Abstract
Background
A wide spectrum of laboratory tests is available to aid diagnosis and classification of equine inflammatory disease.
Objectives
To compare diagnostic efficacy and combined predictive capability of the myeloperoxidase index (MPXI), and plasma fibrinogen, iron and serum amyloid A (SAA) concentrations for the diagnosis of inflammation.
Animals
Twenty‐six hospitalized horses with systemic inflammation (SI), 114 with local inflammation (LI) and 61 healthy horses or those with noninflammatory disease (NI) were included.
Methods
A retrospective study was performed; clinicopathologic data from horses were compared between groups. Receiver‐operator characteristic (ROC) curves were used to evaluate diagnostic efficacy; classification and regression tree analysis (CART) and logistic regression analysis were used to generate diagnostic algorithms.
Results
Horses with SI had significantly higher SAA than horses with LI (P = .007) and NI (P < .001) and lower iron concentrations than horses with LI (P < .001) and NI (P < .001). Fibrinogen concentration was higher in horses with inflammation than in those without inflammation (P = .002). There was no difference between the SI and LI groups. White blood cell count, neutrophil count and MPXI were similar between groups. SAA had the highest accuracy for diagnosing inflammation (area under ROC curve [AUC], 0.83 ± 0.06) and iron and SAA concentration had the highest accuracy for differentiating SI from LI (AUC, 0.80 ± 0.09 and 0.73 ± 0.10 respectively). Predictive modeling failed to generate useful algorithms and classification of cases was moderate.
Conclusions and Clinical Importance
Very high SAA and low iron concentrations may reflect SI, but diagnostic guidelines based on quantitative results of inflammatory markers could not be formulated.
Keywords: Classification and regression tree, Iron, Myeloperoxidase index, Serum amyloid A
Abbreviations
- APP
acute phase protein
- AUC
area under the curve
- CART
classification and regression tree
- EDTA
ethylenediaminetetraacetic acid
- LI
local inflammatory disease
- MPO
myeloperoxidase
- MPXI
myeloperoxidase index
- NI
noninflammatory disease/healthy
- NSAIDs
nonsteroidal anti‐inflammatory drugs
- RI
reference intervals
- ROC
receiver‐operator characteristic
- SAA
serum amyloid A
- Se
sensitivity
- SIRS
systemic inflammatory response syndrome
- SI
systemic inflammation
- Sp
specificity
- TWBC
total white blood cell count
Inflammation is defined as a well‐organized cascade of exudative and cellular changes within vascularized tissue triggered by a variety of causes such as mechanical trauma, neoplasia, tissue necrosis, and infection.1 Inflammation occurs as part of many diseases in horses, either locally or as a systemic reaction—the systemic inflammatory response syndrome (SIRS). Diseases causing SIRS include endotoxemia, localized bacterial infections, septicemia and noninfectious conditions such as ischemia and trauma.2
Early detection and monitoring of SIRS is pivotal for successful case management. Because the leukocyte response in horses is insensitive, recent efforts have focussed on the development and validation of other laboratory tests that might aid in the diagnosis and monitoring of inflammatory disease in horses.3, 4, 5 The neutrophil myeloperoxidase index (MPXI), which is routinely measured by the ADVIA hematology system, recently has been investigated as a marker for systemic inflammation (SI) in horses.6, 7 Acute phase proteins (APP) such as serum amyloid A (SAA) are now available for routine testing.3, 8 A decreased serum or plasma iron concentration has been identified as a good indicator of acute inflammatory disease in horses.9, 10 Although fibrinogen is only a moderate APP in the horse and prone to preanalytical errors such as microclot formation, it is commonly used because of its wide availability and low cost of the heat precipitation method.8 Comparative data investigating the diagnostic efficacy of these laboratory tests in combination for detecting SI is scarce. In 1 study decreased serum iron was found to be a better marker for SI in horses than plasma fibrinogen concentration, however the diagnostic efficacy of the 2 tests used together was not investigated.10 Jacobsen et al found that SAA and serum iron concentration better reflected the course and severity of inflammation postcastration than did total white blood cell count (TWBC) and plasma fibrinogen concentration.4 Similarly, postoperative concentrations of SAA, iron, and fibrinogen were found to differ depending on type of surgery performed, whereas TWBC did not.11
The aims of this study were to compare the differences in results and diagnostic efficacy of MPXI and plasma SAA, iron, and fibrinogen concentrations for diagnosing various equine inflammatory conditions. Furthermore, the use of predictive models based on combinations of these analytes to formulate diagnostic algorithms and develop recommendations for diagnosing inflammatory disease in horses in the field was examined.
