Abstract
Background
Some dogs with primary hypoadrenocorticism (HA) have normal sodium and potassium concentrations, a phenomenon called atypical Addison's disease. The assumption that the zona glomerulosa and aldosterone secretion in these dogs are normal seems widely accepted; however, aldosterone measurements are missing in most published cases.
Objectives
To measure aldosterone in dogs with HA with and without electrolyte abnormalities and to determine the time point of aldosterone peak concentrations during ACTH stimulation.
Animals
Seventy dogs with HA, 22 dogs with diseases mimicking HA, and 19 healthy dogs.
Methods
Prospective study. Blood samples were taken before and 60 minutes after injection of 250 μg ACTH in all dogs. Additional blood samples were taken 15, 30, and 45 minutes after ACTH in 7 dogs with HA and in 22 with diseases mimicking HA.
Results
Baseline and ACTH‐stimulated aldosterone was significantly lower in dogs with HA than in the other groups. Aldosterone was low or undetectable in 67/70 dogs with HA independently of sodium and potassium levels. In 3 dogs, sodium/potassium concentrations were normal; in 1 dog, sodium was normal and potassium decreased. In all 4, ACTH‐stimulated aldosterone concentrations were below the detection limit of the assay. Aldosterone concentrations were not different at 30, 45, or 60 minutes after ACTH administration.
Conclusion and Clinical Importance
Cortisol and aldosterone secretion is compromised in dogs with HA with and without electrolyte abnormalities. The term atypical Addison's disease, used for dogs with primary HA and normal electrolytes, must be reconsidered; other mechanisms allowing normal electrolyte balance without aldosterone should be evaluated in these dogs.
Keywords: adrenal insufficiency, canine, mineralocorticoids
Abbreviations
- cACTH
canine endogenous ACTH
- HA
hypoadrenocorticism
Hypoadrenocorticism (HA) is an uncommon disease in which adrenal steroid hormone secretion falls below the requirement of the animal. The vast majority of dogs suffer from primary HA, also known as Addison's disease. Usually, primary HA results from immune‐mediated destruction of the adrenal cortex, which terminates in absolute deficiency of glucocorticoids and mineralocorticoids. Typically, dogs with primary HA reveal laboratory abnormalities of mineralocorticoid deficiency, eg, decreased sodium and increased potassium concentrations. However, in a small number of dogs with primary disease, serum sodium and potassium concentrations are normal.1, 2 Dogs with primary HA not evidencing mineralocorticoid insufficiency are classified as having atypical Addison's disease.2, 3, 4, 5, 6, 7
In atypical Addison's disease, adrenal destruction is assumed to be confined to the zona fasciculata and reticularis, resulting in isolated glucocorticoid deficiency. Sparing of the zona glomerulosa with disappearance of the zona fasciculata and reticularis was proven histologically in cases with combined hypothyroidism and primary HA, an entity known as polyglandular deficiency syndrome.8, 9 In addition, a retrospective study about adrenocortical inflammation gives further evidence for the existence of segmental sparing of the zona glomerulosa in dogs suspected of HA.10 In humans, however, dissociation between zona glomerulosa and fasciculata function is thought to occur rarely, as increased renin values, indicating compensated failing of the zona glomerulosa, were shown to occur in patients with primary HA and normal electrolytes.11
Sodium and potassium concentrations may also be normal in dogs with secondary HA. Secondary HA in dogs is rare and characterized by ACTH deficiency and lack of glucocorticoid secretion alone.2 Extrapolated from other species, it is assumed that mineralocorticoid production in dogs with secondary HA is not impaired, as it is primarily regulated by the plasma potassium concentration and the renin‐aldosterone‐system.12
The gold standard for diagnosing HA is the ACTH stimulation test. Dogs with HA have low baseline cortisol concentrations and do not show an adequate increase in cortisol after ACTH administration. Because of limited availability of the assay, evaluation of aldosterone is not routinely included during workup of dogs suspected of HA and studies investigating aldosterone concentrations in dogs with HA are scarce. Most consistent seems an insufficient increase or an overall decreased ACTH‐stimulated aldosterone concentration.13, 14 A preliminary study in our canine population showed undetectable ACTH‐stimulated aldosterone concentrations in most dogs with HA.15 In dogs with atypical Addison's disease, ACTH‐stimulated aldosterone concentrations have only been measured in a small number of dogs and ranged from normal to decreased.2, 6 Finally, no studies exist evaluating the time point of maximal aldosterone concentrations after ACTH stimulation in dogs. Cortisol peaks 60 minutes after administration of ACTH.16 Knowing the exact time point of aldosterone peak concentrations seems of importance, as if sampling is not timed around this point, a residual aldosterone secretion of an impaired zona glomerulosa could be missed.
