Abstract
Background
Ultrasonographic appearance of the gastrointestinal (GI) tract of equine neonates has not been completely described.
Objectives
To describe (1) sonographic characteristics of the GI segments in normal nonsedated equine neonates, (2) intra‐ and interobserver variation in wall thickness, and (3) the sonographic appearance of asymptomatic intussusceptions, and (4) to compare age and sonographic findings of foals with and without asymptomatic intussusceptions.
Animals
Eighteen healthy Standardbred foals ≤5 days of age.
Methods
Prospective, cross‐sectional blinded study. Gastrointestinal sonograms were performed stall‐side. Intraobserver variability in wall thickness measurements was determined by calculating the coefficient of variation (CV). The Bland–Altman method was used to assess interobserver bias. Student's t‐test and Fisher's exact test were used to test the association among presence of intussusceptions, age, and selected sonographic findings.
Results
The reference ranges (95% predictive interval) for wall thickness were 1.6–3.6 mm for the stomach, 1.9–3.2 mm for the duodenum, 1.9–3.1 mm for the jejunum, 1.3–2.2 mm for the colon, and 0.8–2.7 mm for the cecum. Intraobserver wall thickness CV ranged from 8 to 21% for the 2 observers for 5 gastrointestinal segments. The interobserver bias for wall thickness measurements was not significant except for the stomach (0.14 mm, P < .05) and duodenum (0.29 mm, P < .05). Diagnostic images of mural blood flow could not be obtained. Asymptomatic intussusceptions were found in 10/18 neonates. Associations between sonographic variables or age and the presence of intussusceptions were not found.
Conclusions and Clinical Importance
Sonographic characteristics of the GI tract of normal Standardbred neonates can be useful in evaluating ill foals. Asymptomatic small intestinal intussusceptions occur in normal Standardbred neonates.
Keywords: Abdominal, Colic, Equine, Neonatology, Ultrasound
Abdominal ultrasonography is a commonly used, noninvasive imaging modality that can assist in the evaluation of equine neonates with clinical problems such as abdominal pain, abdominal distension, nasogastric reflux, diarrhea, and sepsis.1, 2, 3 During evaluation of horses with gastrointestinal (GI) disease, sonographic evaluation of echogenicity, wall thickness, contents, and motility of the GI tract provide useful information to determine diagnosis, prognosis, and treatment, and to monitor response to treatment.3, 4, 5, 6, 7 Ultrasonographic anatomy of the abdomen of normal foals has been previously described.8 However, some potentially relevant aspects such as intestinal wall layering patterns, characteristics of specific intestinal segments, blood flow within the intestinal wall, motility in the nonsedated foal, or the variability in wall thickness measurements have not been explored.
Assessment of intestinal motility is a valuable component of the sonographic evaluation of a horse with GI disease and is affected by sedatives.9, 10, 11 Opioids and alpha‐2 adrenergic drugs are expected to decrease intestinal motility in foals, whereas the effect of benzodiazepines could be the opposite, based on its effects in other species.10, 12, 13, 14 To the best of our knowledge, sonographic evaluation of GI motility of nonsedated foals has not been described.
Sonographic evaluation of GI blood supply correlates with histology and outcome in human neonates and allows the differentiation of focal from diffuse necrosis in the setting of necrotizing enterocolitis.15, 16, 17 Color flow Doppler is useful in pediatric patients to quantify intestinal blood flow and to identify increased or decreased vascularity associated with inflammation or bowel necrosis, respectively.18, 19 The use of color flow Doppler to assess intestinal wall vascularity in foals has not been described.
The initial objectives of the study were to (1) describe echogenicity, wall thickness, motility, contents, and color flow Doppler signal of the different GI segments of normal nonsedated equine neonates and (2) describe the intra‐ and interobserver variation in wall thickness measurements. We also describe the sonographic appearance of intussusceptions in normal neonatal foals, and compare the age, GI wall thickness, small intestinal motility, and presence of meconium between the foals with and without asymptomatic intussusceptions.
Materials and Methods
The study population consisted of a convenience sample of normal Standardbred neonates on a large breeding farm, born during the 2012 season. Foals were eligible and defined as normal if they had a normal gestational duration between 320 and 360 days, were born to a healthy mare, had an uncomplicated delivery, the placenta was judged to be normal by the farm veterinarian, were <7 days of age, had a normal physical examination, and an IgG plasma concentration >800 mg/dL1 at 24 hours of age. A follow‐up phone call 6 months after the ultrasound examinations was used to determine if foals had subsequently exhibited signs of GI disease. The appropriate Institutional Animal Care and Use Committee approved the study.