Materials and Methods
Animals
The data bank of the Clinical Pathology Platform of the University of Veterinary Medicine, Vienna was searched for all equine blood samples where a TWBC, including an absolute neutrophil concentration and MPXI, fibrinogen, iron, and SAA were measured concurrently, over a 2.5 year period. Repeat samples, samples from horses under the age of 1 year and samples from horses for which no clinical information was available (external submissions) were excluded. The clinical records of these horses were examined and animals were divided into 3 groups: noninflammatory (NI), local inflammation (LI), and SI. The NI group included horses without clinical signs of inflammation at the time of blood sampling (eg, healthy horses presented for preoperative examinations, endocrine disease, cardiac insufficiency, renal insufficiency, respiratory, and other diseases in remission without clinical signs and gastrointestinal conditions such as large bowel impaction, colonic retroflexion, or left dorsal displacement). The LI group consisted of animals with an inflammatory process that did not meet the criteria for SI (eg, gastric ulceration, abscesses, and strangles). Animals were allocated to the SI group if ≥2 of the following criteria were met: WBC > 10 × 103/μL or <5 × 103/μL (leukocytosis or leukopenia), rectal temperature >38.5°C, heart rate >60 beats/min and respiratory rate >30 breaths/min.6 Nonsteroidal anti‐inflammatory drug (NSAID) or antimicrobial use in the period up to 24 hours before blood sampling was recorded.
Sample Analysis
Blood samples from all horses were collected during hospitalization at the equine clinic into potassium ethylenediaminetetraacetic acid (EDTA) and lithium heparin tubes. Samples were analyzed within 4 hours of collection. Hematology was performed from the EDTA samples on an automated hematology analyzer (Advia 21201 ) and included a TWBC, white blood cell differential and MPXI. The laboratory reference interval (RI) for the TWBC was 5–10 × 103/μL, for the neutrophil count 3–7 × 103/μL, and for the MPXI 8.5–10.0.6 A Wright‐stained blood smear (Hema‐Tek1) was evaluated and a manual 100‐cell differential count was performed in all cases of leukopenia, leukocytosis or if abnormal scattergrams were present. Heparin plasma was used for the measurement of the biochemistry analytes on an automated chemistry analyzer.2 Fibrinogen was measured using an immunoturbidimetric assay, validated in‐house for horses.3 SAA was measured using an immunoturbidimetric assay4 previously validated in‐house for horses.12 Iron was measured using the photometric FerroZine method.5 Laboratory RIs were 150–220 mg/dL for plasma fibrinogen concentration, 80–240 μg/dL for iron concentration and <10 mg/L for SAA concentration.12 Daily quality control was performed for both analyzers, and results fell within the preset performance goals.
Statistical Analysis
Statistical analysis was carried out using Analyse‐it version 2.266 and SPSS version 19.0.7 The level of significance was set at P < .05. Descriptive statistics were used to describe the patient population. Data were analyzed for normality using the Shapiro–Wilk test. All data except WBC and neutrophils in the NI group were nonnormally distributed; data are presented as median and interquartile range, unless stated otherwise. Analyte results were compared among the 3 groups using a Kruskal–Wallis test with Bonferroni's corrections posthoc. The effect of previous treatment on each analyte in each group was investigated using a Mann–Whitney test. Receiver‐operating characteristic (ROC) curves were employed and area under the curve (AUC) calculated to evaluate diagnostic accuracy, classified as low (0.5 < AUC ≤ 0.7), moderate (0.7 < AUC ≤ 0.9) or high (0.9 < AUC ≤ 1.0).13 ROC curves were compared using the method of DeLong et al.14 The Youden index (differential positive rate) was calculated from the ROC curves to investigate whether optimized cut‐off values (clinical decision limits) for iron, fibrinogen and SAA concentrations could be generated to aid in differentiating SI from LI.15 To investigate the predictive value of the combination of the analytes, 2 additional analyses were performed: a classification and regression tree analysis (CART) and logistic regression. CART analysis is a nonlinear, nonparametric recursive partitioning method that allows for the prediction of the category of the dependent variable based on the values of the independent variables.16 The dataset is split into homogenous subsets using the Gini method, choosing a variable at each split that best partitions the data. The results can be represented as a tree with splits and terminal leaves. The category of inflammation was used as the dependent variable and the laboratory results as predictor variables. For the binary forward logistic regression analysis, the SI and LI categories were grouped together as inflammatory disease compared to NI disease.
