Abstract
Background
Although Chiari‐like malformation (CM) and syringomyelia (SM) have been described in many small breed dogs, the prevalence and clinical manifestations of this complex have not been documented in a large cohort of American Brussels Griffon (ABG) dogs.
Objectives
To characterize the clinical and magnetic resonance imaging (MRI) features of CM and SM in the ABG breed.
Animals
Eighty‐four American Kennel Club registered ABG dogs were recruited.
Methods
Prospective study. Complete histories and neurologic examinations were obtained before MRI. Images were blindly reviewed and calculations were made by using OsiriX. All analyses were performed by Student's t‐test, Spearman's correlation, ANOVA, and chi‐square test where appropriate.
Results
Chiari‐like malformation and SM were present in 65% and 52% of dogs, respectively. Twenty‐eight percent of dogs had neurologic deficits and 20% had neck pain. Mean central canal (CC) transverse height was 2.5 mm with a mean length of 3.6 cervical vertebrae. Neurologic deficits were significantly associated with a larger syrinx (P = .04, P = .08) and syrinx size increased with age (P = .027). SM was associated with a smaller craniocervical junction (CCJ) height (P = .04) and larger ventricles (P = .0001; P < .001).
Conclusions and Clinical Importance
Syringomyelia and CM are prevalent in American Brussels Griffon dogs. Syrinx size is associated with neurologic deficits, CM, larger ventricles, a smaller craniocervical junction height, neurologic deficits, and cerebellar herniation. Fifty‐two percent of dogs with a SM were clinically normal.
Keywords: Central canal, Foramen magnum, Magnetic resonance imaging, Syrinx
Abbreviations
- ABGA
American Brussels Griffon Association
- AKC
American Kennel Club
- CC
central canal
- CCD
central canal dilatation
- CCJ
craniocervical junction
- CHF
Canine Health Foundation
- CKCS
Cavalier King Charles Spaniel
- CM
Chiari‐like malformation
- CSF
cerebrospinal fluid
- FLAIR
fluid attenuated inversion recovery
- FM
foramen magnum
- MRI
magnetic resonance imaging
- SM
syringomyelia
- T1W
T1 weighted
- T2W
T2 weighted
Chiari‐like malformation (CM) and syringomyelia (SM) are recognized in small breed dogs.1, 2, 3, 4, 5 The terminology used to describe these conditions has been adopted from similar disorders in humans. In dogs, CM is characterized by herniation of the cerebellum and medulla into or through the foramen magnum (FM).6 Overcrowding of the caudal cranial fossa has been demonstrated in Cavalier King Charles Spaniels (CKCS) where there is a mismatch between caudal cranial fossa volume and brain parenchyma within leading to cerebellar herniation, medullary kinking, obstruction of the dorsal craniocervical subarachnoid space, and alteration of cerebrospinal fluid (CSF) flow.7, 8, 9, 10, 11 The cause of CM is complex, and studies in dogs demonstrate mixed results. Some have reported no difference in caudal cranial fossa volume in CKCS compared to other brachycephalic or small breed dogs2, 12 or a link between caudal cranial fossa volume and development of SM.7, 11, 13 Other studies have shown that SM‐affected CKCS have a “shallower” caudal cranial fossa compared to mesaticephalic dogs and to CKCS without SM.14 In the CKCS, CM has been associated with premature closure of the spheno‐occipital synchondrosis, which may be a contributing factor for brachycephalic skull conformation and CM.15 Additionally, in CKCS there is an association between increased cerebellar volume and SM.16
Different terminology has been used to describe abnormal CSF‐like fluid accumulation within the spinal cord, but the preferred term is SM.6 SM or a syrinx, which is generally associated with the central canal, can involve the dorsal horn resulting in abnormal processing of sensory information resulting in neuropathic pain.17, 18 In addition, proinflammatory mediators such as substance P produced by nociceptive afferents in the dorsal horn and interleukin‐6 are likely important mediators causing pain in CKCS with SM.19 In dogs, a CC dilatation >2 millimeters is considered to be SM, with larger asymmetrical SM associated with more signs of neuropathic pain.20
In people and dogs, SM has been associated with obstruction of CSF channels through the FM and cerebellar herniation.13, 21, 22, 23, 24 In humans, the reported occurrence of SM in association with CM ranges from 65 to 80%.22 Because of the recognition of the CM/SM complex in dogs, there have been several investigations into its prevalence, pathogenesis, diagnosis, and treatment. CM and SM are best documented in the CKCS, where an association between CM and SM has been reported.25, 26 The prevalence of CM and CM/SM is 95% and 46%, respectively, in this breed,4, 27 and a complex inheritance has been determined.26, 27 This condition also has been documented in a group of Brussels Griffon dogs predominantly from Australia and Europe (where the breed is referred to as the Griffon Bruxellois).5 However, the prevalence and severity of the CM/SM complex in this breed in the United States currently are unknown.