The aims of this study were, first, to compare the aldosterone concentrations of dogs with HA with those of dogs with diseases mimicking HA and healthy dogs; second, to investigate a potential relation between serum electrolyte concentrations in dogs with HA and their aldosterone concentrations; and third, to determine the time point of maximal aldosterone concentration after ACTH stimulation.
Material and Methods
Animals
Seventy client‐owned dogs with newly diagnosed HA were prospectively enrolled between December 1996 and March 2011. Results of 44 dogs had been part of a previous study.15 Work‐up included complete blood count, serum biochemical profile, urinalysis, ACTH stimulation test, abdominal ultrasonography (in particular adrenal glands) in all dogs, and measurement of endogenous ACTH in some dogs. HA was confirmed with an insufficient ACTH‐stimulated cortisol concentration (<1 μg/dL). Dogs with iatrogenic causes of HA (eg, previous steroid or trilostane therapy) were excluded from the study.
Twenty‐two dogs with diseases mimicking HA diagnosed between November 2007 and July 2009 were prospectively enrolled in the study. All dogs had initially been suspected of having HA but been given a different final diagnosis; all had ACTH‐stimulated cortisol concentrations >5 μg/dL. Diseases mimicking HA were associated with clinical signs or laboratory findings routinely seen in dogs with HA, such as vomiting, diarrhea, weakness, lethargy, and hyperkalemia, hyponatremia, or both.
Nineteen privately owned dogs were used as controls. The samples from these dogs had been taken to validate the aldosterone assay.17 The dogs were considered to be healthy on the basis of normal history and physical examination, as well as unremarkable hematology, serum biochemistry profile, urinalysis, and ACTH‐stimulated cortisol concentrations >5 μg/dL.
All procedures were approved by the Cantonal Veterinary Office of Zurich (permission number: 97/2003) and conducted in accordance with guidelines established by the Animal Welfare Act of Switzerland. In addition, informed consent was obtained from the owners of the healthy dogs.
Analytical Procedures
For the ACTH simulation test, blood samples were taken before, and 60 minutes after, intravenous injection of 250 μg synthetic ACTH.1 The ACTH stimulation test was performed in all 3 groups of dogs. Furthermore, an “extended” ACTH simulation test was performed in 7 dogs with HA and 22 dogs with diseases mimicking HA to confirm that aldosterone peak concentrations were not missed by the standard protocol. For the “extended” ACTH simulation test, additional blood samples were taken 15, 30, and 45 minutes after ACTH administration. Cortisol concentrations were measured by chemiluminescence assay.2 Sensitivity of the cortisol assay was 0.2 μg/dL. Aldosterone concentrations were detected by a radioimmunoassay previously validated by our group.3,17 The assay sensitivity was 4 pg/mL. Endogenous ACTH before ACTH stimulation was determined by a chemiluminescence assay.2 Blood was collected into chilled EDTA‐coated tubes placed on ice and centrifuged at 4°C. Cortisol and endogenous ACTH measurements were performed in house twice a week; plasma was stored either at −20°C (cortisol) or at −80°C (ACTH) until assayed. Samples for aldosterone determination were sent off to an external laboratory. In house, the blood sample was immediately centrifuged; the plasma was sent off to the external laboratory on the same day. In the external laboratory, aldosterone measurements were performed once or twice a week; plasma was stored at −20°C until assayed.
Statistical Analysis
Statistical analysis was performed by commercial software by means of nonparametric tests.4,5 Data are expressed as median and range. Differences between groups were tested by the use of the Kruskal‐Wallis H test and Dunn's post‐test. Linear correlation was calculated by Spearman nonparametric correlation. In the extended ACTH stimulation test, differences between the time points were tested by use of Friedman's repeated measures test and Dunn's post‐test. The level of significance was set at P < .05. For values below the detection limit, the mean between 0 and the detection limit was entered for statistical analysis.
