidation was 1 cm or more using thoracic ultrasonography.
Of the 56 calves with ultrasonographic evidence of lung consolidation, only 23 (41.1%) had been treated previously with antimicrobials by the producers. Of the 50 calves without evidence of lung consolidation, 13 (28%) had been treated previously. These numbers were not significantly different (P = .18).
The clinical score was significantly associated with the maximal DEPTH (P = .02), but Spearman's correlation coefficient was low (r s = 0.24; Fig 3). The clinical score also was positively correlated with ΣsDEPTH (r s = 0.25; P= .005; Fig 4).
Figure 3.
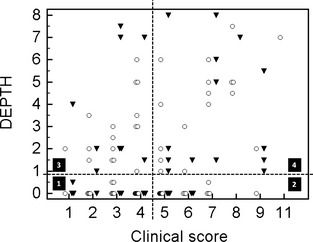
Maximal depth of consolidated lung determined by thoracic ultrasonography in relation to the clinical score of respiratory disease in 106 calves treated or not for bronchopneumonia. The maximal depth of consolidation (DEPTH) in centimeters has been plotted in relation to the Wisconsin Calf Respiratory Score Chart (clinical score) in previously treated (▼) and nontreated (○) calves. The plot has been divided into 4 different quadrants according to threshold of treatment based on the clinical score (if ≥5, this calf should be treated for respiratory problem, quadrants 2 and 4) and the significant consolidation (DEPTH ≥1 cm, quadrants 3 and 4) (arrows). Quadrant 1: Calves with a normal clinical score and absence of clinically relevant consolidation. Quadrant 2: Calves with a high clinical score and no clinically relevant consolidation. Quadrant 3: Calves with significant consolidation and no clinical suspicion of respiratory disease based on the clinical score. Quadrant 4: Calves with significant consolidation and clinical suspicion of respiratory diseases based on their clinical score.
Figure 4.
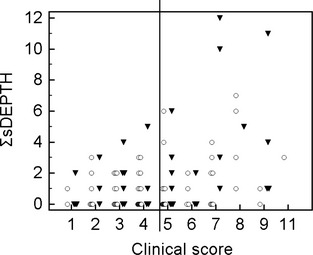
Total number of thoracic sites at which consolidated lung was diagnosed by thoracic ultrasonography in relation with to the clinical score in 106 calves treated or not for bronchopneumonia. The number of sites at which consolidation was detected using ultrasonography (ΣsDEPTH) has been plotted in relation to the Wisconsin Calf Respiratory Score (clinical score) in previously treated (▼) and nontreated (○) calves. The vertical line separates the calves that should or should not be treated according to their clinical score.
The comparison between thoracic auscultation and the ultrasonographic evidence of lung consolidation is summarized in Table 2. The sensitivity of thoracic auscultation was on average poor (from 0 to 16.7%) to detect lung consolidation. Producer diagnostic accuracy had moderate sensitivity to detect calves with significant consolidation (Se = 71.4%) (Table 3).
Table 3.