The aims of our study were to document the prevalence of CM and SM in a large cohort of American Brussels Griffon (ABG) dogs, to define the clinical manifestations associated with the structural abnormalities, and to evaluate associations between CM and SM in this breed.
Materials and Methods
Patient Recruitment
American Kennel Club (AKC) registered ABG dogs >6 months of age were recruited for participation in the study conducted at the University of Georgia, College of Veterinary Medicine (UGA CVM). A 5‐generation pedigree was required for participation and was provided by the owner or obtained from the AKC. Dogs without previous imaging or therapeutic intervention participated to avoid overinclusion of dogs with confirmed disease. Recruitment was assisted by the AKC Canine Health Foundation (CHF), the American Brussels Griffon Association (ABGA), the National Brussels Griffon Association, a UGA CVM website, and an email address dedicated to the study. Clinically affected and unaffected dogs were included, but the majority of participants presented for the purposes of screening for breeding purposes. An attempt was made to recruit geographically diverse individuals of similar and varied ancestry. The costs of participation were covered by the study to prevent exclusion of animals because of financial factors.
History and Evaluation
A complete history was obtained at admission, and included information about frequency and severity of phantom scratching if present, presence of weakness or exercise intolerance, suspicion of pain and its behavioral manifestations, and the presence of previous medical problems. All dogs underwent physical and neurologic examinations. Neurologic examination included evaluation of mental status, gait analysis, presence of postural reactions and spinal reflex deficits, cranial nerve and cutaneous trunci assessment, and presence or absence of pain based on subjective evaluation during palpation of vertebral column. The term neurologic deficit was used to describe dogs with a postural reaction deficit, paresis, proprioceptive ataxia, or some combination of these signs.
Anesthesia and Magnetic Resonance Imaging (MRI)
All dogs had a CBC, serum biochemical analysis, and urinalysis performed before anesthesia for imaging using a 3.0 T MRI unit1 with an 8‐channel phased array extremity coil. Dogs received butorphanol2 (0.2 mg/kg IV) and diazepam3 (0.2 mg/kg IV) as premedication; anticholinergic drugs (atropine4 or glycopyrrolate5) were administered based on heart rate and blood pressure. Anesthesia was induced with propofol6 (4 mg/kg IV or to effect) and was maintained with isoflurane.
Dogs were positioned in sternal recumbency with the head flexed using the same positioning pad to mimic head position in standing dogs.8, 28 Magnetic resonance images of brain and cervical spinal cord from C1 through C7 were obtained in the sagittal and transverse planes using the following pulse sequences: T1‐weighted fluid‐attenuated inversion recovery (T1W FLAIR), T2‐weighted (T2W), and T2W FLAIR. After IV administration of 0.1 mmol/kg of gadopentetate dimeglumine,7 additional T1W FLAIR sequences were obtained. The following MRI parameters were used: field of view of 721 × 530, slice thickness of 2 mm on sagittal images, slice thickness of 3 mm on transverse images with an interslice gap of 0 mm, and matrix size of 512 × 512. T2W images: time to repeat (TR) of 4,000 milliseconds, time to echo (TE) of 87–117.6 milliseconds. For T1W FLAIR images: TR of 2,596 milliseconds, TE of 10 milliseconds. For T2W FLAIR images: TR of 9,502 milliseconds, TE of 127 milliseconds. An atlanto‐occipital‐CSF collection and analysis were performed after the MRI while under anesthesia in those dogs whose owner consented.
Morphologic Examination
All images were reviewed by a resident in an ACVIM‐approved neurology residency (ACF), an ACVIM board‐certified neurologist (SRP), and a veterinary student (EH), using a commercially available DICOM viewer.8 The reviewers were blinded to the clinical status of the dogs.
For CM evaluation:
The presence of CM was subjectively assessed by recording the presence of cerebellar deviation and cerebellar herniation.
CM also was graded using a version of the current British Veterinary Association (BVA) grading scheme8 (Table 1, Fig 1).