Foals were restrained and the ventral abdomen was clipped from the xiphoid to the inguinal region and laterally to the costochondral junctions using a #40 blade. The clipped region was cleaned with alcohol and ultrasonic coupling gel applied. Foals were allowed to nurse until the time of the examination. Foals were not sedated before or during the examination.
The ventral abdomen was scanned stall‐side with the foal manually restrained in standing position and using portable ultrasound equipment.2 All sonograms were performed by one of the authors. The time used to perform the sonographic examinations was recorded. A wide band‐width 7.0 MHz (3.0–10.0 MHz) convex array transducer was used to perform a survey of the abdomen to evaluate location, motility, and intestinal contents. Three still images and 3 clips of 3 seconds duration of each segment of the GI tract (stomach, duodenum, jejunum, small colon, cecum, and colon) were saved digitally on the ultrasound machine. All images were saved at the highest frequency that allowed visualization of the viscus being evaluated. The stomach was located in the ventral abdomen by identifying the presence of rugae and its relationship with adjacent viscera.1, 8 The duodenum was identified between the ventral aspects of the right lobe of the liver and the right dorsal colon or ventral to the right kidney.8 The jejunum was identified as intestine with a rounded appearance in different locations than the duodenum but with a similar luminal diameter and longer mesentery. The ileum was identified by the characteristic thicker appearance of the muscularis layer and the presence of a thin hyperechoic line in the center of the muscularis layer corresponding to connective tissue separating the longitudinal and circular muscular layers.1 The cecum was identified in the right ventral paralumbar fossa and ventral abdomen by its location and relationship with the cecal vasculature, visualization of the cecal apex and by its relationship with the right colon. The right dorsal and ventral colon was identified medial and ventral to the right lobe of the liver.20, 21, 22 The small colon was identified by observing intestine with a sacculated appearance dorsal to the bladder.1 A wide band‐width 7.5 MHz (6.2–11.0 MHz) linear array transducer was used to obtain high resolution images of the bowel wall for each segment of intestine. These images were used for measuring intestinal wall thickness and to assess the layering pattern. Three images of highest quality for each segment of intestine were digitally stored for measuring wall thickness. Two of the authors, independently and blinded, measured wall thicknesses using the linear measurement function imbedded in the ultrasound equipment software. Wall thicknesses were measured by placing the cursors at the outer edge of the serosal surface and at the mucosal to GI contents interface. Measurements were not obtained where there was folding or contraction of the intestinal wall, or where meconium was present.
Distinct layering of the GI wall was defined as presence of alternating hypoechoic and echoic layers.23 The number of layers observed was recorded. Motility of each intestinal segment was evaluated by the ultrasonographer throughout the examination and was categorized as continuous, intermittent, or absent. Continuous motility was defined as continuous rhythmic contractions. Intermittent motility was defined as the presence of rhythmic contractions followed by periods of absent contractions. Intestinal segments were categorized as having absent motility if there were no visible intestinal contractions.
Gastrointestinal contents were classified based on the luminal pattern (fluid, gas, mucous, or alimentary) as previously described.1, 24 A mucous pattern is the appearance of the bowel segment in a collapsed state and with echogenic contents (without acoustic shadowing). A fluid pattern is the presence of anechoic or hypoechoic luminal contents. A gas pattern appears as a hyperechoic interface with acoustic shadowing. The alimentary pattern was subdivided into meconium or milk. Meconium was hypoechoic and speckled with a ball or log—like shape. Milk was defined as echogenic fluid or fluid with mixed echogenicity with larger echogenic accumulations consistent with milk clots.1
Color flow Doppler interrogation was performed using a wide band‐width 7.5 MHz (6.2–11.0 MHz) linear array transducer. The initial color flow Doppler settings were adapted from those used in an earlier study of human neonates.16 From these settings, the pulse repetition frequency was lowered until the flow signal or aliasing was observed. Velocity was initially set at 0.11 m/s and decreased progressively. Doppler gain settings were increased until maximal Doppler signal or flash artifacts were observed.