Results
In total, 274 blood samples including TWBC, neutrophil count, MPXI, fibrinogen, iron, and SAA were submitted from September 2011 to January 2014. Results of 50 repeat blood samples from 32 horses and an additional 18 samples from horses <1 year of age as well as 5 external submissions were excluded. Data from 201 horses finally were used in the study.
The population consisted of 80/201 (41%) females and 121/201 (59%) males of which 101 were geldings. Median age was 12 years (range, 1–36 years). Of the 201 horses used in the study, 26 were classified as SI, 114 as LI and 61 as NI. Horses in the SI group suffered most commonly from lymphoreticular disease including guttural pouch infections, lymphoma and lymphadenitis (6), inflammatory intestinal diseases (10), anaplasmosis (4), respiratory disease (4), and wounds (4) alone or in combination with other disorders. Diseases most commonly present in the LI group were gastrointestinal including dental (52), respiratory (27), lymphoreticular (eg, strangles, lymphadenitis, lymphoma) (24) and musculoskeletal disease (21), wounds (10) and neoplasia (10), alone or in combination. NI group horses had noninflammatory gastrointestinal disease (31), respiratory (6), endocrine (6), other (12), or no disease (6).
Group differences are given in Table 1. There was no significant difference in MPXI among the groups. Significant differences were found for SAA (LI and SI versus NI, P < .001; LI versus SI, P = .007) and iron concentrations (P < .001) between all 3 groups. Fibrinogen concentrations in the SI and LI group differed significantly from the NI group (P < .001).
Table 1.
Median and (IQR) of TWBC, neutrophil concentration, MPXI, fibrinogen, SAA, and iron concentration in horses with systemic, local, and noninflammatory disease.
| Analyte | Reference Interval | Horses with Systemic Inflammation (n = 26) | Horses with Local Inflammation (n = 114) | Horses with No Inflammation (n = 61) |
|---|---|---|---|---|
| TWBC (×103/μL) | 5.0–10.0 | 10.3 (3.3–15.5) | 7.8 (6.1–9.9) | 7.5 (6.1–8.4) |
| Neutrophils (×103/μL) | 3.0–7.0 | 7.0 (2.2–13.4) | 5.0 (3.8–7.0) | 4.9 (4.0–6.3) |
| MPXI | 8.5–10.0 | 10.5 (6.5–14.7) | 11.0 (6.5–14.7) | 10.3 (6.4–14.2) |
| Fibrinogen (mg/dL) | 150–220 | 224 (180–265)a | 181 (140–242)a | 128 (103–162)b |
| SAA (mg/L) | <10 | 1583 (688–4000)a | 343 (37–1609)b | 5.6 (1.8–14.5)c |
| Iron (μg/dL) | 80–240 | 56 (41–85)a | 105 (65–150)b | 132 (104–196)c |
For each analyte, values with different superscripts are significantly different from other values, values with no or the same superscripts are not different, across inflammatory status groups (P < .05).
TWBC, total white blood cell count; MPXI, myeloperoxidase index; SAA, serum amyloid A.
Of the horses with SI, 14/26 (54%) had fibrinogen concentrations above the RI compared to 38/114 (33%) in the LI and 2/61 (3%) in the NI group. Regarding SAA, 25/26 (96%) of horses in the SI group, 90/114 (79%) of horses in the LI group and 18/61 (30%) of horses in the NI group had increased SAA concentrations. Hypoferremia was present in 18/26 (69%) of horses with SI, 38/114 (33%) of horses with LI, and 6/61 (10%) horses with NI.
Receiver‐operator characteristic curves were constructed, firstly for inflammatory (SI and LI) versus NI disease, and secondly for SI versus LI. SAA concentration had the highest albeit moderate AUC for diagnosing inflammation, followed by fibrinogen concentration (Table 2; Fig 1). Accuracy of SAA was significantly higher than that of iron and MPXI (P = .006, P < .001). The differentiation of SI from LI was moderately accurate if SAA or iron concentration were used; fibrinogen concentration and MPXI showed poor diagnostic accuracy (Table 2; Fig 2). The AUCs of the latter ROCs were not significantly different except for iron versus MPXI (P = .008). Sensitivity (Se) and specificity (Sp) of values outside of the RI for diagnosing inflammation are shown in Table 3. Furthermore, the optimal cut‐off values indicated by the Youden index and the corresponding Se and Sp for differentiating SI versus LI disease for these optimal values are shown in Table 3.
Table 2.