Table 1.
Chiari‐like malformation grading definitions adapted from the British Veterinary Association / Kennel Club Chiari‐like malformation Health Scheme.
| BVA Grade | Cerebellar Changes |
|---|---|
| Grade 0 | No CM |
| Grade 1 | Cerebellar indented (not rounded). Indentation by supraoccipital bone, but signal consistent with CSF between caudal cerebellar vermis and FM |
| Grade 2 | Cerebellum impacted or herniated into FM |
BVA, British Veterinary Association; CM, Chiari‐like malformation; CSF, cerebrospinal fluid, FM, foramen magnum.
Figure 1.
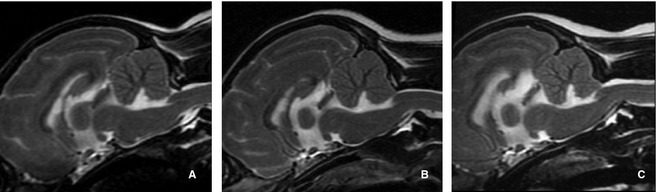
Sagittal T2‐weighted magnetic resonance images illustrating the Chiari‐like malformation (CM) grades used in this study based on British Veterinary Association grading scheme (Table 1). (A) CM grade 1 has no malformation with a normal shaped cerebellum. (B) CM grade 2 has an indented cerebellum but signal consistent with cerebrospinal fluid exists between the caudal cerebellar vermis and the foramen magnum (FM). (C) CM grade 3 exhibits a cerebellum impacted or herniated into the FM.
For syrinx evaluation:
Central canal or syrinx dorsoventral heights (mm) in the sagittal and transverse planes were measured by using OsiriX 3.69 (Fig 2).
SM was graded using a version of the current BVA grading scheme8 (Table 2).
Syrinx length was measured in units of cervical vertebral body length with the third cervical vertebra used as reference.
Figure 2.
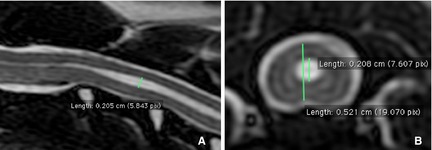
Sagittal T2‐weighted magnetic resonance images illustrating central canal, syrinx dorsoventral height (mm), or both in the sagittal (A) and transverse (B) planes measured by using OsiriX 3.6i.9
Table 2.
Syringomyelia grading definitions adapted from the British Veterinary Association / Kennel Club Chiari‐like malformation Health Scheme.
| BVA Grade | CC/Syrinx Characteristics | Age |
|---|---|---|
| Grade 0 | Normal (No CC dilatation, presyrinx or syrinx) | Any age |
| Grade 1 | CC dilatation <2 mm | ≥6 years |
| Grade 2 | CC dilatation ≥2 mm | <6 years |
| Grade 3 | Syrinx/presyrinx/CC dilatation ≥2 mm | Any age |
BVA, British Veterinary Association; CC, central canal.
For intracranial fluid compartments:
At the level of the interthalamic adhesion, the area of the lateral ventricles was calculated as a percentage of the cerebral area by measuring the area of the cerebral hemispheres in the transverse plane and the area of the lateral ventricles in the transverse and sagittal planes (% ventricular area; Fig 3).
The lateral ventricles then were subjectively graded on a scale of 1–3 using the following definitions: Grade 1: <20% of the cerebrum, Grade 2: >20% but <50%, Grade 3 >50% (Fig 4).
Quadrigeminal diverticula were recorded as present or absent.
Figure 3.
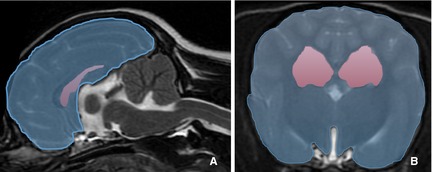
At the level of the interthalamic adhesion, the area of the lateral ventricles was calculated as a percentage of the cerebral area by measuring the area of the cerebral hemispheres in the transverse plane and the area of the lateral ventricles in the transverse and sagittal planes (% ventricular area).
Figure 4.