Because of the recognition of small intestinal intussusceptions, three of the authors independently and blindly reviewed the digitally saved images and clips. The authors had different levels of experience, ranging from 6 months to 30 years of ultrasound experience. Intussusceptions were identified in cross‐section if intestine with a classically described target‐like or doughnut appearance was observed, and in longitudinal section when the classically described sandwich sign was observed.25, 26, 27, 28, 29 One of the authors (MA) measured wall thickness of the intussusceptum and the intussuscipiens for each foal.
Statistical Analysis
Results for wall thicknesses were reported as mean and standard deviation. Intraobserver variation in wall thickness measurements was assessed by calculation of the coefficient of variation (CV). Interobserver variation was assessed using the method described by Bland and Altman.30 Interobserver bias for each segment of bowel was calculated as the mean difference between the 2 observer's measurements for each image, and the limits of agreement were calculated as a ±1.96 × s, where s is the standard deviation of the bias. Interobserver bias for wall thickness was tested for significance using two‐way analysis of variance with repeated measures. A 95% predictive interval (reference range) using the combined measurements for observer A and B was calculated for each bowel segment, by calculating the population standard deviation (SD) using the subject SD, and multiplying by the one‐tailed 97.5% t n‐1 quantile for the Student's t distribution, where n − 1 is the number of degrees of freedom for the number (n) subjects in the study. Age and gastrointestinal thicknesses for foals with and without intussusceptions were compared using Student's t‐test. The presence of meconium and motility category was compared in foals with and without intussusceptions using Fisher's exact test. A value of P < .05 was used to determine significance and only foreseen associations were tested. Wall thickness measurements between the intussusceptum, intussuscipiens, and average jejunal measurements were compared using analysis of variance for repeated measures, with Tukey's test used for posthoc comparisons.
Results
Eighteen foals were enrolled in the study. Ten were male and 8 were female. Fourteen were less than 24‐hour old, 3 foals were 1‐day old and 1 foal was 5‐day old. None of the foals had signs of abdominal discomfort or required treatment for GI disease in the 6 months after the examination. The echogenicity, wall thickness, motility, and contents of the different GI segments are described in Table 1 and images are shown in Figures 1, 2. Wall layering was not observed in the jejunum (Fig S1) or duodenum of any foal, but was present in the stomach, colon, cecum, and small colon. Duodenal motility was intermittent, whereas jejunal motility was continuous or intermittent. We were unable to obtain diagnostic images of large intestinal motility or color flow Doppler in any gastrointestinal segment in any foal. Small colon wall thickness was not measured as it was not clearly identified in 11/18 foals and in the other 7, meconium was present within it. The intraobserver CV, the bias in interobserver variability for wall thickness measurements and the reference ranges for each bowel segment are reported in Table 2. Interobserver bias in wall thickness measurements was not significant with the exception of interobserver variability in stomach (0.14 mm, P < .05) and duodenum (0.29, P < .05).
Table 1.
Ultrasonographic characteristics of gastrointestinal segments in Standardbred neonates
| Viscus | Viscus Identified (n/18) | Layering Observed (n) | Number of Layers (n) | Wall Thickness Mean ± SD | Motility | Contents |
|---|---|---|---|---|---|---|
| Stomach | 16/18 | 16 | 5 (16) | 2.6 ± 0.5 | NA | Milk and gas 16/16 |
| Duodenum | 18/18 | 0 | NA | 2.6 ± 0.5 | I: 18/18 | Fluid |
| Jejunum | 18/18 | 0 | NA | 2.4 ± 0.4 |
C: 10/18 I: 8/18 |
Fluid |
| Ileum | 0/18 | NA | NA | NA | NA | NA |
| Cecum | 18/18 | 18 | 3 (10); 4 (2); 5 (6) | 1.7 ± 0.5 | Not detected | Gas 18/18 |
| Colon | 18/18 | 18 | 3 (4); 4 (2); 5 (12) | 1.8 ± 0.3 | Not detected |
Meconium 11/18 Heterogenous 7/18 |
| Small colon | 18/18 | 7 | 3 (7) | Not measured | Not detected | Meconium 6/18 |
SD, standard deviation; NA, not applicable; n, number of individuals; I, intermittent; C, continuous.
Figure 1.