AUC for receiver‐operating characteristic curve analysis for horses with inflammatory versus noninflammatory, and systemic versus local inflammatory disease. When comparing SAA, fibrinogen and iron, the only significant difference in AUC was found for SAA versus iron (P = .0016) for inflammation versus noninflammation.
| Analyte | Inflammatory Versus NonInflammatory Disease | Systemic Versus Local Inflammation |
|---|---|---|
| SAA | 0.83 (0.77–0.89) | 0.80 (0.72–0.89) |
| Fibrinogen | 0.79 (0.72–0.75) | 0.63 (0.52–.075) |
| Iron | 0.71 (0.63–0.78) | 0.73 (0.63–0.83) |
| MPXI | 0.51 (0.43–0.59) | 0.53 (0.40–0.65) |
95% confidence intervals in parentheses.
AUC, area under the curve; SAA, serum amyloid A.
Figure 1.
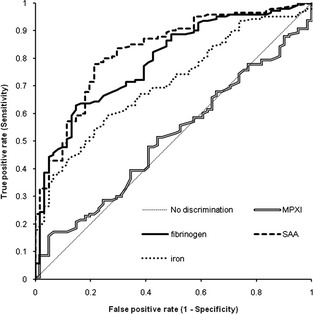
Receiver‐operating characteristic curves for myeloperoxidase index (MPXI), fibrinogen, serum amyloid A (SAA), and iron for discerning horses with from those without inflammatory disease.
Figure 2.
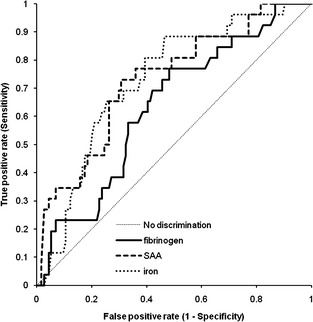
Receiver‐operating characteristic curves for fibrinogen, serum amyloid A (SAA), and iron for discerning horses with systemic from those with local inflammatory disease.
Table 3.
In the first part: Se and Sp for abnormal fibrinogen, iron, and SAA concentrations for the diagnosis of inflammatory status. In the second part: Optimal cut‐off values based on Youden indices with corresponding Se and Sp for horses with SI versus LI disease.
| Analyte | Concentration Outside Reference Interval | Se | Sp |
|---|---|---|---|
| Fibrinogen | >220 mg/dL | 37% (29–46) | 97% (89–100) |
| Iron | <80 μg/dL | 40% (32–49) | 90% (78–96) |
| SAA | >10 mg/L | 82% (75–88) | 71% (57–82) |
| Optimum cut‐off for SI versus LI | |||
| Fibrinogen | >181 mg/dL | 77% (56–91) | 52% (42–61) |
| Iron | <102 μg/dL | 89% (70–98) | 54% (44–63) |
| SAA | >2030 mg/L | 46% (27–6) | 82% (73–88) |
Se and Sp: 95% confidence intervals in parentheses.
Se, sensitivity; Sp, specificity; SAA, serum amyloid A; SI, systemic inflammation; LI, local inflammation.
The CART analysis produced a tree with 5 levels (preset) with 25 nodes of which 13 were end‐nodes. CART correctly classified 75.1% (151/201) of patients to 1 of the 3 groups, as shown in Table 4. However, less than one‐third of the SI cases were predicted correctly, with all but 1 of the incorrectly classified cases being assigned to the LI group. Prediction was better, but not optimal, for the LI and NI groups.
Table 4.
Results of the CART analysis showing the number of cases in each disease category correctly classified by the model.
| Classified by CART as | Correctly Classified (%) | |||
|---|---|---|---|---|
| NI | LI | SI | ||
| True value of dependent variable | ||||
| NI | 44 | 16 | 1 | 72.1 |
| LI | 15 | 99 | 0 | 86.8 |
| SI | 1 | 17 | 8 | 30.8 |
| Total % | 29.9 | 65.7 | 4.5 | 75.1 |
CART, classification and regression tree; NI, noninflammatory; SI, systemic inflammation; LI, local inflammation.
Logistic regression analysis showed that of the 4 analytes examined, SAA and fibrinogen were significant predictors of inflammatory versus noninflammatory disease (SAA; β = 0.001, P = .012, OR = 1.001; fibrinogen β = 0.014, P = .001, OR = 0.995). With the final regression model (Table 5), 56% of NI cases were correctly predicted to be NI whereas 87% of the horses with inflammation (SI and LI) were correctly predicted. Overall, the model correctly predicted 78% of the disease statuses, slightly more than found with the CART analysis.
Table 5.