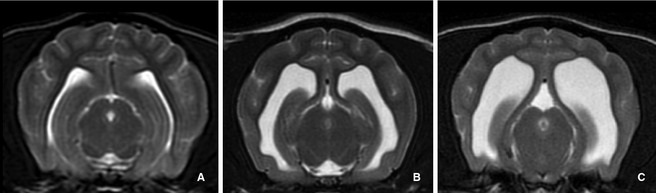
The lateral ventricles were subjectively graded using transverse T2‐weighted magnetic resonance images at the level of the rostral mesencephalon; a scale of 1 to 3 was assigned using the following definitions: (A) Grade 1: <20% of the cerebrum, (B) Grade 2: >20% but <50%, (C) Grade 3 >50%.
For craniocervical junction (CCJ) evaluation:
The length of the cerebellar protrusion into or beyond the FM was measured (Fig 5A).
The height of the CCJ was measured and expressed as a percentage of caudal cranial fossa height for standardization (CCJ/caudal cranial fossa percentage; Fig 5B). Height was measured from the caudal aspect of the FM at the level of the rostral border of the arch of the atlas as this was found to be the most reliable and consistent landmark in all patients. Height measurements were made perpendicular to the skull base.
Figure 5.
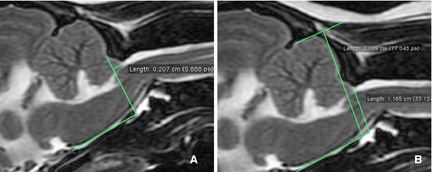
The length of the cerebellar protrusion beyond the foramen magnum was measured (A). The height of the craniocervical junction was measured and expressed as a percentage of caudal cranial fossa height for standardization (CCJ/caudal cranial fossa percentage). Height was measured from the caudal aspect of the foramen magnum at the level of the rostral border of arch of atlas. Height measurements were made perpendicular to the skull base (B).
Statistical Analysis
All analyses were performed by SAS V 9.2.10 Student's t‐tests were used to compare the mean syrinx size and extent values among groups with and without skull abnormalities, spinal pain, and neurologic signs. The folded form F statistic was used to test if variances were equal among groups. If unequal, Satterwaithe's approximation for degrees of freedom for the Student's t‐test was used. Spearman's correlation was used to test for correlations of age with syrinx size and extent values. Analysis of variance (ANOVA) was used to compare the means of syrinx sizes and extent values among all 4 sex and neuter statuses. A chi‐square test was used to test for an association between skull and CCJ abnormalities and presence of spinal pain or neurologic signs. It also was used to test for an association between presence of 1 or 2 skull and craniocervical junction abnormalities and presence of spinal pain or neurologic signs.
All hypothesis tests were 2‐sided and the significance level was α = 0.05. Chi‐square tests were implemented by using PROC FREQ10 in SAS 9.2., Student's t‐tests were implemented using PROC TTEST10 in SAS 9.2., ANOVA in PROC GLM10 in SAS 9.2., and correlations by using PROC CORR10 in SAS 9.2.
Results
Eighty‐four ABG dogs from diverse geographic regions in the United States participated in the study, including 32 males and 52 females. The mean age was 50 months (range, 8–135; median, 42.5). Although over half of the dogs (56%; 47/84) were >3 years of age, 8 dogs (9.5%) were <2 years and 1 dog was <1 year (8 months). Thirty‐four dogs (40%) had pain, neurologic deficits, or both detected on examination. Of these dogs, 24 (28.4%) had neurologic deficits and 17 (20.2%) had neck pain detected on palpation; 7 dogs (8.3%) had both. Fifty‐two percent (18/34) of dogs with pain, neurologic deficits, or both detected on clinical examination were considered to be normal by their owners. Owners suspected CM/SM or reported clinical signs consistent with CM/SM in 23.8% (20/84); the remaining 76.2% pet or breeding dogs presented for screening. Phantom scratching was not reported in any of the dogs and no other cranial cervical junction abnormalities (eg, dorsal angulation of the dens, atlanto‐axial subluxation) were identified.
Chiari‐Like Malformation
Chiari‐like malformation was present in 65% of the dogs; 39 dogs (47%) had cerebellar deviation, 55 dogs (65%) had cerebellar herniation, and 22 dogs (26%) had both.
Syringomyelia
Forty‐four (52%) of dogs had a CC/syrinx height of >2 mm and 66 dogs (78.5%) had CC/syrinx height >1 mm. Although dogs with CM had a larger SM size (P = .02), 6 dogs (7%) had SM >2 mm without any evidence of CM. The mean of the maximum dorsoventral CC height was 2.34 mm (range, 0–7.23 mm; median, 1.86 mm) in the sagittal plane and 2.5 mm (range, 0–7.6 mm; median, 2.05) in the transverse plane. The mean SM length was 3.6 cervical vertebrae (range, 1–7; median, 3). Cerebellar deviation was associated with a longer syrnix (P < .01).