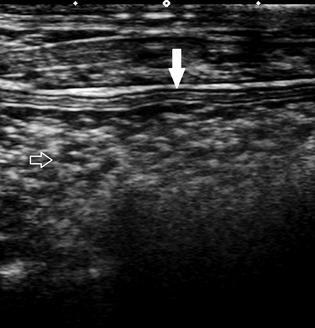
Image of the colon. Layering within the colon wall (solid arrow) and echoic ingesta (outlined arrow). Each diamond‐shaped marker represents 1 cm.
Figure 2.
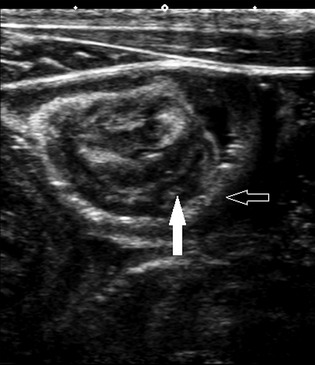
Asymptomatic jejuno‐jejunal intussusception showing the intussusceptums (solid arrow) and intususcipiens (outlined arrow). Each diamond‐shaped marker represents 1 cm.
Table 2.
Intra‐ and interobserver variation in wall thickness measurements
| Tissue | Intra‐Obs CV (%) | Interobs | Reference Range (mm) (95% Predictive Interval) | ||
|---|---|---|---|---|---|
| Obs A | Obs B | Bias (A–B) | Limits of Agreement (mm) | ||
| Stomach | 8.0 | 9.8 | −0.14a | −1.3 to 0.14 | 1.6–3.6 |
| Duodenum | 14.5 | 14.2 | 0.29a | −0.6 to 1.2 | 1.9–3.2 |
| Jejunum | 12.5 | 9.9 | −0.04 | −0.8 to 0.7 | 1.9–3.1 |
| Colon | 13.5 | 11.8 | −0.15 | −0.7 to 0.4 | 1.3–2.2 |
| Cecum | 12.5 | 21.3 | −0.12 | −0.7 to 0.5 | 0.8–2.7 |
CV, coefficient of variation; Obs, observer.
Indicates a statistically significant (P < .05) difference.
In 10/18 foals, jejuno‐jejunal intussusceptions were identified (Fig 2). Intussusceptions were identified by the sonographer during the ultrasound examination, and confirmed in at least 2 digitally stored still images and 1 video clip from each case by 3 investigators reviewing the images. There was no difference in age, intestinal wall thicknesses, duodenal or jejunal motility or presence of meconium between foals with and without intussusceptions (Table 3). The intussuscipiens was significantly (P < .01) thinner (1.9 ± 0.1 mm) than the normal jejunum (2.5 ± 0.1 mm). There were no differences between normal jejunal measurements and the intussusceptum (2.6 ± 0.9 mm). None of the measurements were over the upper end of the reference range described for the overall population (3.1 mm) and in 4 cases, the intussuscipiens was 0.1–0.2 mm thinner than the lower end of the reference range for the general population (1.9 mm).
Table 3.
Comparison of ultrasonographic findings in equine neonates with and without intussusception (mean ± SD)
| Measurement | Intussusception | No Intussusception | P value |
|---|---|---|---|
| Gastric thickness (mm) | 2.44 ± 0.3 | 2.75 ± 0.6 | .02 |
| Duodenal thickness (mm) | 2.61 ± 0.18 | 2.49 ± 0.43 | .39 |
| Duodenal motility intermittent | 11/11 | 7/7 | 1 |
| Jejunal thickness (mm) | 2.5 ± 0.14 | 2.51 ± 0.37 | .82 |
| Jejunal motility intermittent | 4/11 | 4/7 | .63 |
| Cecal thickness (mm) | 1.66 ± 0.31 | 1.89 ± 0.54 | .25 |
| Colonic thickness (mm) | 1.78 ± 0.18 | 1.7 ± 0.2 | .38 |
| Meconium present | 9/11 | 4/7 | .32 |
| Age (hours) | 26.4 ± 30.4 | 23 ± 11.2 | .78 |
In 1 foal, the appearance of the duodenum was suggestive of a pyloric‐duodenal or duodeno‐duodenal intussusception: the wall of the duodenum was hypoechoic to echoic without distinct layering and a section of the wall appeared to invaginate into the lumen or be thickened (5.2 mm). This diagnosis was not suspected at the time the ultrasonographic examination was performed and additional images or loops were not available for review. This foal was clinically normal.