Results of the binary logistic regression analysis showing the number of cases in each disease category correctly predicted by the model.
| Predicted as | Correctly Classified (%) | ||
|---|---|---|---|
| Noninflammatory | Inflammatory | ||
| True value of dependent variable | |||
| Noninflammatory disease | 34 | 27 | 55.7 |
| Inflammatory disease | 18 | 122 | 87.1 |
| Total % | 77.6 | ||
In total, 88 horses (23 NI, 48 LI, 17 SI) had received NSAIDs and 46 (4 NI, 28 LI, 14 SI) had received antimicrobials. Neutrophils were found to be lower in horses with SI receiving NSAIDs (treated 3.0 × 103/μL [range, 2.0–9.4], nontreated 13.4 × 103/μL [range, 7.8–14.6], P = .029). There were similar significant differences in horses in the SI group that received antimicrobials for TWBC (treated 4.8 × 103/μL [range, 2.9–11.0], nontreated 14.8 × 103/μL [range, 7.7–17.2], P = .020), and neutrophil concentration (treated 3.4 × 103/μL [range, 2.0–8.2], untreated 13.1 × 103/μL [range, 5.3–15.0], P = .013). (Mann–Whitney U‐test, data presented as median and interquartile range.) Twelve SI horses received both treatments.
Discussion
The detection and differentiation of LI from SI can be difficult in horses. There are situations in which a quick assessment of inflammatory status is critical for effective treatment and accurate prognosis. Significant changes of plasma fibrinogen, iron, and SAA concentrations were found in the SI, LI, and NI groups in this study, as well as in others.10 However, none of the analytes studied, either alone or in combination, was good enough to reliably assess inflammatory status in general or to differentiate LI from SI.
Because of its retrospective nature, this study has several limitations. Automated and cost‐effective testing for SAA became routinely available in the clinical pathology laboratory (and therefore the Equine Hospital) in September 2011. After introduction, not all eligible horses were tested for all study parameters, thus some sample bias could not be prevented. Horses that appeared in rather poor clinical condition were more likely to be chosen for testing of all four analytes. Later, clinicians selected all analytes more frequently for evaluation of less severely ill patients. The population also represents a referral population. This study therefore consists of a convenience sample, and horses with severe clinical disease may be overrepresented.
Neutrophil concentrations were lower in SI horses receiving NSAID or antimicrobial treatments. No differences in SAA, fibrinogen, or iron concentrations were found. Treatment may have led to modulation of inflammatory markers but this would reflect the change in inflammatory status and not a direct effect of the drug (eg, binding or breakdown). A change in inflammatory status would have lead to modulation of clinical signs and would have been classified appropriately. Because of the retrospective nature of the study, this possibility cannot be evaluated further.
The impact of transport stress also must be considered. SAA concentration was found in 1 study to be increased 48 hours after transport in horses, but the magnitude of increase was approximately 4 mg/L and concentrations remained within the RI.17 Although statistically significant, this change is small and of no clinical relevance.
Total white blood cell count and neutrophil counts are insensitive markers of inflammation in horses and often are found to be within the RI despite the presence of inflammatory disease, as shown in this and other studies.4, 6, 11, 18, 19
Myeloperoxidase index is a measure of neutrophil myeloperoxidase (MPO) content, with high MPXI values indicating a population with a high MPO content (eg, band neutrophils), a low or negative MPXI indicating neutrophil degranulation or an MPO deficiency. In pilot studies, MPXI was decreased in horses with sepsis and normal TWBC compared to healthy horses and those with LI, and increased in neutropenic septic foals compared to other groups of sick foals.6, 7 In this study, this index was not different among different groups or helpful in discriminating different types of inflammation. MPXI seems to be dependent on the stage of the myelopoietic response and degree of intravascular degranulation, which was not evaluated here. In addition, the results of MPXI in the NI group suggest the laboratory RIs, reported in a previous study, may not be accurate.6 Future prospective studies examining MPXI in a healthy population and the kinetics of MPXI over the course of various inflammatory states may provide more insight.
Fibrinogen is a moderate APP with concentrations increasing 2–10 times within 24–72 hours after the start of inflammation.8 Borges et al reported Se of 82% for increased fibrinogen concentration to detect SI. Furthermore, mean fibrinogen concentrations were significantly different among horses with systemic, local, and no inflammatory disease in that study.10 A second study reported Se of 59% and Sp of 51% of an increased fibrinogen concentration to discriminate between inflammatory and noninflammatory disease.19 Both of these previous studies used the heat precipitation method and not a turbidimetric method to measure fibrinogen concentration, and RIs differed (100–200 mg/dL10 versus >400 mg/dL19), making further comparisons difficult. In our study, only 52/140 (Se 37%; 95% CI, 29–46%) of horses with inflammatory disease (54% of which had SI) had an increased fibrinogen concentration, reflecting its poor Se as an inflammatory marker. Fibrinogen had a Sp for inflammation of 97% (95% CI, 89–100%), but was unsuitable for further discrimination between LI and SI. The AUC of the ROC curve indicated a moderate diagnostic accuracy for detecting inflammation.13 The time course of inflammation could not be examined in this study, but most of the horses were referred and most likely had inflammatory conditions of more than 24 hours duration and presumably had enough time to mount a fibrinogen response. Furthermore, fibrinogen concentrations have been reported not to differ between horses with acute, subacute, and chronic disease.10 In summary, increased fibrinogen concentration is specific for inflammation but not useful for further differentiation, whereas a fibrinogen concentration within the RI does not rule out inflammation.