Results
Animals
In the dogs with HA, age ranged from 1 to 11 years (median, 5 years) and body weight from 4.2 to 65.8 kg (median: 21.4 kg). There were 30 males (18 castrated) and 40 females (27 spayed). Fifty‐six purebred dogs and 14 mixed‐breed dogs were included. No breed seemed overrepresented.
In the dogs with diseases mimicking HA, age ranged from 1 to 13 years (median, 10 years) and body weight from 4.5 to 45.7 kg (median: 12.9 kg). There were 13 males (5 castrated) and 9 females (5 spayed). Eighteen purebred dogs and 4 mixed‐breed dogs were included. The final diagnoses reached were acute gastroenteritis (8), chronic‐intermittent gastroenteritis (4), protein‐losing enteropathy (1), intestinal adenocarcinoma (1), gastric ulceration (1), colitis (1), idiopathic megaesophagus (1), liver mass (1), hypercalcemia of unknown origin (2), chronic renal failure (1), and dehydration of unknown origin (1).
In the healthy dogs, age ranged from 1 to 14 years (median, 11 years) and body weight from 7.8 to 30 kg (median: 24.4 kg). There were 10 males (5 castrated) and 9 females (4 spayed). Fourteen purebred dogs and 5 mixed‐breed dogs were included.
Cortisol and Aldosterone Concentrations
Baseline cortisol concentrations in dogs with HA, with diseases mimicking HA, and healthy dogs ranged from <0.2 to 1.1 μg/dL (median, <0.2 μg/dL), from <0.2 to 6.8 μg/dL (median, 2.1 μg/dL), and from <0.2 to 3.5 μg/dL (median, 0.7 μg/dL), respectively. ACTH‐stimulated cortisol concentrations in dogs with HA, with diseases mimicking HA, and healthy dogs ranged from <0.2 to 0.8 μg/dL (median, <0.2 μg/dL), from 6.4 to 37.0 μg/dL (median, 9.5 μg/dL), and from 5.1 to 9.0 μg/dL (median, 6.9 μg/dL), respectively. Baseline and ACTH‐stimulated cortisol concentrations were significantly lower in dogs with HA than in dogs with diseases mimicking HA and healthy dogs (P < .0001/P < .0001). There was no difference between the baseline and ACTH‐stimulated cortisol concentrations of dogs with diseases mimicking HA and healthy dogs (Fig 1).
Figure 1.
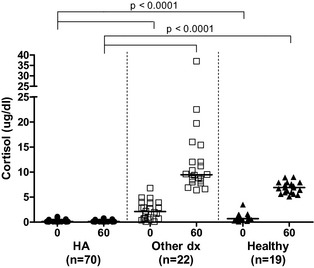
Baseline (0) and ACTH‐stimulated (60) serum cortisol concentrations in dogs with HA (HA, n = 70), dogs with diseases mimicking HA (other dx, n = 22), and healthy dogs (healthy, n = 19).
Baseline aldosterone concentrations in dogs with HA, with diseases mimicking HA, and healthy dogs ranged from <4 to 158 pg/dL (median, <4 pg/dL), from <4 to 556 pg/dL (median, <4 pg/dL), and from <4 to 157 pg/dL (median, 12 pg/dL), respectively. ACTH‐stimulated aldosterone concentrations in dogs with HA, with diseases mimicking HA, and healthy dogs ranged from <4 to 253 pg/dL (<4 pg/dL), from <4 to 1670 pg/dL (154 pg/dL), and from 46 to 602 pg/dL (187 pg/mL), respectively. Baseline and ACTH‐stimulated aldosterone concentrations were significantly lower in dogs with HA than in dogs with diseases mimicking HA and healthy dogs (P < .0001/P < .0001). There was no difference between the baseline and ACTH‐stimulated aldosterone concentrations of dogs with diseases mimicking HA and healthy dogs (Fig 2).
Figure 2.
