Abstract
Background
Boxer dogs are predisposed to congenital and adult onset cardiac diseases. Breed‐specific reference values for M‐mode and Doppler echocardiographic measurements previously have been established. Left ventricular (LV) end‐systolic (ESV) and end‐diastolic volumes (EDV) can be measured by M‐mode or two‐dimensional methods, such as Simpson's method of discs (SMOD). Reference ranges for SMOD‐derived LV volumes are lacking.
Objectives
To determine reference intervals for EDV and ESV in Boxer dogs.
Animals
Previously collected data from 85 healthy Boxers (37 males and 48 females) were used for analysis.
Methods
Simpson's method of discs‐derived EDV and ESV were measured using offline analysis by 1 observer, in both the right parasternal and the left apical views. Measurements were compared between both views and between male and female dogs using a t‐test. Reference intervals were established using the mean + 2 × SD.
Results
Measurements obtained from both views showed good agreement, and mean EDVI and ESVI, indexed to body surface area (BSA), were calculated. Reference intervals were 49–93 mL/m² for EDVI, and 22–50 mL/m² for ESVI. EDV and ESV were significantly higher in males compared with females, when indexing to BSA, but not when indexing to body weight.
Conclusion and Clinical Importance
The upper limit for ESVI exceeds the previously suggested cut‐off of 30 mL/m² for detection of systolic dysfunction. The reference intervals generated in this study should be useful clinically in the assessment of LV size and function in Boxer dogs.
Keywords: Boxer breed, Echocardiography, Reference values, Two‐dimensional volume measurement, Ultrasound
Abbreviations
- 2D
two‐dimensional
- 3D
three‐dimensional
- ASE
American Society of Echocardiography
- BSA
body surface area
- BW
body weight
- CV
coefficient of variation
- DCM
dilatory cardiomyopathy
- EDV
end‐diastolic volume
- EDVI
end‐diastolic volume index
- EF
ejection fraction
- ESV
end‐systolic volume
- ESVI
end‐systolic volume index
- FS
fractional shortening
- LVIDd
left ventricular inner diameter in diastole
- LVIDS
left ventricular inner diameter in systole
- LV
left ventricle
- pulm
pulmonic valve
- SD
standard deviation
- SMOD
biplane Simpson's method of discs
- Vmax
maximal velocity
- VPCs
ventricular premature complexes
Boxer dogs are predisposed to congenital cardiac diseases, namely aortic and pulmonic stenosis, as well as adult onset cardiomyopathies, such as arrhythmogenic right ventricular cardiomyopathy and idiopathic dilated cardiomyopathy (DCM).1, 2, 3, 4 Therefore, cardiac screening is highly recommended in this breed. To provide breeders and owners with optimal screening programs, development of breed‐specific reference values is important. Indeed, previous studies have shown a smaller left ventricular (LV) outflow tract, higher aortic blood flow velocities, and increased LV wall thickness in Boxers compared with non‐Boxer dogs.5, 6
Although 24‐hour Holter ECG monitoring remains the gold standard for diagnosis and monitoring of the arrhythmias associated with all forms of Boxer cardiomyopathy, echocardiography is the most important tool to identify LV dimensions and function. M‐mode echocardiography is the most widespread method to measure LV dimensions, but has limitations. It is a one‐dimensional technique, relying on geometric assumptions that may not be accurate in disease states. The Teichholz method for calculation of LV volumes results in inaccuracies as a result of the geometric assumptions required to convert a linear measurement to a three‐dimensional (3D) volume.7, 8 According to the American Society of Echocardiography (ASE), the current recommendation for two‐dimensional (2D) volume measurement in humans is the biplanar Simpson's method of discs (SMOD).9 In dogs, SMOD correlated well with 3D measurements of LV volume, whereas the Teichholz formula overestimated LV volume with a factor 2.10 SMOD measurements have been shown to be more sensitive than M‐mode echocardiography for early detection of occult cardiomyopathy in Doberman Pinschers.11 SMOD reference values have been published for the Doberman Pinscher11 and the Great Dane,12 but not for Boxer dogs. Because of the substantial variability in echocardiographic measurements in dogs of different somatotypes,13 and the particular interest of cardiac screening in the Boxer breed, the primary objective of this study was to establish breed‐specific SMOD reference values in healthy Boxer dogs. Secondary aims were (1) to determine the agreement between SMOD values obtained in the right parasternal long‐axis and the left apical 4‐chamber view, (2) to compare measurements between male and female dogs, and (3) to investigate indexation of end‐diastolic and end‐systolic volumes (EDV and ESV) to body surface area (BSA) or body weight (BW).