Inflammatory hypoferremia occurs because of the up‐regulation of hepcidin by inflammatory cytokines as well as direct effects of interferon‐ϒ and tumor necrosis factor‐α, which result in iron sequestration and decreased iron availability.20 A low iron concentration has been shown to be a reliable marker of inflammation in horses.4, 9, 10, 21 In addition, the concentration of iron was found to be correlated with the severity or course of inflammation in several studies.4, 10, 11 These findings are supported by this study, in which lower iron concentrations were found in the SI group compared to the LI group and NI groups.
Serum amyloid A is a major APP in horses and its physiology and application in the use of inflammatory disease have been well‐described.8, 22 As expected, SAA in this study was much higher in the horses with inflammatory disease as compared to those without inflammation. Increased SAA however had moderate diagnostic accuracy and Sp was lower and Se higher for the diagnosis of inflammation than previously reported.19 Just under one‐third of patients in the NI group had increased SAA—this may be explained by an acute phase reaction which resolves without any overt clinical signs. The clinical significance and therapeutic consequences of this are unclear and should be investigated in prospective studies. The difference in SAA concentration between the SI and LI groups corroborates the results of other studies.4, 5, 11, 18, 23, 24, 25 SAA was significantly higher in SI than in LI but had a moderate AUC for the diagnosis of SI. This observation is likely because of the fact that although median SAA concentrations were very different among the groups, there was a large degree of overlap in the ranges. SAA increases very quickly within 6–12 hours after the inflammatory stimulus, peaking at 48 hours and decreasing rapidly after synthesis ceases, because of its short plasma half‐life.26 Some patients may have been in transition from LI to SI or vice versa. Overall, this data suggests that SAA concentrations may not always correlate with the presence or absence, character, and duration of inflammation suggested by clinical examination. A reason for this finding is that it is often impossible to determine the exact onset of a disease process and modulating effects of previous treatments before presentation at a referral clinic.
High biological variation also may be a reason for the wide range in results. Biological variation for SAA in horses has not been investigated, but is reported to be high in healthy humans.27 The low baseline SAA concentrations in healthy horses suggest a low interindividual variation. However the magnitude of the SAA response among individual horses has been shown to be highly variable in experimental situations and may be because of differences in inflammatory genes among individuals.18
The findings reported here suggest that a single SAA measurement is likely to indicate the presence or absence of inflammation, whereas duration or severity cannot be assessed. Fibrinogen and iron were more specific than SAA for diagnosing inflammation but showed poor Se and are not optimal screening tests. A combination of SAA with fibrinogen or iron might be useful in the assessment of apparently clinically healthy individuals. Increased SAA in combination with iron and fibrinogen concentrations within the RI could indicate a momentary acute phase reaction which may not necessarily transform into manifest clinical disease. The acute phase reaction is a protective mechanism and may be successful before clinical signs develop.
Although this study showed significant group differences in inflammatory markers, assessment of the inflammatory status of the individual patient remains difficult. Additional analyses were carried out to investigate whether interpretative guidelines could be formulated. Optimal cut‐off values for SAA, fibrinogen, and iron for differentiating SI from LI were determined by calculating the Youden index based on the respective ROC analyses. The Youden index (Se − [1 − Sp]) was calculated at various concentrations. The highest Youden index indicates the analyte concentration with the highest combined Se and Sp and can be used to set clinical decision limits.15 When the optimal cut‐off values differentiating SI and LI disease were examined, the cut‐off values for iron and fibrinogen were found to be within their respective RIs, with low Sp but higher Se. Because these cut‐offs of iron and fibrinogen lie within the RI, using them to diagnose SI versus LI is confusing and unwarranted.