Sensitivity, specificity, and predictive values of thoracic auscultation, individual treatment administration by the producer, and/or calf respiratory score to predict lung consolidation detected by thoracic ultrasonography in 106 dairy calves from farms with enzootic pneumonia
| Ultrasonographic Lung Findings | Consolidated | Normal | Total | Se | Sp | PPV | NPV |
|---|---|---|---|---|---|---|---|
| Auscultation A | |||||||
| Abnormal | 1 | 1 | 2 | ||||
| Normal | 30 | 180 | 210 | ||||
| Total | 31 | 181 | 212 | 3.2 | 99.5 | 50.0 | 85.7 |
| Auscultation B | |||||||
| Abnormal | 5 | 5 | 10 | ||||
| Normal | 25 | 177 | 202 | ||||
| Total | 30 | 182 | 212 | 16.7 | 97.3 | 50.0 | 87.6 |
| Auscultation C | |||||||
| Abnormal | 0 | 0 | 0 | ||||
| Normal | 18 | 194 | 212 | ||||
| Total | 18 | 194 | 212 | 0.0 | 100.0 | NA | 91.5 |
| Auscultation D | |||||||
| Abnormal | 2 | 3 | 5 | ||||
| Normal | 55 | 152 | 207 | ||||
| Total | 57 | 155 | 212 | 5.2 | 98.7 | 60.0 | 73.4 |
| Treatment | |||||||
| Treated | 23 | 13 | 36 | ||||
| Not treated | 33 | 37 | 70 | ||||
| Total | 56 | 50 | 106 | 41.1 | 74.0 | 63.9 | 52.9 |
| Scorea | |||||||
| Score ≥5 | 31 | 21 | 52 | ||||
| Score ≤4 | 25 | 29 | 54 | ||||
| Total | 56 | 50 | 106 | 55.4 | 58.0 | 59.6 | 53.7 |
| Producer accuracy | |||||||
| Sick | 40 | 29 | 69 | ||||
| Scorea + Treatment | |||||||
| Not sick | 16 | 21 | 37 | ||||
| Total | 56 | 50 | 106 | 71.4 | 42.0 | 58.0 | 56.8 |
Lung auscultation was considered abnormal if crackles, wheezes, or absence of respiratory noises was noted.
Se, sensitivity; Sp, specificity; PPV, positive predictive value; NPV, negative predictive value; NA, not applicable.
Calf respiratory scoring chart.
Four of the 106 calves died within 30 days after ultrasonographic examination. Areas under the ROC curve were 0.809 (95% CI, 0.721–0.879) and 0.743 (95% CI, 0.648–0.823) for ΣsDEPTH and DEPTH, respectively, for predicting death within 1 month after examination (Fig 5). However, this difference was not significant (P = .06). The optimal diagnostic cutoff for ΣsDEPTH was 7 consolidated sites (Se = 75%; 95% CI, 19.4–99.4%; Sp = 100%; 95% CI, 96.4–100%) and 4.5 cm on DEPTH (Se = 75%; 95% CI, 19.4–99.4%; Sp = 84.3%; 95% CI, 81.5–94.5%).
Figure 5.
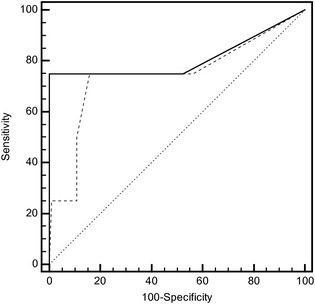
Comparison of receiver operating characteristics curves for the number of consolidated sites and the maximal depth of consolidation (DEPTH) seen during thoracic ultrasonography as a predictor of death within 30 days. The ROC curve of the number of consolidated sites (ΣsDEPTH) is indicated with the continuous line and the maximal depth of consolidation (DEPTH) is indicated by the noncontinuous line. The line of identity is represented by the dotted line. Only 4 events of interest (deaths) occurred among the 106 calves.
Discussion
The BRD complex is a medical challenge in veterinary medicine because clinical diagnosis is difficult, especially because of a lack of gold standard diagnostic tests.5, 8 Thoracic ultrasonography was used in this study in order to assess lesions secondary to lung infection. It has been proven to be a reliable tool to assess lung consolidation as well as the extent of lesions in calves15, 16, 18 and can be done easily in a field setting.4 , 19 However, it is important to remember that consolidated lung is not systematically associated with lung infection. It can also be found in cases of lung infarction or atelectasis.23 Atelectasis can be because of compression associated with pleural effusion or can be secondary to airway obstruction with gradual air resorption within the affected part of the lung.23 Ultrasonographically, compression atelectasis is suspected when massive amounts of pleural fluid are seen surrounding the consolidated lung. However, these findings cannot be differentiated from pleuropneumonia. Obstructive atelectasis can be differentiated from consolidation secondary to lung infection when a bronchogram is observed moving simultaneously with breathing movements.24 In cases of infection, a dynamic bronchogram can be observed if a gas/tissue interface “moves” during breathing. In cases of obstructive atelectasis, the observed bronchogram is “static” and does not change in relation to breathing movements. In a study of human patients, the observation of a dynamic bronchogram had a sensitivity of 61% and a specificity of 94% for detecting pneumonia.24 The design of the study did not allow systematic recording of the presence of dynamic or static bronchograms. The absence of pleural effusion excluded compression atelectasis in the calves examined in this study. It remains unknown how many calves had obstructive atelectasis rather than consolidation because of infection, but we believe that this number has a high probability of being low. In fact, pneumonia is by far the most common lung disease in replacement dairy calves25 and pure atelectasis is uncommon in these animals. Both lesions lead to nonfunctional portions of lung that cannot be used for ventilation and may therefore have a deleterious impact on calf health. Future studies should be performed to assess the impact of lung consolidation (because of infection or atelectasis) on clinical (clinical score, relapse rate, mortality rate) and subclinical outcomes (average daily gain, risk of being culled before the end of first lactation).