Materials and Methods
Study Population
Medical records of the Cardiology Department, Ludwig‐Maximilian University, Munich from 2004 to 2012 were searched for files of healthy Boxer dogs. Inclusion criteria were the absence of clinical signs according to the owner, no relevant abnormalities on physical examination, an unremarkable 2‐minute 6‐lead ECG, no abnormalities on the continuous ECG during the echocardiographic examination, and a complete echocardiographic examination stored in the offline database. Dogs with overt cardiovascular signs noted by the owner (eg, exercise intolerance, syncope, dyspnea) were excluded. Further exclusion criteria were ventricular premature complexes or other arrhythmias on the 2‐minute 6‐lead ECG or continuous ECG during echocardiography, a peak aortic velocity ≥2.3 m/s,14 a peak pulmonic velocity >2 m/s, or echocardiographic signs of LV dilatation (based on previously established reference values for left ventricular inner diameter in diastole [LVIDd] and left ventricular inner diameter in systole [LVIDs]), myocardial dysfunction (based on subjective assessment of systolic function, LVIDs above upper limit, and fractional shortening [FS] <25%), or some combination of these findings.1 , 15, 16 Data extracted from patient files included the following: age, body weight, sex, examination date, mean heart rate, LVIDd, LVIDs, FS, ejection fraction, peak aortic, and peak pulmonic blood flow velocities.
Echocardiographic Procedures
All dogs were examined without sedation in right and left lateral recumbency. All echocardiographic examinations were performed using the same commercially available high frame rate ultrasound system equipped with a 2.0/4.3 MHz probe with simultaneous ECG recording.2 Minimal requirements for echocardiographic examinations were as follows: presence of 2D echoloops of at least 3 cardiac cycles of the right parasternal 4‐chamber view with cardiac apex and the left apical 4‐chamber view with cardiac apex, longitudinal 4‐chamber and transverse M‐mode images, color Doppler images of all 4 cardiac valves, and pulsed wave, continuous wave Doppler blood velocities of aortic and pulmonic valves, or both.
Using SMOD, EDV and ESV were measured by offline image analysis software3 by a single cardiology resident (PS) under supervision of a cardiology diplomate (GW). SMOD measurements were performed on the right parasternal long‐axis 4‐chamber view and the left apical 4‐chamber view. Selection of end‐diastolic frames corresponding to the onset of QRS (ie, at the time of mitral valve closure) and end‐systolic frames (corresponding to the last frame before mitral valve opening) were selected using frame‐by‐frame analysis. The LV area was measured by tracing the endocardial border on each selected image, maximal LV length was measured from the middle of a line connecting the 2 mitral annuli to the endocardial border of the LV apex (Fig 1), followed by automatic calculation of LV volumes by the ultrasound machine. SMOD‐derived end‐diastolic and end‐systolic LV volumes were indexed to BSA (EDVI and ESVI, respectively). Nonindexed volumes are abbreviated as EDV and ESV.
Figure 1.
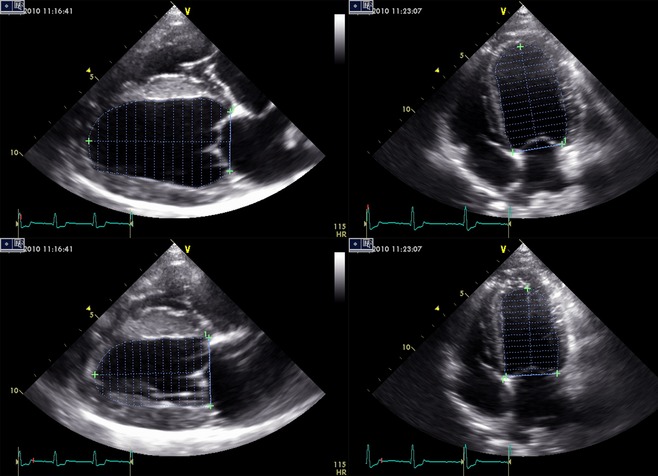
Simpson's method of discs measurements performed on the right parasternal long‐axis and the left apical 4‐chamber view, in diastole and systole. The left ventricular (LV) area was measured by tracing the endocardial border; maximal LV length was measured from the middle of a line connecting the 2 mitral annuli to the endocardial border of the LV apex.