The second analysis used to generate a diagnostic algorithm for equine inflammation was the CART analysis. CART analysis has been widely used in human medicine and has been suggested for veterinary clinical decision‐making, but few studies have used this method to good effect.16, 28, 29 In our study, however, CART could not clearly differentiate among the 3 study groups. The predictive accuracy of the tree was poor, with the model incorrectly predicting more than one‐ half the cases of SI as LI and just over 10% of inflammatory disease cases as NI. Numbers of cases in the SI group however was low, and the model may be strengthened in the future by increasing sample size.
A logistic regression model for the diagnosis of inflammatory versus noninflammatory disease was examined and performed fairly well, predicting 87% of inflammatory disease cases as such. The combination of analytes as used in the model appears therefore to have good Se for predicting inflammation, but poor Sp. This finding again is probably because of the overlap in values among the groups.
Increases in fibrinogen and decreases in iron concentrations are specific but not necessarily sensitive markers for inflammatory disease in horses. Increases in SAA concentrations were not as specific for clinical inflammation in this study as has been reported elsewhere.19 Significant differences were demonstrated in these analytes among disease groups but it was not possible to generate diagnostic algorithms or clinical decision limits to differentiate between LI and SI. This could be because of limited sample numbers as well as kinetics and interindividual variation in the magnitude of reaction to an inflammatory stimulus. A prospective study involving larger numbers of animals should be considered for the future.
Acknowledgment
Conflict of Interest Declaration: The authors disclose no conflict of interest.
The work was performed at the University of Veterinary Medicine, Vienna, Austria.
A part of this article, with preliminary data and a smaller sample size, was presented at the European Society of Veterinary Clinical Pathology Annual Congress, Berlin, November 2013, as a short oral abstract.
Footnotes
Siemens Healthcare, Vienna, Austria
Cobas 6000; Roche, Vienna, Austria
DakoCytomation Polyclonal Rabbit Anti‐Human Fibrinogen, Glostrup, Denmark
LZ Test “Eiken” SAA; Mast, Merseyside, UK
Iron2; Roche, Vienna, Austria
Analyse‐it, Leeds, UK
SPSS Statistics; IBM Corporation, Armonk, NY
References
- 1. Ackerman MR. Acute inflammation In: McGavin MD, Zachary JF, eds. Pathologic Basis of Veterinary Disease, 4th ed St. Louis, MO: Mosby; 2007:101–152. [Google Scholar]
- 2. Carr EA. The systemic inflammatory response syndrome In: Robinson NE, Sprayberry KA, eds. Current Therapy in Equine Medicine, 6th ed St Louis, MO: Elsevier Health Sciences; 2009:862–866. [Google Scholar]
- 3. Jacobsen S, Andersen PH. The acute phase protein serum amyloid a (SAA) as a marker of inflammation in horses. Equine Vet Edu 2007;19:38–46. [Google Scholar]
- 4. Jacobsen S, Jensen JC, Frei S, et al. Use of serum amyloid A and other acute phase reactants to monitor the inflammatory response after castration in horses: A field study. Equine Vet J 2005;37:552–556. [DOI] [PubMed] [Google Scholar]
- 5. Hultén C, Demmers S. Serum amyloid A (SAA) as an aid in the management of infectious disease in the foal: Comparison with total leucocyte count, neutrophil count and fibrinogen. Equine Vet J 2002;34:693–698. [DOI] [PubMed] [Google Scholar]
- 6. Schwarz BC, van den Hoven R, Schwendenwein I. Diagnostic value of the neutrophil myeloperoxidase index in horses with systemic inflammation. Vet J 2012;191:72–78. [DOI] [PubMed] [Google Scholar]
- 7. Piviani M, Segura D, Monreal L, et al. Neutrophilic myeloperoxidase index and mean light absorbance in neonatal septic and nonseptic foals. Vet Clin Pathol 2011;40:340–344. [DOI] [PubMed] [Google Scholar]
- 8. Crisman MV, Kent Scarratt W, Zimmerman KL. Blood proteins and inflammation in the horse. Vet Clin North Am Equine Pract 2008;24:285–297. [DOI] [PubMed] [Google Scholar]
- 9. Smith JE, Cipriano JE. Inflammation‐induced changes in serum iron analytes and ceruloplasmin of Shetland ponies. Vet Pathol 1987;24:354–356. [DOI] [PubMed] [Google Scholar]