Discussion
This study investigated a number of sonographic variables of the gastrointestinal tract in nonsedated foals that had not been previously described, including wall layering, wall thickness, interobserver variability in wall thickness measurements, and a qualitative description of motility. The use of color Doppler to assess mural blood flow was attempted but not possible. This is the first report of asymptomatic intussusceptions in neonatal foals. We describe the sonographic appearance of asymptomatic intussusceptions and compare age and sonographic finding in foals with and without intussusceptions.
Equine neonates showed distinct layering of the stomach and large intestine, and indistinct layering of the small intestine. Rapid changes occur in the first days of life, including marked changes in total and relative thickness of the different histologic layers with the muscularis layer changing quantitatively the most in this period.31, 32 The reason why the layering of the stomach and large intestine was distinct in all cases, but not in any case for the small intestine, is uncertain. Perhaps the detail of the images was not sufficient or the frequency of the transducers was too low. Higher frequency (up to 20.0 MHz) transcutaneous or transendoscopic transducers have been used to allow detailed description of normal adult human GI wall layering.23, 33 This, however, would not explain the differences in layering recognition between different intestinal segments. The effects of early postnatal maturation on the sonographic appearance of the GI tract in any species have not been reported. Different patterns of abnormal intestinal wall echogenicity have been shown to correlate with specific disease processes in children with colitis.34 Histologic to sonographic correlation in foals with GI disease could be useful in equine neonatal medicine.34
Wall thicknesses obtained in this study are largely consistent with previous literature8 with the exception of the stomach (mean is 1 mm thicker than previously reported). Small magnitude differences that have questionable clinical significance were also seen when comparing measurement ranges for each region of the GI tract between the 2 studies (upper end of the range for jejunal measurements was 0.8 mm thicker than previously reported for small intestinal wall measurements). The studies are different in the sonographic windows used (ventral abdomen versus full abdomen), breed distribution (Standardbreds versus heterogenous population), equipment, ultrasonographers, and administration of sedation (nonsedated foals versus different sedation protocols). Any or a combination of these factors could be responsible for the small magnitude variation. The specific reference ranges for different small and large intestinal segments had not been previously described in equine neonates.
Interobserver bias was significant for gastric and duodenal wall thicknesses. Despite the statistical significance, the overall bias (Table 2) is unlikely to be clinically significant. In adult horses, the reported interobserver variability is overall larger than in neonates and highest for cecal measurements (0.7 mm).20 The differences in interobserver variability between segments of the GI tract between adults and neonates could be because of differences in development, contents, or the better image quality allowed by the thin body wall.
Because of the movement of nonsedated foals (foal activity and rapid respiratory motion), quantification of GI motility (contractions/unit of time described in the literature for adult horses) was not attempted.35, 36 A qualitative description, as described in a previous study in equine neonates, was chosen.8 In the study reported here, small intestinal motility was intermittent or continuous, whereas it had been described as uniformly continuous in a previous study.8 The differences could be because of effects of sedation in the previous study, the subjective impression of the operators, or the fact that jejunal and duodenal motility were assessed separately in the current study.
Finding jejuno‐jejunal intussusceptions in 10/18 normal foals was unexpected, as asymptomatic intussusceptions had not been previously reported in horses. Jejuno‐jejunal intussusceptions are the most common type of intussusception in foals with abdominal pain, but intussusceptions are reported to uniformly require surgical correction in horses.27, 28, 37 Dysrhythmic peristaltic activity has been proposed as the cause of intussusception in horses and people. Asymptomatic small bowel intussusceptions occur in humans and dogs and can resolve spontaneously (up to 20% in people38 and 8% in dogs39), suggesting that this process might be underreported.26, 38, 39, 40 Sonographic signs that have been associated with transient, nonclinical small intestinal intussusceptions are the absence of identifiable intestinal lesions, normal wall thickness, length of less than 3.5 cm, normal undilated proximal bowel, normal vascularity on color Doppler, respected layering, and the intussusception being compressible.40, 41 We did not recognize pathologic lead points, increased wall thickness, or altered echogenicity in any of the asymptomatic intussusceptions we observed. Compressibility, length or mesenteric, and mural blood flow in the area of the intussusceptions were not evaluated. Stomach wall thickness was thinner in foals with intussusceptions when compared to those without an intussusception. We could speculate that delayed gastric emptying (caused or parallel to the intussusceptions) and consequent gastric wall stretching could cause this difference in thickness. However, the average difference between stomach wall thickness of 0.3 mm between foals with and without intussusceptions is unlikely to be clinically or diagnostically significant. The wall thickness of the intussuscipiens was 0.1–0.2 mm thinner than the lower end of the reference range for normal jejunal wall thickness in 4 cases and the average wall thickness of the intussuscipiens was less than the average jejunal wall thickness. Clinical intussusceptions in humans41 are characterized by an increased thickness of both intussusceptum and intussuscipiens, and the marginal thinning observed in foals with asymptomatic intussusceptions is of uncertain relevance. No other differences in the sonographic variables compared were found in foals with or without intussusceptions. Though age was not significantly different between foals with or without intussusception, most (14/18) of the foals in this study were less than 24 hours of age and no foal was older than 5 days of age. It is possible that asymptomatic intussusceptions are a normal occurrence in foals of this age range because of initial development of gastrointestinal function and motility.