Statistical Analysis
The data were analyzed by commercially available software.4 All data were graphically inspected and tested for normality using the Shapiro‐Wilk test. All variables except age were normally distributed. Results are presented as mean ± SD, except for age, which is presented as median (range). Measurements obtained from the right parasternal and left apical views were compared using a paired t‐test. Limits of agreement between EDV right parasternal and EDV left apical, and between ESV right parasternal and ESV left apical were analyzed using Bland‐Altman plots.17 The intraobserver coefficient of variation (CV) for EDV and ESV measurements obtained in both views was calculated as the square root of the variance divided by the mean of 3 repeated measurements, multiplied by 100. Body weight, LVIDd, LVIDs, EDVI right parasternal and left apical, ESVI right parasternal and left apical, V max aorta, and V max pulm were compared between male and female dogs using a t‐test. Level of significance was set at a P value <.05. The relationships of EDV and ESV with BSA and BW were graphically examined using a scatter plot graph.
Results
Eighty‐five Boxer dogs met the inclusion criteria. Most of dogs (53/85) were presented for aortic stenosis screening. The remaining dogs (32/85) were referred for other various reasons. Thirty‐seven dogs were male and 48 dogs were female. Results for each investigated variable are presented in Table 1, for the complete group and for male and female dogs separately.
Table 1.
Results for the investigated variables in the complete group, and in male and female Boxer dogs separately
| Variable | Complete Group | Male Dogs | Female Dogs |
|---|---|---|---|
| Number of dogs | 85 | 37 | 48 |
| Age (years) | 1.6 (1.0–14.5) | 1.6 (1.0–14.5)* | 1.6 (1.0–10.8)* |
| BW (kg) | 29.8 ± 4.6 | 32.2 ± 4.4** | 26.8 ± 2.6** |
| Heat rate (bpm) | 97 ± 18 | 100 ± 19 | 94 ± 15 |
| LVIDd (mm) | 39.6 ± 3.4 | 40.8 ± 3.0** | 38 ± 3.2** |
| LVIDs (mm) | 27.3 ± 3.7 | 28.1 ± 3.9* | 26.1 ± 3.0* |
| Fractional shortening (%) | 31.3 ± 6.4 | 31.2 ± 7.1 | 31.3 ± 5.5 |
| V max aorta (m/s) | 1.80 ± 0.27 | 1.79 ± 0.30 | 1.8 ± 0.24 |
| V max pulm (m/s) | 1.24 ± 0.25 | 1.18 ± 0.24 | 1.30 ± 0.26 |
| EDVI right parasternal (mL/m²) | 70 ± 10 | 73 ± 10* | 67 ± 11* |
| EDVI left apical (mL/m²) | 72 ± 11 | 74 ± 10 | 69 ± 12 |
| ESVI right parasternal (mL/m²) | 36 ± 7 | 38 ± 7* | 33 ± 7* |
| ESVI left apical (mL/m²) | 37 ± 8 | 38 ± 7* | 35 ± 7* |
| Mean EDVI (mL/m²) | 71 ± 11 | 73 ± 10* | 68 ± 11* |
| Mean EDV indexed to BW (mL/kg) | 2.3 ± 0.4 | 2.3 ± 0.3 | 2.3 ± 0.4 |
| Mean ESVI (mL/m²) | 36 ± 7 | 38 ± 7* | 34 ± 7* |
| Mean ESV indexed to BW (mL/kg) | 1.2 ± 0.2 | 1.2 ± 0.2 | 1.2 ± 0.2 |
| Ejection fraction (%) | 49 ± 7 | 48 ± 8 | 50 ± 6 |
BW, body weight; LVIDd, left ventricular inner diameter in diastole; LVIDs, left ventricular inner diameter in systole; V max, maximal velocity; pulm, pulmonary artery; EDVI, end‐diastolic volume index; ESVI, end‐systolic volume index.