- 10. Borges AS, Divers TJ, Stokol T, et al. Serum iron and plasma fibrinogen concentrations as indicators of systemic inflammatory diseases in horses. J Vet Intern Med 2007;21:489–494. [DOI] [PubMed] [Google Scholar]
- 11. Jacobsen S, Nielsen JV, Kjelgaard‐Hansen M, et al. Acute phase response to surgery of varying intensity in horses: A preliminary study. Vet Surg 2009;38:762–769. [DOI] [PubMed] [Google Scholar]
- 12. Swancar‐Haid P. Implementierung eines serum amyloid A (SAA) assays—als früher Marker entzündlicher Erkrankungen beim Pferd. [dissertation]. Vienna, Austria: Fachhochschule Wien; 2011. [Google Scholar]
- 13. Gardner IA, Greiner M. Receiver‐operating characteristic curves and likelihood ratios: Improvements over traditional methods for the evaluation and application of veterinary clinical pathology tests. Vet Clin Pathol 2006;35:8–17. [DOI] [PubMed] [Google Scholar]
- 14. DeLong ER, DeLong DM, Clarke‐Pearson DL. Comparing the areas under two or more correlated receiver operating characteristic curves: A nonparametric approach. Biometrics 1988;44:837–845. [PubMed] [Google Scholar]
- 15. Jensen AL, Poulsen JSD. Evaluation of diagnostic tests using relative operating characteristic (ROC) curves and the differential positive rate. An example using the total serum bile acid concentration and the alanine aminotransferase activity in the diagnosis of canine hepatobiliary diseases. J Vet Med Series A 1992;39:656–668. [DOI] [PubMed] [Google Scholar]
- 16. Stärk KDC, Pfeiffer DU. The application of non‐parametric techniques to solve classification problems in complex data sets in veterinary epidemiology—An example. Intell Data Anal 1999;3:23–35. [Google Scholar]
- 17. Casella S, Fazio F, Giannetto C, et al. Influence of transportation on serum concentrations of acute phase proteins in horse. Res Vet Sci 2012;93:914–917. [DOI] [PubMed] [Google Scholar]
- 18. Andersen SA, Petersen HH, Ersbøll AK, et al. Vaccination elicits a prominent acute phase response in horses. Vet J 2012;191:199–202. [DOI] [PubMed] [Google Scholar]
- 19. Belgrave RL, Dickey MM, Arheart KL, et al. Assessment of serum amyloid A testing of horses and its clinical application in a specialized equine practice. J Am Vet Med Assoc 2013;243:113–119. [DOI] [PubMed] [Google Scholar]
- 20. Wessling‐Resnick M. Iron homeostasis and the inflammatory response. Annu Rev Nutr 2010;30:105–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brosnahan MM, Erb HN, Perkins GA, et al. Serum iron parameters and acute experimental EHV‐1 infection in horses. J Vet Intern Med 2012;26:1232–1235. [DOI] [PubMed] [Google Scholar]
- 22. Cray C, Zaias J, Altman NH. Acute phase response in animals: A review. Comp Med 2009;59:517–526. [PMC free article] [PubMed] [Google Scholar]
- 23. Cohen ND, Chaffin MK, Vandenplas ML, et al. Study of serum amyloid A concentrations as a means of achieving early diagnosis of Rhodococcus equi pneumonia. Equine Vet J 2005;37:212–216. [DOI] [PubMed] [Google Scholar]
- 24. Copas VE, Durham AE, Stratford CH, et al. In equine grass sickness, serum amyloid A and fibrinogen are elevated, and can aid differential diagnosis from non‐inflammatory causes of colic. Vet Rec 2013;172:395. [DOI] [PubMed] [Google Scholar]
- 25. Pollock PJ, Prendergast M, Schumacher J, et al. Effects of surgery on the acute phase response in clinically normal and diseased horses. Vet Rec 2005;156:538–542. [DOI] [PubMed] [Google Scholar]
- 26. Eckersall PD, Bell R. Acute phase proteins: Biomarkers of infection and inflammation in veterinary medicine. Vet J 2010;185:23–27. [DOI] [PubMed] [Google Scholar]
- 27. Melzi d'Eril G, Anesi A, Maggiore M, et al. Biological variation of serum amyloid A in healthy subjects. Clin Chem 2001;47:1498–1499. [PubMed] [Google Scholar]
- 28. Porter RS, Leblond A, Lecollinet S, et al. Clinical diagnosis of West Nile Fever in Equids by classification and regression tree (CART) analysis and comparative study of clinical appearance in three European countries. Transbound Emerg Dis 2011;58:197–205. [DOI] [PubMed] [Google Scholar]
- 29. Kirtz G, Leschnik M, Hooijberg E, et al. In‐clinic laboratory diagnosis of canine babesiosis (Babesia canis canis) for veterinary practitioners in Central Europe. Tierärztliche Praxis Kleintiere 2012;40:87–94. [PubMed] [Google Scholar]