The study has several limitations. Large intestinal motility could not be evaluated and diagnostic color Doppler signals of mural blood flow could not be obtained. It is possible that different ultrasound equipment or equipment settings, or sedating the foals, might help in obtaining diagnostic color flow Doppler readings and this should be further investigated. The study design called for only partial examinations and the entire GI tract was not imaged. Serial sonographic examinations of the foals with intussusceptions were not performed to confirm that these were transient and how quickly they resolved, or whether they were dynamic and reoccurred. In addition, further investigation into the presence of asymptomatic intussusceptions in neonatal foals in the first few days of life is necessary. We only examined Standardbred foals and therefore conclusions might only be applicable to this breed. However, we are unaware of GI structural or functional traits specific to the Standardbred breed.
In conclusion, the sonographic characteristics of the GI tract of normal Standardbred neonates described here can be useful to clinicians evaluating ill foals and to researchers studying equine neonatal GI disease. Diagnostic images of mural blood flow using color flow Doppler could not be obtained with this technique in nonsedated foals. The intraobserver CV of the wall thickness was small and not significantly different in all cases. The interobserver variation was not significant for jejunum, colon, and cecum, but statistically significant for gastric and duodenal measurements. Asymptomatic small intestinal intussusceptions are frequent in normal Standardbred equine neonates and this should be considered when making treatment decisions in foals with intussusceptions.
Supporting information
Fig S1. Image of jejunum. Echoic fluid ingesta (outlined arrow) and lack of wall layering (solid arrow). Each diamond‐shaped marker represents 1 cm.
Acknowledgments
The authors thank Winbak Farm and Dr Sarah Mackey for allowing examination of their foals and for providing assistance during the sonograms. The authors thank Universal Solutions, Inc for providing the ultrasound equipment.
Conflict of Interest Declaration: Ultrasound equipment used was loned by Universal Imaging. 299, Adams Street, Bedford Hills, New York 10507, USA. Tel: (914) 666‐6200.
Sonograms were performed at Winbak Farm, 155 Yearling Row, Chesapeake City, MD, 21915.
Footnotes
SNAP Foal IgG Test IDEXX Laboratories, Inc, Westbrook, ME
Toshiba VIAMO. Universal Solutions, Inc, Bedford Hills, NT
References
- 1. Reef VB. Pediatric abdominal ultrasonography In: Reef VB, ed. Equine Diagnostic Ultrasound. Philadelphia, PA: WB Saunders Company; 1998:364–403. [Google Scholar]
- 2. Porter M, Ramirez P. Equine neonatal thoracic and abdominal ultrasonography. Vet Clin Equine 2005;21:407–429. [DOI] [PubMed] [Google Scholar]
- 3. de Solis Navas C, Palmer JE, Boston RC, Reef VB. The importance of ultrasonographic pneumatosis intestinalis in equine neonatal gastrointestinal disease. Equine Vet J 2012;44(Suppl 41):64–68. [DOI] [PubMed] [Google Scholar]
- 4. Pease AP, Scrivani PV, Erb HN, Cook VL. Accuracy of increased large‐intestine wall thickness during ultrasonography for diagnosing large‐colon torsion in 42 horses. Vet Radiol Ultrasound 2004;45:220–224. [DOI] [PubMed] [Google Scholar]
- 5. Beccati F, Pepe M, Gialletti R, et al. Is there a statistical correlation between ultrasonographic findings and definitive diagnosis in horses with acute abdominal pain? Equine Vet J Suppl 2011;39:98–105. [DOI] [PubMed] [Google Scholar]