Normally distributed variables are presented as mean ± SD; only age was not normally distributed and is presented as median (range). A single asterisk (*) indicates a significant difference between male and female dogs with a P value <.05, and a double asterisk (**) indicates a significant difference with a P value <.001.
End‐diastolic volume index and ESVI measurements obtained from the right parasternal long‐axis view were significantly smaller than those obtained from the left apical 4‐chamber view (EDVI, P = .004; ESVI, P < .001). However, when comparing the mean difference and the 95% confidence interval (CI) of the measurements of both sides, the difference was not clinically relevant. The mean difference between the left apical and right parasternal view for EDVI and ESVI was 1 mL/m². The 95% CI was 68–72 mL/m² for EDVI right parasternal, and 69–74 mL/m² for EDVI left apical; the 95% CI of ESVI right parasternal was 34–37 mL/m², and that of ESVI left apical was 35–39 mL/m². The Bland‐Altman graphs in Figure 2 display the differences between EDVI and ESVI obtained from the right parasternal long‐axis view and the left apical 4‐chamber view plotted against average values of EDVI and ESVI. The limits of agreement represent the mean difference ± 2 × SD and are shown as dotted lines. These plots show good agreement between both views. The mean intraobserver CV for repeated measurements of EDVI right parasternal was 4.2%, for ESVI right parasternal, it was 5.7%, for EDVI left apical 5.1%, and for ESVI left apical 5.6%.
Figure 2.
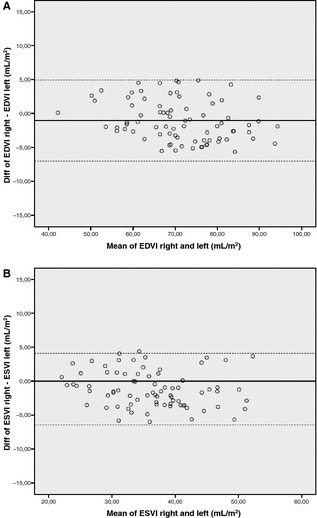
Bland‐Altman graphs illustrating good agreement between measurements of EDVI (A) and ESVI (B) obtained in the right parasternal and left apical view. Diff, difference; EDVI right, end‐diastolic volume index right parasternal view; EDVI left, end‐diastolic volume index left apical view; ESVI right, end‐systolic volume index right parasternal view; ESVI left, end‐systolic volume index left apical view.
Because they showed good agreement, mean values for EDVI and ESVI obtained in the right parasternal and left apical view were calculated. Table 2 presents minimum and maximum values, and reference intervals, based on the mean value ± 2 × SD,18 for mean EDVI and mean ESVI in the complete group and for male and female Boxer dogs separately. Teichholz‐derived volumes for mean EDV indexed to BSA were similar to SMOD‐derived values, namely 71 mL/m² in the complete group, 72 mL/m² in males, and 69 mL/m² in females. Teichholz‐derived volumes for mean ESV indexed to BSA were slightly lower than the SMOD‐derived volumes, namely 29 mL/m² in the complete group, 30 mL/m² in male dogs, and 28 mL/m² in female dogs.
Table 2.
Maximum and minimum values, and reference intervals for mean EDVI and ESVI (in mL/m²) in the complete group of Boxer dogs (n = 85) and in males (n = 37) and females (n = 48) separately
| Mean EDVI | Mean ESVI | Mean EDVI | Mean ESVI | |||
|---|---|---|---|---|---|---|
| Male | Female | Male | Female | |||
| Minimum | 42 | 22 | 51 | 42 | 24 | 22 |
| Maximum | 94 | 52 | 94 | 89 | 52 | 47 |
| Reference interval: mean ± 2SD | 49–93 | 22–50 | 53–93 | 46–90 | 24–52 | 20–48 |
EDVI, end‐diastolic volume index; ESVI, end‐systolic volume index; SD, standard deviation.