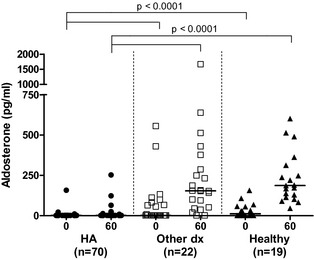
Baseline (0) and ACTH‐stimulated (60) serum aldosterone concentrations in dogs with HA (HA, n = 70), dogs with diseases mimicking HA (other dx, n = 22), and healthy dogs (healthy, n = 19).
Sodium, Potassium, and Aldosterone Concentrations in Dogs with HA
Sodium and potassium concentrations of the 70 dogs with HA ranged from normal to severely abnormal (sodium 116–160 mmol/L, median: 137 mmol/L, reference range: 151–160; potassium 3.3–11.3 mmol/L, median: 7.3 mmol/L, reference range: 4.2–5.4) (Fig 3). No correlation was found between ACTH‐stimulated aldosterone concentrations and sodium levels. A weak negative correlation was detected between ACTH‐stimulated aldosterone concentrations and potassium values (r = −0.32, P = .005).
Figure 3.
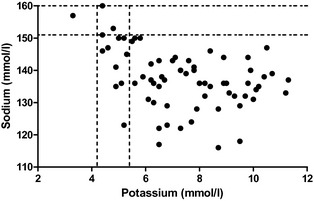
Serum sodium and potassium concentrations in dogs with HA (n = 70). The dotted lines represent the reference range for sodium and potassium concentrations.
In 64/70 dogs with HA, baseline and ACTH‐stimulated aldosterone concentrations were below the detection limit of the assay. In 6/70 dogs, ACTH‐stimulated aldosterone was either lower than normal or normal (3 dogs each: range: 9–253 pg/mL, median: 45 pg/mL, reference range: 46–514). Hyponatremia was present in all 6 dogs (124–150 mmol/L, median: 145.5 mmol/L) and hyperkalemia in 3 dogs (4.9–7.7 mmol/L, median: 5.5 mmol/L).
In 3/70 dogs with HA, both sodium and potassium concentrations were normal and in 1/70 dogs, sodium was normal and potassium decreased. ACTH‐stimulated aldosterone concentrations were below the detection limit of the assay in all 4 dogs. In 2 dogs, treatment was initiated with prednisolone. One of them developed hyperkalemia after 6 months, requiring additional mineralocorticoid supplementation. The other dog continued to have normal electrolytes throughout a 2‐year observational period. Of the other 2 dogs, treatment of one was initiated and maintained with gluco‐ and mineralocorticoids and the other was lost to follow‐up.
“Extended” ACTH Stimulation Test
In dogs with diseases mimicking HA, aldosterone concentrations 15, 30, 45, and 60 minutes after ACTH stimulation ranged from <4 to 1042 pg/mL (median, 115 pg/mL), from <4 to 1094 pg/mL (median, 160 pg/mL), from <4 to 1184 pg/mL (median, 180 pg/mL), and from <4 to 1670 pg/mL (median, 154 pg/mL), respectively. Aldosterone concentrations 15, 30, 45, and 60 minutes after ACTH stimulation were significantly higher than baseline values (P < .0001). Values collected 15 minutes after ACTH injection were significantly lower than those after 45 minutes (P = .002) (Fig 4).
Figure 4.
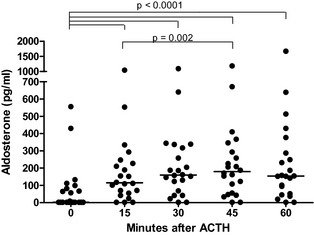
Baseline (0) and ACTH‐stimulated (15, 30, 45, 60) serum aldosterone concentrations in dogs with diseases mimicking HA (n = 22).
All ACTH‐stimulated aldosterone concentrations in the dogs with HA were below the detection limit, except for a 60‐minute sample in 1 dog.
Discussion
Little data exist about aldosterone concentrations in dogs with HA. The first objective of this study was therefore to compare baseline and ACTH‐stimulated aldosterone concentrations of dogs with HA with those of dogs with diseases mimicking HA and healthy dogs. The results of our study are in agreement with earlier studies finding that dogs with HA have significantly lower baseline and ACTH‐stimulated aldosterone concentrations than dogs with diseases mimicking HA and healthy dogs, but that overlapping values among the 3 groups are possible.13, 15, 18 Complete separation of healthy dogs and dogs with HA can be achieved by calculating the ratio between plasma renin activity and the baseline serum aldosterone concentration.18 However, measurement of plasma renin activity has limited availability and was not a part of our routine work‐up in this study.