Central canal dilatation (CCD) >1 mm was found to be significantly associated with the following variables: presence of CM, CM grade, and cerebellar herniation (P = .04). Dogs with CCD > 1 mm and >2 mm also had a smaller CCJ (lower CCJ/caudal cranial fossa percentage; P = .01 and P = .04, respectively), a higher ventricular grade (P = .001, P = .0001), and a higher % ventricular area (P = .001, P < .0001). However, only a syrinx >2 mm was associated with the presence of neurologic deficits (P = .03). A positive correlation existed between age and syrinx size (P = .027).
Neurologic Deficits
The presence of neurologic deficits was significantly associated with increased syrinx size (sagittal height, P = .02; transverse height, P = .04; extent, P = .08), as neurologic deficits were present in 39% of dogs with syrinx >2 mm and only 18% of dogs with CCD < 2 mm (SM > 2 mm: 17/44, 39%; SM not >2 mm: 7/40, 18%; P = .032). Only 3 dogs (12.5%) had neurologic deficits and no SM; 2 of these dogs had CM. One dog with neurologic deficits and pain had no SM or CM.
Ventricles
A positive correlation also was found between ventricular area and ventricular grade with SM size (%, P < .001; grade, P < .001), extent (%, P < .001; grade, P = .088), percentage of spinal cord (%, P = .001; grade, P = .001), and grade (%, P = .001; grade, P < .001).
Craniocervical Junction Height
Dogs with smaller CCJ ratios had larger SM heights (sagittal, P = .0031; axial, P = .002), longitudinal extent (P = .002), percent (P = .038), and grade (P = .01).
CSF Evaluation
Atlanto‐occipital CSF collection was performed in 45/84 (53%) of dogs in the study. Mean total nucleated cell count (TNCC) was 4.97/μL (range, 0–39/μL; median 3/μL). CSF white blood cell count was higher in dogs with CCD > 1 mm (P = .0015). The dog with TNCC of 39/μL had no signs of pain or neurologic deficits. This dog had SM > 2 mm and no CM; therefore, an inflammatory cause of SM could not be excluded.
Discussion
Prevalence of CM/SM Complex
Based on our study, SM and CM are prevalent in ABG dogs, with CM occurring in 65% of these dogs. SM was present in 52–78% of dogs depending on whether a syrinx was defined as >2 mm or >1 mm, respectively. These results are similar to a previous study evaluating a group of Brussels Griffon dogs where the prevalence of CM and CM with concurrent SM was 60% and 61%, respectively. Although SM was more prevalent in our study compared to 37% in the previous study population (10% or 6/56 dogs from the United States)5; CM and SM are most prevalent in the CKCS with a prevalence of 95% and 46%, respectively.4
Association between CM and SM
The presence of SM is highly associated with the presence of CM, where the reported occurrence of SM in association with CM type 1 ranges from 65 to 80% in people.22 As in this study, there also is a well‐described association between the presence of CM and SM in dogs.29, 30, 31, 32 The coexistence of CM and SM is thought to be related to CSF flow dynamics and alteration of flow at the FM.21, 24, 33, 34 We not only found an overall association among the presence of CM with SM but also with the components of CM, cerebellar herniation and deviation. This significant association between CM, CM grade, and cerebellar herniation was seen beginning with at CCD > 1 mm.
We found that ABG dogs with CM/SM had a smaller CCJ ratio than dogs without the disease. A smaller CCJ ratio also was associated with a longer and wider syrinx and a more severe SM grade. We chose to measure the height of the CCJ at the rostral border of the atlas, which could include the atlanto‐occipital ligament rather than the ventral aspect of the supraoccipital bone.28 This measurement seemed to more accurately represent outflow from the cranial cavity, especially because occipital bone dysplasia has been demonstrated in association with CM occipital dysplasia,35, 36 resulting in an increased measurement for FM height. We also attempted to minimize variation by standardizing the CCJ height by expressing it as a ratio to total caudal cranial fossa height. Ultimately, a decreased CCJ height could predispose to obstruction of CSF flow and compression of CSF channels.