When comparing male and female dogs, age (P = .021), body weight (P < .001), LVIDd (P < .001), LVIDs (P = .011), EDVI right parasternal (P = .011), ESVI right parasternal (P = .008), ESVI left apical (P = .044), but not EDVI left apical (P = .052), mean EDVI (P = .04) and mean ESVI (P = .04) were significantly higher in male dogs. When LVIDd and LVIDs were indexed using BW1/316, the difference was no longer statistically significant (P = .6 for both measurements). Mean EDV and ESV are plotted against BSA and BW in Figure 3. The squares represent male dogs and the circles female dogs. This figure illustrates that the range of BW was larger in the male compared with the female dogs (24.4–45 kg in males compared with 22.5–34 kg in females). Furthermore, it shows that the correlations of mean EDV and ESV with BW are slightly higher than the correlation with BSA. When indexing using BW, instead of BSA, EDV right parasternal (P = .54), ESV right parasternal (P = .20), and ESV left apical (P = .51), mean EDV (P = .81) and mean ESV (P = .51) are no longer significantly different between male and female dogs.
Figure 3.
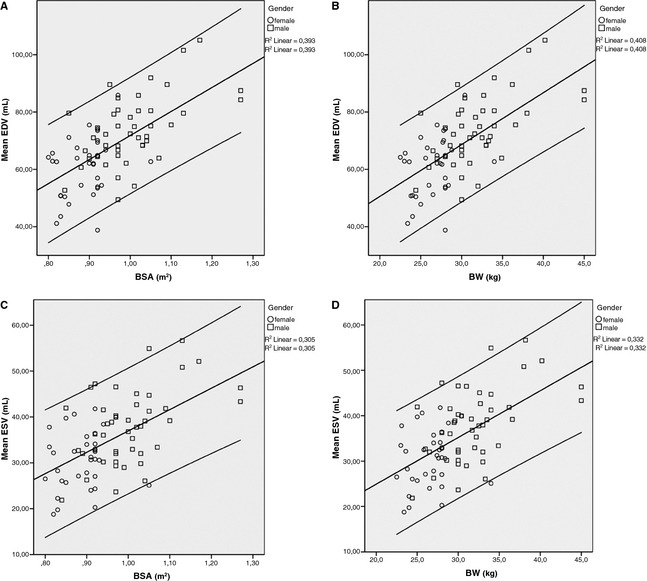
Scatter plot presenting the correlation of mean EDV (A,B) and ESV (C,D) with BSA (A,C) and BW (B,D). For both mean EDV and ESV, the R² value is slightly higher for BW than for BSA. The squares represent male dogs and the circles female dogs. The line in the middle represents the mean and the outer lines represent the 95% CI of EDV and ESV values. EDV, end‐diastolic volume; ESV, end‐systolic volume; BW, body weight; BSA, body surface area.
Discussion
This study is the first to present reference intervals for diastolic and systolic LV volumes using SMOD specifically in Boxer dogs. Echocardiographic estimation of LV volumes can be determined by M‐mode‐derived geometric methods, such as the Teichholz method, or 2D planimetric methods, such as SMOD. An interesting and unexpected finding in this study was that in healthy Boxer dogs, the volumes calculated by Teichholz measurements and the volumes derived by SMOD were quite comparable. This is in contrast to findings by Tidholm and Serres, which showed that Teichholz measurements overestimated the volumes by a factor of 2.10, 19 Differences between the Teichholz and SMOD estimations of LV volume may be more pronounced in disease states than in healthy dogs.8 In addition, Teichholz‐derived volumes and 2D and 3D volume estimations also have been shown to differ more as EDV increases in dogs with heart disease.10 Another explanation might be the elliptical heart form of Boxer dogs, compared to that of small breed dogs such as Dachshunds or Cavalier King Charles Spaniels, and the Teichholz formula may be better suited in dogs with elliptical heart geometry. This variable needs to be further evaluated in future studies.