This study indicated that in dairy herds in which BRD is enzootic, the prevalence of lung consolidation can be high (more than 1 of 2 calves in this study) in preweaned calves. Lung consolidation is not a normal ultrasonographic finding in healthy calves.15 Fewer than 50% of calves with lung lesions were diagnosed as being sick, and were treated by the farmer. This may demonstrate poor recognition of this condition because of its subclinical course or lack of adequate monitoring. The latter may be because of the fact that the farms are small with no one focusing only on calf health management. Interestingly, consolidation can be observed in very young calves. The youngest consolidated calves were ≤15 days of age in 5 of 13 herds. The aim of this study was not to specifically look for the youngest animals. When ≥10 preweaned calves were present on a farm, we recruited the 10 oldest in order to increase the chances of finding consolidated lungs. However, our results show that pneumonia can occur in preweaned calves and consolidation can be observed in very young animals, thus putting emphasis on BRD monitoring during the whole preweaning period.
Surprisingly, 2 of 3 herds without anticipated BRD problems had calves with ultrasonographic evidences of lung consolidation which also shows that lung lesions may be an underdiagnosed problem in Québec dairy farms. Only 4 of 9 consolidated calves would have been detected using the CRSC on the day of examination.
Despite the high prevalence of lung consolidation and the fact that the calves were young, and thus potentially could be more easily auscultated than older calves or adult cattle, the sensitivity of auscultation was poor to detect lung consolidation. Very few studies have been performed concerning the validity of thoracic auscultation for the diagnosis of pneumonia in veterinary medicine. A Scottish study showed that in sheep, when compared with both ultrasonography and necropsy, severe lung lesions can be missed easily using thoracic auscultation.11 In humans, a French study performed in a hospital setting with better auscultation conditions than on a farm, the performance of thoracic auscultation was poor for detection of alveolar consolidation (sensitivity = 36%).26 The available evidence concerning the use of lung sounds in a clinical setting is a recurrent debate in the human medical literature.27, 28
A limitation of this study is that we did not include large airway (eg, bronchial) sounds in the list of abnormal findings during lung auscultation. These also are recognized as suggestive of alveolar consolidation.26 This could have contributed to the low sensitivity of lung auscultation. It also may be argued that, in this study, the gold standard used (presence of lung consolidation) may occur later in the pathophysiologic process of lung infection or can be because of lung atelectasis without lung infection per se. This may have decreased the apparent sensitivity of thoracic auscultation and also may decrease specificity (ie, abnormal sounds in nonconsolidated calves). However, the high specificity of auscultation (from 97.3 to 100%) showed that abnormal sounds occur infrequently in nonconsolidated calves. Ultrasonographic evidence of lung consolidation may appear as soon as 2 hours after experimental infection with Mannheimia haemolytica.7
Other studies have shown that lung lesions are highly correlated with impaired growth performance in beef calves,6, 20, 21 but also have been linked with decreased average daily gain in dairy calves.4 Because of the lack of a gold standard for accurately defining BRD‐affected animals, Bayesian analyses were used in a recent study to determine the accuracy of clinical inspection by pen checkers and lung lesions observed at harvest.8 The sensitivity and specificity of clinical inspection (Se = 61.8%; 97.5% PI, 55.7–68.4%; Sp = 62.8%; 97.5% PI, 60.0–65.7%) were lower than for lung lesions at harvest (Se = 77.4%; 97.5% PI, 66.2–87.3%; Sp = 89.7%; 97.5% PI, 86.0–93.8%). Adding ultrasonography to the clinical examination of calves potentially should increase the sensitivity of lung lesion detection and may help to more accurately detect BRD because it is a good estimate of the extent of lung lesions.14, 18 Furthermore, this method avoids delaying results to the end of the feeding period for beef calves or to necropsy.