In our study, aldosterone concentrations were low or undetectable in most dogs with HA independently of the degree of sodium and potassium abnormalities. It has been postulated by some authors that aldosterone secretion in dogs with primary HA and normal sodium and potassium concentrations may be normal.6, 7 The finding that sodium and potassium can be normal despite undetectable low ACTH‐stimulated aldosterone concentrations was striking. The question arose whether a potential residual aldosterone peak could occur early during the ACTH stimulation test and would therefore be missed by blood sampling only 60 minutes after ACTH administration. Hence, our protocol for the ACTH stimulation test was modified and additional blood samples 15, 30, and 45 minutes after ACTH injection were included. However, values after 60 minutes did not differ from values after 15, 30, or 45 minutes in healthy dogs and no aldosterone was detectable after 15, 30, or 45 minutes in dogs with HA, rendering the assumption of a missed aldosterone peak unlikely. As aldosterone concentrations after 60 minutes did not differ from those after 30 or 45 minutes, we can state that aldosterone measurements can be performed at the same time point (60 minutes) as cortisol determination, if 250 μg synthetic ACTH is used for stimulation.
Some of the published cases of so‐called atypical Addison's disease in dogs developed electrolyte abnormalities in the follow‐up period.2, 3, 4, 7 In our study, 2 cases with primary HA and normal electrolyte concentrations could be followed up. One dog developed sodium and potassium abnormalities after 6 months. The other dog could be followed up for 2 years and was still stable on glucocorticoid therapy only. The observation that most dogs develop electrolyte changes sooner or later led to the assumption that the immune‐mediated destruction of the adrenal cortex is first confined to the zona reticularis/fasciculata and in a later phase of disease spreads to the zona glomerulosa. However, aldosterone measurements in our study, as well as in earlier studies, demonstrate that normal electrolytes do not necessarily reflect a normally functioning zona glomerulosa.1, 2
In human medicine, normal sodium and potassium concentrations are found in 10% and normal potassium in 25% of patients with primary HA.19 The assumption that the zona glomerulosa and the aldosterone secretion are still intact in these patients has been degraded. Increased renin concentrations were found in all of those patients, indicating compensation for a failing zona glomerulosa.11 Therefore, in humans, it is currently assumed that a dissociation between zona glomerulosa and fasciculata function occurs very rarely.11 However, segmental sparing of the zona glomerulosa with atrophy of the zona reticularis and fasciculata has been documented in dogs.8, 9, 10 In addition, a very recent case report documents a dog with hypoaldosteronism preceding hypocortisolemia.20 This demonstrates that failing of 1 layer of the adrenal cortex can occur in dogs. It is most likely that these are rare cases. In future, renin measurements should be included in the work‐up of dogs with primary HA and normal electrolyte concentrations, to completely assess the function of the zona glomerulosa.
Possible mechanisms allowing a normal potassium balance without aldosterone, which are discussed in human medicine, are a high tubular flow rate with high delivery of potassium to the collecting duct, an increased sensitivity of the tubule to aldosterone caused by up‐regulation of the receptor, or both. Furthermore, hyperkalemia itself increases potassium excretion.14, 19 In veterinary medicine, it has been postulated that an animal with HA may be able to maintain potassium balance if the sodium intake is sufficient to maintain extracellular fluid volume and distal tubular flow rate.21 We have no detailed information about the feeding habits of these dogs. Most of these dogs were fed commercial pet food. Further information about the consumption of additional salty food (eg, treats and chews) was not available.