Although SMOD is the current ASE‐recommended method for measurement of LV volumes in humans and seems to be very accurate compared with other methods in dogs, studies describing reference ranges are scarce.19, 20 Boxer dogs are particularly prone to heart disease and breed‐specific M‐mode and Doppler reference ranges have been generated for this breed.1 ,[ 5, 6, 14 ] In this study, suggested reference intervals for SMOD‐derived EDVI and ESVI based on data of 85 Boxer dogs are 49–93 mL/m² and 22–50 mL/m², respectively. These reference ranges are comparable to a previous study in Doberman Pinschers, where upper limits of normal for EDVI and ESVI were 95 mL/m² and 55 mL/m², respectively.11 Furthermore, the SMOD‐derived ESVI reference interval, based on data of 40 Great Danes, was 21.9–47.0 mL/m² in a recent study.12 Based on these 3 studies, SMOD‐derived LV volumes, indexed to BSA, show considerable agreement in Boxers, Doberman Pinschers, and Great Danes. All of these breeds are predisposed to idiopathic DCM, for which diagnostic criteria have been proposed by the ESVC taskforce.21 In the ESVC recommendations, it is stated that “an ESV‐I over 80 mL/m² offers unequivocal evidence for systolic dysfunction, although this may be an excessively conservative value, as the normal canine ESV‐I has been suggested to be <30 mL/m².” The upper limit of the reference intervals for ESVI in Doberman Pinschers, in Great Danes, and in this study is clearly lower than the 80 mL/m² but higher than the 30 mL/m², suggested in the ESVC paper.11, 12 This would indicate, as previously suggested,11 that either ESVI is higher in these 3 breeds compared with other breeds, suggesting intrinsic systolic dysfunction, or the cut‐off value of 30 mL/m² is too low. In contradiction to this study, the range of SMOD‐derived ESVI was below the cut‐off of 30 mL/m² (9.5–25.4 mL/m²) in a previous study including a group of 24 healthy smaller breed dogs (<10 kg).19 Because of the large overlap between normal and abnormal values in the ESVC guidelines and several studies describing ESVI >30 mL/m²,11, 12 further study in dogs of different breeds is warranted to determine SMOD‐derived ESVI cut‐offs.
A characteristic of the Boxer breed, which could theoretically affect afterload and thus ESVI, is the smaller diameter of the aortic annulus compared with other breeds.5, 6 Although Boxers were found to have increased indices of LV wall thickness compared with healthy non‐Boxer dogs, this was independent of LV cavity size,5 and thus unlikely to substantially affect ESVI in this study.
When comparing the SMOD values for EDVI and ESVI derived from the right parasternal and left apical views, there was good agreement between both views, with a mean difference of 1 mL/m². A previous study also demonstrated good agreement between measurements obtained from these 2 views.11 For accurate measurements, it is crucial to optimize LV length and include the anatomical LV apex. Although technically more demanding than the right parasternal view, it is possible that LV length was optimized better in the left apical view in this study, producing higher measurements. As previously suggested, it is advisable to measure LV volume from both sides and use either the highest measurement or the mean of both views for patient assessment.11 The intraobserver CVs for repeated measurements of EDVI and ESVI in the right parasternal and left apical views ranged from 4.2 to 5.7%. Although this indicates somewhat higher variability compared with previous studies,11, 19 these CVs still indicate good‐to‐excellent repeatability.
Several studies have described different M‐mode reference ranges for male and female dogs, for example, in Greyhounds, German Shepherds, and Great Danes.12, 22, 23 In this study, both LVIDd and LVIDs were significantly smaller in female dogs, which had lower body weight than male dogs. Comparable to the study in Great Danes, this difference was no longer statistically significant when allometric scaling was applied (indexing by BW1/3). Mean EDVI and ESVI, indexed to BSA, also were significantly higher in male dogs, although the difference between both sexes was relatively small. When indexing EDV and ESV to BW, the difference between male and female dogs was no longer statistically significant. A possible reason for this finding is the fact that absolute numbers become smaller and variation between genders is minimized, when indexing to body weight compared with BSA (eg, mean body weight in males was 32.2 kg, whereas mean BSA was 1.0 m²). This may be responsible for the lack of statistically significant difference between males and females when indexing to BW. As stated in the paper by Cornell et al, it seems logical that cardiac volumes, such as EDV and ESV, should be linearly related to BW, whereas cross‐sectional cardiac areas should relate linearly to BSA.16 When plotting mean EDV and mean ESV against BSA and BW, the correlation was highest for BW. These findings suggest that different EDVI and ESVI reference ranges should be used for male and female Boxer dogs, when indexing to BSA, or that measurements should be indexed to BW.