Most of the available studies on ultrasonography concerning thoracic examination in cases of BRD in cattle are mainly descriptive.13, 15, 16, 17 Comet‐tail artifacts, pleural thickening, and pleural effusion have been mentioned as possible signs of lung disease.17 Comet‐tail artifacts were frequently observed in this study in healthy calves and all but 2 animals had at least 1 thoracic ultrasonographic site with this artifact. In human medicine29 as well as in previous work in cattle,16 diffuse comet‐tail artifacts have been associated with diffuse parenchymal lung diseases such as emphysema. Our study therefore suggests that these can also be found without lung consolidation or clinical score compatible with BRD. The number of comet‐tails per site was not assessed in this study, but it also is associated with emphysematous disease in humans.29
Interestingly, approximately 60% of calves with consolidated lungs had never received any antibiotic treatment for BRD by the producer. Using a clinical score of ≥5 on the day of ultrasound examination, 71.4% of the calves would have been classified as sick which shows that this simple score could be an interesting practical tool to implement on farms. On the other hand, 30% of nonconsolidated calves had been previously treated by the producers. These cases are difficult to classify as either false positive cases or as effectively treated calves with no ultrasonographic sequela of respiratory disease. Adding the clinical score assessment of the calves increased sensitivity, but also decreased the specificity when compared with lung consolidation findings. The complementary nature of these 2 tools is evident. Previous experimental studies using Pasteurella multocida‐induced pneumonia have shown that the evolution of clinical signs, spirometric signs and ultrasonographic lesions are not closely correlated.18 Severely damaged lungs can be observed with minimal impact on breathing dynamics or spirometric values, and on the other hand, calves with severe respiratory signs may have minimal lung lesions on ultrasonography.18 It was not the aim of this study to directly compare the diagnostic accuracy of the CRSC to assess BRD in dairy calves given that the score and ultrasonography (which was used as a comparator in this study) do not focus on the same stage of the disease. The CRSC was designed to focus on early diagnosis of BRD with typical clinical signs that can be easily implemented on dairy farms. One could say that the CRSC would be more sensitive to detect pneumonia than ultrasound, which focuses on a later stage of the disease with severe lung damage. However, a recent study on experimental M. haemolytica pneumonia showed that the clinical score may lack sensitivity to detect infected calves early in the disease process.7 Another value of ultrasonography is its ability to detect calves with severe lung changes, which are likely to experience poor growth.6
This study indicated that a majority of calves with ultrasonographic evidence of lung consolidation were not previously detected as sick by the producers. A limitation of this study is that it is not known to what extent lung consolidation can be an indicator of active BRD which requires specific treatment. It is not possible to be sure that antimicrobial treatment for every case with significant consolidation would have a beneficial effect on the affected calves. This aspect was beyond the scope of our observational study, but it definitely raises interesting future research questions. We believe that simple thoracic ultrasonographic examination can be a useful tool to monitor health management of dairy calves. It can be used as an indicator of adequate BRD surveillance by farm staff and for identifying consolidated calves without any previous history of BRD treatment (based on daily monitoring by staff).
The number of consolidated sites as well as the maximal depth of consolidated lung appeared to be potential predictors of death within 1 month after the examination. Because the number of adverse outcomes was low in this study (only 4 deaths), one cannot definitely speculate on the relevance of this finding or on the cutoff obtained as shown by the wide 95% CI of the sensitivity. However, this needs to be confirmed by other studies using ultrasonography findings as prognostic markers for survival or growth in calves.