Moreover, dogs with HA and normal serum electrolyte concentrations could suffer from secondary HA. Measurement of cACTH concentrations can help to distinguish between primary and secondary HA, as cACTH is high in the first, but low in the latter form. Endogenous ACTH concentrations could only be measured in a small number of cases (5/70) in this study. The reasons for this were no assay availability during the earlier years of the study and presentation of dogs at night or at the weekend, which precluded correct blood handling for ACTH measurement. However, undetectable ACTH‐stimulated aldosterone concentrations in most of the dogs in this study indicate primary HA. Aldosterone production is primarily regulated by the plasma potassium concentration and the renin‐aldosterone‐system and not the pituitary ACTH secretion. Extrapolated from other species, it is therefore assumed that in cases of secondary HA, aldosterone secretion should not be impaired in dogs.12 Measurement of cACTH would have been of particular interest in the 3 dogs with ACTH‐stimulated aldosterone concentrations in the reference range. Unfortunately, in none of those dogs, endogenous ACTH concentrations were measured. Therefore, we cannot exclude that one of these dogs may have had a secondary HA. One other dog had a detectable but low ACTH‐stimulated aldosterone concentration. Endogenous ACTH concentration was severely elevated (704 pg/mL) in this dog indicating primary HA.
The value of aldosterone determinations during the routine diagnostic work‐up of HA has to be questioned. Most dogs with HA, independently of their electrolyte concentrations, have undetectable ACTH‐stimulated aldosterone concentrations. It could therefore be argued that almost all primary HA dogs may benefit from mineralocorticoid supplementation. The main reason not to start mineralocorticoid supplementation in a dog with primary HA and normal electrolytes would be the high expense of the medication. In our clinic, dogs with normal electrolyte concentrations are started on glucocorticoid therapy and regular rechecks are scheduled with the owner. However, owners should be aware that fluid losses because of vomiting could lead to an acute deterioration of their dog's condition and that a modification in therapy might be necessary in the later course of the disease. In future, determination of the plasma renin activity in these dogs should be intended to better classify their hormonal need.
In summary, aldosterone concentrations in dogs with HA seem to be compromised independent of their electrolyte concentrations. Therefore, normal sodium and potassium concentrations in dogs with HA may not reflect a normal function of the zona glomerulosa.
Acknowledgments
The authors gratefully acknowledge all colleagues at the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty University of Zurich for their contribution of cases.
Conflict of Interest Declaration: Authors disclose no conflict of interest.
The work was done at the Clinic for Small Animal Internal Medicine, Vetsuisse Faculty University of Zurich, Winterthurerstrasse 260, 8057 Zurich, Switzerland.
The study was presented as an abstract at the ACVIM Forum, Denver, USA, June 15–18, 2011
Footnotes
Synacthen; Novartis Pharma Schweiz AG, Bern, Switzerland
DPC Immulite 1000; Siemens Schweiz AG, Zurich, Switzerland
Coat a Count; Siemens Schweiz AG
GraphPad Prism5; GraphPad Software, San Diego, CA
SPSS 18.0 for Windows; SPSS Inc, Chicago, IL
References
- 1. Peterson ME, Kintzer PP, Kass PH. Pretreatment clinical and laboratory findings in dog with hypoadrenocorticism: 225 cases (1979–1993). J Am Vet Med Assoc 1996;208:85–91. [PubMed] [Google Scholar]
- 2. Thompson AL, Scott‐Moncrieff JC, Anderson JD. Comparison of classic hypoadrenocorticism with glucocorticoid‐deficient hypoadrenocorticism in dogs: 46 cases (1985–2005). J Am Vet Med Assoc 2007;230:1190–1194. [DOI] [PubMed] [Google Scholar]