This study has several limitations. A 24‐hour Holter ECG recording was not an inclusion criterion and therefore it is not possible to be certain that none of the dogs belonged to the concealed (“arrhythmias only”) Harpster form of dilatory cardiomyopathy. However, most of the dogs were very young (95% CI between 2.4 and 3.7 years) and therefore it is unlikely that they were already affected. On the other hand, the fact that most were young dogs presented for aortic stenosis screening also implies that the dogs in this study were not a completely representative sample for the population. The cardiac ultrasound examinations were recorded by several echocardiographers, which could be a possible source of variability, although interechocardiographer variability in SMOD in our institution has been described to be as low as 5.7–6.5% for the right parasternal view, and 2.4–5.8% for the left apical view.11 In addition, all measurements in this study were performed by 1 observer. Inclusion of a group of Boxers with idiopathic DCM would have allowed calculation of optimal cut‐off values for SMOD‐derived LV volumes, and their respective sensitivity and specificity, to distinguish between normal and affected dogs.
In conclusion, this study is the first to report SMOD‐derived reference ranges for EDV and ESV in the Boxer dog. The upper limit of the reference interval for ESVI (51 mL/m²) in this study exceeds the previously suggested cut‐off of 30 mL/m² for detection of systolic dysfunction. Therefore, future studies are warranted in Boxer dogs with DCM and other breeds to investigate the ideal cut‐off for detection of systolic dysfunction. This study also showed a significant difference in EDVI and ESVI between male and female dogs, when indexing to BSA. This difference may be minimized by indexing EDV and ESV to BW. Although SMOD is the ASE‐recommended method for measurement of LV volumes in humans and is increasingly used in dogs, reference ranges are scarce. Therefore, the reference intervals generated in this study are of clinical importance in the assessment of LV size and function in Boxer dogs.
Acknowledgments
Conflict of Interest Declaration: Authors disclose no conflict of interest.
Footnotes
Herrtage M. Echocardiographic measurements in the normal Boxer. European Society of Veterinary Internal Medicine Congress Proceedings, 1994;172 (abstract)
Vivid 7; General Electric Medical Systems, Wauhesha, WI
EchoPac Clinical Workstation Software, General Electric Medical Systems, Wauhesha, WI
SPSS Statistics 19; IBM Deutschland, Ehninge, Germany
References
- 1. Bussadori C, Quintavalla C, Capelli A. Prevalence of congenital heart disease in Boxers in Italy. J Vet Cardiol 2001;3:7–11. [DOI] [PubMed] [Google Scholar]
- 2. Chetboul V, Trolle JM, Nicolle A, et al. Congenital heart diseases in the Boxer dog: A retrospective study of 105 cases (1998–2005). J Vet Med A Physiol Pathol Clin Med 2006;53:346–351. [DOI] [PubMed] [Google Scholar]
- 3. Harpster NK. Boxer cardiomyopathy In: Kirk R, ed. Current Veterinary Therapy VIII. Philadelphia, PA: W.B. Saunders; 1983:329–337. [Google Scholar]
- 4. Kittleson MD. Primary myocardial disease leading to chronic myocardial failure In: Kittleson MD, Kienle RD, eds. Small Animal Cardiovascular Medicine. St. Louis, MO: Mosby; 1998:316–346. [Google Scholar]
- 5. Cunningham SM, Rush JE, Freeman LM, et al. Echocardiographic ratio indices in overtly healthy Boxer dogs screened for heart disease. J Vet Intern Med 2008;22:924–930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Koplitz SL, Meurs KM, Bonagura JD. Echocardiographic assessment of the left ventricular outflow tract in the Boxer. J Vet Intern Med 2006;20:904–911. [DOI] [PubMed] [Google Scholar]