Six dogs (7%) had SM without any evidence of CM. Although reported to more prevalent, this scenario has been described previously in 22% (5/22 dogs with SM) of Brussels Griffon dogs.5 The presence of SM without CM has been seen in dogs with enlargement of the entire ventricular system and is thought to be secondary to obstruction at the FM by something other than CM (eg, arachnoiditis) or an imbalance between CSF production and absorption.37, 38, 39 CSF flow studies of these patients may help elucidate alterations in CSF flow dynamics at the cervicomedullary junction that may be contributing to the pathophysiology of syrinx development without overt evidence of CM. In addition, volume reduction in the jugular foramen has been demonstrated in CKCS with SM and may result in increased intracranial pressure leading to turbulent flow of CSF in the subarachnoid space creating SM independent of cerebellar herniation.40 We found a positive correlation between ventricular area and ventricular grade with SM size, longitudinal extent, SM percentage of spinal cord, and SM grade, which could indicate a problem with CSF absorption and secondary enlargement of the entire ventricular system.
Clinical Signs and Morphologic Abnormalities
There is considerable variation in clinical signs associated with CM and SM in CKCS41 and humans.31, 42, 43, 44, 45, 46
Pain
The most common clinical sign in both species is cervical pain or intermittent, nonspecific neuropathic pain.23, 47, 48, 49, 50, 51, 52 Interestingly, pain was not the most common clinical finding in our patients, which may be a result of our study population because most dogs were presented for screening purposes rather than for clinical suspicion of disease. Although not reported by any of our owners, a classical sign is phantom scratching at the neck and shoulders which may represent paresthesia or allodynia.18, 23 Cervical pain was only identified in 20% of our patients and was less common than weakness. In addition, 47% (8/17) of these dogs were thought to be clinically normal by their owners, indicating possible overinterpretation of the examination because of the inclusion of very subtle or subjective abnormalities. Alternatively, clinical signs of pain can easily be overlooked in veterinary patients. SM in clinically normal dogs is common and reported in up to 70% of older CKCS.4
Magnetic resonance imaging‐calculated syrinx width has been shown to be the strongest predictor of pain, with a wide syrinx being significantly associated with discomfort.20 A syrinx of >6.4 mm caused clinical signs in 95% of CKCS.20 We did not find an association between pain and syrinx size in our study population.
The mechanism underlying development of pain associated with SM is thought to be related to the location of the syrinx within the dorsal horn of the spinal cord, especially if asymmetrical, damaging the superficial laminae and their connections to the spinothalamic tracts.18, 19, 20 Pain associated with CM also may be secondary to compression of the brainstem or first cervical nerve.44, 45 Recent studies also have demonstrated the importance of proinflammatory mediators such as substance P (produced by nociceptive afferents in the dorsal horn) and interleukin‐6 in CKCS with SM with persistent pain.19 Although the average TNCC was not increased in our dogs, CSF white blood cell count was higher in dogs with CCD > 1 mm. This finding is consistent with a previous study that found dogs with SM had higher TNCC than dogs without CM.53
Neurologic Dysfunction
Additional signs include varying degrees of paresis and ataxia, atrophy of appendicular musculature of the thoracic limbs, and scoliosis of the cervical vertebral column.23, 48, 49 Neurologic deficits ranging from general proprioceptive ataxia to postural reaction deficits were the most common clinical signs in our study. These signs were found in 28.4% (24/84) of these dogs and were significantly associated with increased syrinx size and longitudinal extent. Some dogs have no apparent clinical signs,54 as seen in 52% of these dogs with a syrinx >2 mm. Ultimately, the question regarding normal CC size is unanswered because any dilatation of the CC should be considered abnormal. Our data supports a cut‐off of 2 mm above which 39% of dogs had deficits, but 18% of dogs with CCD < 2 mm also had neurologic deficits. In addition, we found similar associations when a syrinx size cut‐off of 1 mm was used. Although CCD of >1 mm but <2 mm was not associated with neurologic deficits, it was associated with CM and its components including CM grade, cerebellar herniation, smaller CCJ ratio, and larger ventricular size. This information should not be ignored especially because SM size may increase with age.36
Controversy exists over treatment options for CM/SM in dogs. As in half of our patients with SM, many dogs with CM/SM are clinically normal, making decisions regarding treatment options confusing. Although CM/SM was prevalent in our study population, only about 25% of dogs had neurologic deficits and fewer (20%) had neck pain. In addition, 52% of the dogs with clinical signs on examination did not have any clinical signs reported by the owner. These findings indicate that treatment may not be warranted in all dogs with MRI evidence of CM/SM because <50% may manifest clinical signs of the condition. However, we also found that increased age was significantly associated with a larger syrinx suggesting SM size may be progressive as has been shown in the CKCS,36 which could ultimately result in a clinically affected patient over time. Unfortunately, without a longitudinal study, the occurrence of disease progression in our study population is unknown.