- 3. Rogers W, Straus J, Chew D. Atypical hypoadrenocorticism in three dogs. J Am Vet Med Assoc 1981;179:155–158. [PubMed] [Google Scholar]
- 4. Lifton SJ, King LG, Zerbe CA. Glucocorticoid deficient hypoadrenocorticism in dogs: 18 cases (1986–1995). J Am Vet Med Assoc 1996;209:2076–2081. [PubMed] [Google Scholar]
- 5. Sadek D, Schaer M. Atypical Addison's disease in the dog: A retrospective survey of 14 cases. J Am Anim Hosp Assoc 1996;32:159–163. [DOI] [PubMed] [Google Scholar]
- 6. Dunn KJ, Herrtage ME. Hypocortisolaemia in a Labrador Retriever. J Small Anim Pract 1998;39:90–93. [DOI] [PubMed] [Google Scholar]
- 7. Mansfield CS. Glucocorticoid deficiency (atypical hypoadrenocorticism) and exocrine pancreatic insufficiency in a German Shepherd Dog. Aust Vet Prac 2000;30:2–5. [Google Scholar]
- 8. Kooistra HS, Rijnberk A, van den Ingh TS. Polyglandular deficiency syndrome in a Boxer dog: Thyroid hormone and glucocorticoid deficiency. Vet Q 1995;17:59–63. [DOI] [PubMed] [Google Scholar]
- 9. Adissu HA, Hamel‐Jolette A, Foster RA. Lymphocytic adenohypophysitis and adrenalitis in a dog with adrenal and thyroid atrophy. Vet Pathol 2010;47:1082–1085. [DOI] [PubMed] [Google Scholar]
- 10. Frank CB, Valentin SY, Scott‐Moncrieff JCR, Miller MA. Correlation of inflammation with adrenocortical atrophy in canine adrenalitis. J Comp Path 2013;149:268–279. [DOI] [PubMed] [Google Scholar]
- 11. Oelkers W, Diederich S, Bähr V. Diagnosis and therapy surveillance in Addison's disease: Rapid adrenocorticotropin (ACTH) test and measurement of plasma ACTH, renin activity, and aldosterone. J Clin Endocrinol Metab 1992;75:259–264. [DOI] [PubMed] [Google Scholar]
- 12. Gyton AC, Hall JE. Adrenocortical hormones In: Hall JE, ed. Textbook of Medical Physiology, 12th ed Philadelphia, PA: Saunders Elsevier; 2011:921–937. [Google Scholar]
- 13. Willard MD, Refsal K, Thacker E. Evaluation of plasma aldosterone concentrations before and after ACTH administration in clinically normal dogs and in dogs with various diseases. Am J Vet Res 1987;48:1713–1718. [PubMed] [Google Scholar]
- 14. Golden DL, Lothrop CD Jr. A retrospective study of aldosterone secretion in normal and adrenopathic dogs. J Vet Intern Med 1988;2:121–125. [DOI] [PubMed] [Google Scholar]
- 15. Müller C, Boretti FS, Wenger M, et al. Untersuchung der Aldosteronkonzentration vor und nach ACTH‐Applikation bei 44 Hunden mit Hypoadrenokortizismus (Investigation of the aldosterone concentration before and after ACTH application in 44 dogs with hypoadrenocorticism). Kleintierpraxis 2007;52:216–224. [Google Scholar]
- 16. Behrend EN, Kemppainen RJ, Bruyette DS, et al. Intramuscular administration of a low dose of ACTH for ACTH stimulation testing in dogs. J Am Vet Med Assoc 2006;229:528–530. [DOI] [PubMed] [Google Scholar]
- 17. Sieber‐Ruckstuhl NS, Boretti FS, Wenger M, et al. Cortisol, aldosterone, cortisol precursor, androgen and endogenous ACTH concentrations in dogs with pituitary‐dependant hyperadrenocorticism treated with trilostane. Domest Anim Endocrinol 2006;31:63–75. [DOI] [PubMed] [Google Scholar]
- 18. Javadi S, Galac S, Boer P, et al. Aldosterone‐to‐renin and cortisol‐to‐adrenocorticotropic hormone ratios in healthy dogs and dogs with primary hypoadrenocorticism. J Vet Intern Med 2006;20:556–561. [DOI] [PubMed] [Google Scholar]
- 19. Shiah CJ, Wu KD, Tsai DM, et al. Diagnostic value of plasma aldosterone/potassium ratio in hypoaldosteronism. J Formos Med Assoc 1995;94:248–254. [PubMed] [Google Scholar]
- 20. McGonigle KM, Randolph JF, Center SA, Goldstein RE. Mineralocorticoid before glucocorticoid deficiency in a dog with primary hypoadrenocorticism and hypothyroidism. Am Anim Hosp Assoc 2013;49:54–57. [DOI] [PubMed] [Google Scholar]
- 21. DiBartola SP, De Morais HA. Disorders of potassium: Hypokalemia and hyperkalemia In: DiBartola SP, ed. Fluid, Electrolyte, and Acid‐Base Disorders in Small Animal Practice, 4th ed St. Louis, MO: Elsevier Saunders; 2012:92–119. [Google Scholar]