Being able to find a gold standard test for early diagnosis and prognosis of BRD remains a major challenge for both the beef and dairy cattle industries. This study was unfortunately a 1‐day observational study and we could not assess differences in accuracy of the diagnostic methods used (eg, auscultation, clinical score, and ultrasonography) depending on the stage of the disease (acute versus chronic), treatment status (treated versus nontreated), or severity (relapsed cases or cases with future poor growth). Ultrasonography can be a useful tool to assess the extent of lung lesions in calves with or without typical acute clinical signs of BRD. Interestingly, subclinical pneumonia also can be detected in well‐managed dairy herds without previous history of BRD in replacement calves. Future studies should focus on the economic impact of subclinical pneumonia in dairy calves and on diagnostic tools to mitigate its effects as well as their use in dairy farms.
Acknowledgments
We would like to acknowledge EI‐Medical Imaging, Loveland, Colorado for providing the ultrasonographic units and facilitating this research project and Merck Animal Health for funding the project. We thank Dr Rebecca Vandormael for her critical reading of the manuscript.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Footnotes
Calf Respiratory Scoring Chart http://www.vetmed.wisc.edu/dms/fapm/fapmtools/8calf/calf_respiratory_scoring_chart.pdf (accessed March 21, 2012)
Veterinary Stethoscope; 3M Littmann, 3M Canada Inc, London, ONT, Canada
Ibex Pro; EI Medical, Loveland, CO
Ollivett TL, Burton AJ, Bicalho RC, Nydam DV. Use of rapid thoracic ultrasonography for detection of subclinical and clinical pneumonia in dairy calves. American Association of Bovine Practitioners Proceedings, 2011;44:148 (abstract)
SAS 9.2, Cary, NC
MedCalc bvba, version 12.4.0, Mariakerke, Belgium
Ollivett TL, et al. Thoracic ultrasonography after experimental Mannheimia haemolytica infection in dairy calves. American College of Veterinary Internal Medicine, Seattle, WA, 2013 (abstract)
References
- 1. Mohd Nor N, Steeneveld W, Mourits MCM, Hogeven H. Estimating the costs of rearing young dairy cattle in the Netherlands using a simulation model that accounts for uncertainty related to diseases. Prev Vet Med 2012;216:214–224. [DOI] [PubMed] [Google Scholar]
- 2. McGuirk SM. Disease management in dairy calves and heifers. Vet Clin North Am Food AnimPract 2008;24:139–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stanton A. Challenges and opportunities for managing respiratory disease in dairy calves. Anim Health Res Rev 2009;10:113–115. [DOI] [PubMed] [Google Scholar]
- 4. Waltner‐Toews D, Martin SW, Meek AH. The effect to early calfhood disease on survivorship and age at first calving. Can J Vet Res 1986;50:314–317. [PMC free article] [PubMed] [Google Scholar]
- 5. Leruste H, Brscic M, Heutinck LF, et al. The relationship between clinical signs of respiratory system disorders and lung lesions at slaughter in veal calves. Prev Vet Med 2012;105:93–100. [DOI] [PubMed] [Google Scholar]
- 6. Wittum TE, Woollen NE, Perino LJ, Littledike ET. Relationships among treatment for respiratory tract disease, lesions evident at slaughter, and rate of weight gain in feedlot cattle. J Am Vet Med Assoc 1996;209:814–818. [PubMed] [Google Scholar]
- 7. Step DL, Krehbiel CR, Depra LHA, et al. Effects of commingling beef calves from different sources and weaning protocols during a forty‐two‐day receiving period on performance and bovine respiratory disease. J Anim Sci 2008;86:3146–3158. [DOI] [PubMed] [Google Scholar]
- 8. White BJ, Renter DG. Bayesian estimation of the performance of using clinical observations and harvest lung lesions for diagnosing bovine respiratory disease in post‐weaned beef calves. J Vet Diagn Invest 2009;21:446–453. [DOI] [PubMed] [Google Scholar]