- 6. Sheats MK, Cook VL, Jones SL, et al. Use of ultrasound to evaluate outcome following colic surgery for equine large colon volvulus. Equine Vet J 2010;42:47–52. [DOI] [PubMed] [Google Scholar]
- 7. Busoni V, De Busscher V, Lopez D, et al. Evaluation of a protocol for fast localised abdominal sonography of horses (FLASH) admitted for colic. Vet J 2011;188:77–82. [DOI] [PubMed] [Google Scholar]
- 8. Aleman M, Gillis CL, Nieto JE, et al. Ultrasonographic anatomy and biometric analysis of the thoracic and abdominal organs in healthy foals from birth to age 6 months. Equine Vet J 2002;34:649–655. [DOI] [PubMed] [Google Scholar]
- 9. Cruz FSF, Carregaro AB, Machado M, Antonow RR. Sedative and cardiopulmonary effects of buprenorphine and xylazine in horses. Can J Vet Res 2011;75:35–41. [PMC free article] [PubMed] [Google Scholar]
- 10. Freeman S, England GCW. Effect of romifidine on gastrointestinal motility assessed by transrectal ultrasonography. Equine Vet J 2001;6:570–576. [DOI] [PubMed] [Google Scholar]
- 11. Sanchez CL, Elfenbein JR, Roberston SA. Effect of acepromazine, butorphanol, or N‐butylscolammonium bromide on visceral and somatic nociception and duodenal motility in conscious horses. Am J Vet Res 2008;69:579–565. [DOI] [PubMed] [Google Scholar]
- 12. Merritt AM, Burrow JA, Hartless CS. Effect of xylazine, detomidine, and a combination of xylazine and butorphanol on equine duodenal motility. Am J Vet Res 1998;59:619–623. [PubMed] [Google Scholar]
- 13. Fargeas MJ, Fioramonti J, Bueno L. Time‐related effects of benzodiazepines on intestinal motility in conscious dogs. J Pharm Pharmacol 1984;36:130–132. [DOI] [PubMed] [Google Scholar]
- 14. Castedal M, Bjornsson E, Abrahamsson H. Effects of midazolam on small bowel motility in humans. Aliment Pharmacol Ther 2000;14:571–577. [DOI] [PubMed] [Google Scholar]
- 15. Silva CT, Daneman A, Navarro OM, et al. Correlation of sonographic findings and outcome in necrotizing enterocolitis. Pediatr Radiol 2007;3:274–282. [DOI] [PubMed] [Google Scholar]
- 16. Faingold R, Daneman A, Tomlinson G, et al. Necrotizing enterocolitis: Assessment of bowel viability with color Doppler US. Radiology 2005;235:587–594. [DOI] [PubMed] [Google Scholar]
- 17. Epelman M, Daneman A, Navarro OM, et al. Necrotizing enterocolitis: Review of state‐of‐the‐art imaging findings with pathologic correlation. Radiographics 2007;27:285–305. [DOI] [PubMed] [Google Scholar]
- 18. Dietrich CF, Jedrzejczyk M, Ignee A. Sonographic assessment of splanchnic arteries and the bowel wall. Euro J Radiol 2007;64:202–212. [DOI] [PubMed] [Google Scholar]
- 19. Quillin SP, Sieger MJ. Gastrointestinal inflammation in children: Color Doppler ultrasonography. J Ultrasound Med 1994;13:751–756. [DOI] [PubMed] [Google Scholar]
- 20. Bithell S, Habershon‐Butcher JL, Bowen IM, Hallowell GD. Repeatability and reproducibility of transabdominal ultrasonographic intestinal wall thickness measurements in Thoroughbred horses. Vet Radiol Ultrasound 2010;51:647–651. [DOI] [PubMed] [Google Scholar]
- 21. Ness SL, Bain FT, Zantingh AJ, et al. Ultrasonographic visualization of colonic mesenteric vasculature as an indicator of large colon right dorsal displacement or 180° volvulus (or both) in horses. Can Vet J 2012;53:378–382. [PMC free article] [PubMed] [Google Scholar]
- 22. Sisson S, Grossman JO. Anatomy of the Domestic Animals, 5th ed Philadelphia, PA: W.B. Saunders; 1975. [Google Scholar]