- 7. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. Eur J Echocardiogr 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 8. Teichholz LE, Kreulen T, Herman MV, et al. Problems in echocardiographic volume determinations: Echocardiographic‐angiographic correlations in the presence of absence of asynergy. Am J Cardiol 1976;37:7–11. [DOI] [PubMed] [Google Scholar]
- 9. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: A report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005;18:1440–1463. [DOI] [PubMed] [Google Scholar]
- 10. Tidholm A, Westling AB, Hoglund K, et al. Comparisons of 3‐, 2‐dimensional, and M‐mode echocardiographical methods for estimation of left chamber volumes in dogs with and without acquired heart disease. J Vet Intern Med 2010;24:1414–1420. [DOI] [PubMed] [Google Scholar]
- 11. Wess G, Maurer J, Simak J, et al. Use of Simpson's method of disc to detect early echocardiographic changes in Doberman Pinschers with dilated cardiomyopathy. J Vet Intern Med 2010;24:1069–1076. [DOI] [PubMed] [Google Scholar]
- 12. Stephenson HM, Fonfara S, Lopez‐Alvarez J, et al. Screening for dilated cardiomyopathy in Great Danes in the United Kingdom. J Vet Intern Med 2012;26:1140–1147. [DOI] [PubMed] [Google Scholar]
- 13. Morrison SA, Moise NS, Scarlett J, et al. Effect of breed and body weight on echocardiographic values in four breeds of dogs of differing somatotype. J Vet Intern Med 1992;6:220–224. [DOI] [PubMed] [Google Scholar]
- 14. Schober KE, Fuentes VL. Doppler echocardiographic assessment of left ventricular diastolic function in 74 boxer dogs with aortic stenosis. J Vet Cardiol 2002;4:7–16. [DOI] [PubMed] [Google Scholar]
- 15. Baumwart RD, Meurs KM, Atkins CE, et al. Clinical, echocardiographic, and electrocardiographic abnormalities in Boxers with cardiomyopathy and left ventricular systolic dysfunction: 48 cases (1985–2003). J Am Vet Med Assoc 2005;226:1102–1104. [DOI] [PubMed] [Google Scholar]
- 16. Cornell CC, Kittleson MD, Della Torre P, et al. Allometric scaling of M‐mode cardiac measurements in normal adult dogs. J Vet Intern Med 2004;18:311–321. [DOI] [PubMed] [Google Scholar]
- 17. Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 18. Sterne JK, Kirkwood BR. The normal distribution In: Sterne JK, Kirkwood BR, eds. Essential Medical Statistics. Oxford: Blackwell Publishing Ltd.; 2003:42–49. [Google Scholar]
- 19. Serres F, Chetboul V, Tissier R, et al. Comparison of 3 ultrasound methods for quantifying left ventricular systolic function: Correlation with disease severity and prognostic value in dogs with mitral valve disease. J Vet Intern Med 2008;22:566–577. [DOI] [PubMed] [Google Scholar]
- 20. Serres F, Pouchelon JL, Poujol L, et al. Plasma N‐terminal pro‐B‐type natriuretic peptide concentration helps to predict survival in dogs with symptomatic degenerative mitral valve disease regardless of and in combination with the initial clinical status at admission. J Vet Cardiol 2009;11:103–121. [DOI] [PubMed] [Google Scholar]
- 21. Dukes‐McEwan J, Borgarelli M, Tidholm A, et al. Proposed guidelines for the diagnosis of canine idiopathic dilated cardiomyopathy. J Vet Cardiol 2003;5:7–19. [DOI] [PubMed] [Google Scholar]
- 22. Kayar A, Gonul R, Or ME, et al. M‐mode echocardiographic parameters and indices in the normal German Shepherd Dog. Vet Radiol Ultrasound 2006;47:482–486. [DOI] [PubMed] [Google Scholar]
- 23. Lonsdale RA, Labuc RH, Robertson ID. Echocardiographic parameters in training compared with non‐training Greyhounds. Vet Radiol Ultrasound 1998;39:325–330. [DOI] [PubMed] [Google Scholar]