- 9. Wilkins PA, Woolums AR. Diagnostic for the respiratory system In: Smith BP, ed. Large Animal Internal Medicine, 4th ed St. Louis, MO: Mosby‐Elsevier; 2009:490–492. [Google Scholar]
- 10. Curtis RA, Viel L, McGuirk SM, et al. Lung sounds in cattle, horses, sheep and goats. Can Vet J 1986;27:170–172. [PMC free article] [PubMed] [Google Scholar]
- 11. Scott PR, Collie D, McGorum B, Sargison N. Relationship between thoracic auscultation and lung pathology detected by ultrasonography in sheep. Vet J 2010;186:53–57. [DOI] [PubMed] [Google Scholar]
- 12. Reef VB, Boy MG, Reid CF, Elser A. Comparison between diagnostic ultrasonography and radiography in the evaluation of horses and cattle with thoracic disease: 56 cases (1984–1985). J Am Vet Med Assoc 1991;198:2112–2118. [PubMed] [Google Scholar]
- 13. Braun U, Pusterla N, Flückiger M. Ultrasonographic findings in cattle with pleuropneumonia. Vet Rec 1997;141:12–17. [DOI] [PubMed] [Google Scholar]
- 14. Rabeling B, Rehage J, Döpfer D, Scholz H. Ultrasonographic in calves with respiratory diseases. Vet Rec 1998;143:468–471. [DOI] [PubMed] [Google Scholar]
- 15. Jung C, Bostedt H. Thoracic ultrasonographic technique in newborn calves and description of normal and pathological findings. Vet Radiol Ultrasound 2004;45:331–335. [DOI] [PubMed] [Google Scholar]
- 16. Flöck M. Diagnostic ultrasonography in cattle with thoracic disease. Vet J 2004;167:272–280. [DOI] [PubMed] [Google Scholar]
- 17. Babkine M, Blond L. Ultrasonography of the bovine respiratory system and its practical application. Vet Clin North Am Food AnimPract 2009;25:633–649. [DOI] [PubMed] [Google Scholar]
- 18. Reinhold P, Rabeling B, Günther H, Schimmel D. Comparative evaluation of ultrasonography and lung function testing with the clinical signs and pathology of calves inoculated experimentally with Pasteurella multocida . Vet Rec 2002;150:109–114. [DOI] [PubMed] [Google Scholar]
- 19. Buczinski S, Forté G, Bélanger AM. Ultrasonographic assessment of the thorax as a fast technique to assess pulmonary lesions in dairy calves with bovine respiratory disease. J Dairy Sci 2013;96:1–6. [DOI] [PubMed] [Google Scholar]
- 20. Thompson PN, Stone A, Schultheiss WA. Use of treatment records and lung lesion scoring to estimate the effect of respiratory disease on growth during early and late finishing periods in South African feedlot cattle. J Anim Sci 2006;84:488–498. [DOI] [PubMed] [Google Scholar]
- 21. Bryant LK, Perino LJ, Griffin D, et al. A method for recording pulmonary lesions of beef calves at slaughter, and the association of lesions with average daily gain. Bovine Pract 1999;33:163–173. [Google Scholar]
- 22. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 23. Sartori S, Tombesi P. Emerging roles for transthoracic ultrasonography in pulmonary diseases. World J Radiol 2010;2:203–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lichtenstein D, Mézière G, Seitz J. The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasia. Chest 2009;135:1421–1425. [DOI] [PubMed] [Google Scholar]
- 25. Ames TR. Dairy calf pneumonia. The disease and its impact. Vet Clin North Am Food Anim Pract 1997;13:379–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lichtenstein D, Goldstein I, Mourgeon E, et al. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004;100:9–15. [DOI] [PubMed] [Google Scholar]
- 27. Benbassat J, Baumal R. Narrative review: Should teaching of the respiratory physical examination be restricted only to signs with proven reliability and validity? J Gen Intern Med 2010;25:865–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wipf JE, Lipsky BA, Hirschmann JV, et al. Diagnosing pneumonia by physical examination: Relevant or relic? Arch Intern Med 1999;159:1082–1087. [DOI] [PubMed] [Google Scholar]
- 29. Reiβig A, Kroegel C. Transthoracic sonography of diffuse parenchymal lung disease. J Ultrasound Med 2003;22:173–180. [DOI] [PubMed] [Google Scholar]