- 23. Odegaad S, Nesje LB, Kimmey MB. High‐frequency ultrasonographic imaging of the gastrointestinal wall. Expert Rev Med Devices 2012;9:263–273. [DOI] [PubMed] [Google Scholar]
- 24. Pennick DG. Gastrointestinal tract In: Nyland TG, Matoon JS, eds., Small Animal Diagnostic Ultrasound. Philadelphia, PA: W.B. Saunders; 2002:207–230. [Google Scholar]
- 25. Fontaine‐Rodgerson G, Rodgerson D. Diagnosis of small intestinal intussusceptions by transabdominal ultrasonography in 2 adult horses. Can Vet J 2001;42:378–380. [PMC free article] [PubMed] [Google Scholar]
- 26. Jain P, Heap SW. Intussusception of the small bowel discovered incidentally by computed tomography. Aust Radiol 2006;50:171–174. [DOI] [PubMed] [Google Scholar]
- 27. Nelson BB, Brounts SH. Intussusception in horses. Compend Contin Educ Vet 2012;34:E1–E5. [PubMed] [Google Scholar]
- 28. Bernard WV, Reef VB, Reimer JM, et al. Ultrasonographic diagnosis of small‐intestinal intussusceptions in 3 foals. J Am Vet Med Assoc 1989;194:395–397. [PubMed] [Google Scholar]
- 29. Ayaz U, Dilli A, Ayaz S, Api A. Ultrasonographic findings of intussusceptions in pediatric cases. Med Ultrason 2011;13:272–276. [PubMed] [Google Scholar]
- 30. Bland JM, Altman DG. Statistical methods for assessing agreement between two measures of clinical measurement. Lancet 1986;1:307–310. [PubMed] [Google Scholar]
- 31. Commare CE, Tappenden KA. Development of the infant intestine: Implications for nutrition support. Nutr Clin Pract 2007;22:159–173. [DOI] [PubMed] [Google Scholar]
- 32. Del Conto C, Oevermann A, Burgener IA, et al. Gastrointestinal tract mucosal histomorphometry and epithelial cell proliferation and apoptosis in neonatal and adult dogs. J Anim Sci 2010;88:2255–2264. [DOI] [PubMed] [Google Scholar]
- 33. Kimmey MB, Martin RW, Haggitt RC, et al. Histologic correlates of gastrointestinal ultrasound images. Gastroenterology 1989;96:433–441. [DOI] [PubMed] [Google Scholar]
- 34. Baud C, Saquintaah M, Veyrac C, et al. Sonographic diagnosis of colitis in children. Euro Radiology 2004;14:2105–2129. [DOI] [PubMed] [Google Scholar]
- 35. Hendrickson EHS, Malone ED, Sage AM. Identification of normal parameters for ultrasonographic examination of the equine large colon and cecum. Can Vet J 2007;48:289–291. [PMC free article] [PubMed] [Google Scholar]
- 36. Epstein K, Short D, Parente E, et al. Gastrointestinal ultrasound in normal adult ponies. Vet Radiol Ultrasound 2008;49:282–286. [DOI] [PubMed] [Google Scholar]
- 37. Gift LJ, Gaughan EM, DeBowes RM, et al. Jejunal intussusception in adult horses: 11 cases (1981–1991). J Am Vet Med Assoc 1993;202:110–112. [PubMed] [Google Scholar]
- 38. Lindor RA, Bellolio MF, Sadosty AT, et al. Adult intussusceptions: Presentation, management, and outcomes of 148 patients. J Emerg Med 2012;43:1–6. [DOI] [PubMed] [Google Scholar]
- 39. Patsikas MN, Papazoglou LG, Adamama‐Moraitou KK. Spontaneous reduction of intestinal intussusception in five young dogs. J Am Anim Hosp Assoc 2008;44:41–47. [DOI] [PubMed] [Google Scholar]
- 40. Mateen MA, Saleem S, Chandrasekhar Rao P, et al. Transient small bowel intussusceptions: Ultrasound findings and clinical significance. Abdom Imaging 2006;31:410–416. [DOI] [PubMed] [Google Scholar]
- 41. Kim JH. Ultrasound features of transient small bowel intussusceptions in pediatric patients. Korean J Radiol 2004;5:178–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig S1. Image of jejunum. Echoic fluid ingesta (outlined arrow) and lack of wall layering (solid arrow). Each diamond‐shaped marker represents 1 cm.