There are several limitations to our study. Pain was recorded as present or not as indicated by subjective discomfort on palpation of the entire spine, which can lead to overinterpretation in some dogs. Also, dogs had a MRI of the head and neck through the C7 vertebrae, because this is the most common location of SM in dogs with CM, but the thoracolumbar vertebral column was not imaged. Therefore, extension of a syrinx caudally or presence of other conditions such as intervertebral disk disease was not evaluated. Another limitation was that CSF analysis was only performed in approximately 50% of patients, meaning a primary inflammatory process was not ruled out in every patient. An additional consideration is that head position was not standardized with a fixed positioning device and may have varied between patients. Head position has been shown to affect degree of cerebellar herniation and CSF space between the cerebellum and brainstem28 with a flexed angle of 100–138 degrees allowing more consistent ability to evaluate CSF flow at the cervicomedullary junction.8 We made an attempt to minimize variation by using similar positioning pads for all dogs and positioning dogs in sternal recumbency in a flexed position, but we did not overflex the neck into an unnatural position. In addition, our measurements were based on 2‐dimensional measurements, which are less accurate than volumetric evaluation.
Finally, our method of measuring height near the FM differed from previously published methods as we chose to measure the height of the CCJ at the rostral border of the atlas, which may have included the atlanto‐occipital ligament rather than the ventral aspect of the supraoccipital bone.28 We chose this method because it allowed for more consistent measurements among patients because the borders of the atlanto‐occipital ligament and the ventral aspect of the supraocciptial bone can be difficult to determine in some patients, especially if they have occipital dysplasia. In addition, we attempted to minimize variation by standardizing the CCJ height by expressing it as a ratio to total caudal cranial fossa height. This junction seems a more accurate representation of cranial cavity outflow in these patients especially as occipital bone dysplasia has been demonstrated in some dogs and may increase the measured FM height, but this method may not represent the true FM height and makes these results difficult to compare to those of previous studies.
In conclusion, SM and CM are prevalent in the ABG breed, with CM occurring in 65% of dogs and SM (defined as >2 mm) in 52% of dogs. At a lower cut‐off of >1 mm, the prevalence of SM was 78.5%. In the ABG breed, syrinx size is associated with CM, larger ventricles, a small CCJ percentage, neurologic deficits, and cerebellar herniation. Although dogs with a longer syrinx in the cervical spinal cord more often have neurologic deficits, SM size was not associated with pain. Importantly, >50% of dogs with SM were clinically normal. Hopefully, studies such as this one will lead to a better understanding of the prevalence and pathogenesis of the CM/SM complex and its associated neurologic signs. Such information is crucial before making treatment suggestions.
Acknowledgments
The Bioimaging Research Center University of Georgia, Penny Knowler, Meg Prior, Greg Lawson, Mark Grigalunas, Raul Peralta, Lee Pieterse, Ruth Pereira, Tim Jarrett, Kim Mason, Sami Al‐Nadaf, Amanda Torres, Christy Chessman, Lisa Reno, and Dr Deborah Keys.
Funding provided by the American Kennel Club Canine Health Foundation Grant #1004, the American Brussels Griffon Association (ABGA), and the National Brussels Griffon Association, For the Love of Ollie Friends of Lola Fund and Lee and Frank Pieterse.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
This study was presented as an abstract at the 2011 ACVIM Forum, Denver, CO.
This study was performed at the University of Georgia, College of Veterinary Medicine.
Footnotes
GE 3.0T Signa HDx; GE Healthcare, Milwaukee, WI
Butorphanol tartrate; Torbugesic, Fort Dodge, Fort Dodge, IA
Diazepam, Valium; Hospira, Lake Forest, IL
Atropine sulfate; Med Pharmex, Pomona, CA
Glycopyrrolate; Baxter Healthcare Corp, Deerfield, IL
PropoFlo; Abbott Laboratories, North Chicago, IL
Gadopentetate dimeglumine, Magnevist; Bayer HealthCare Pharmaceuticals, Wayne, NJ
Osiri x 3.6 Pixmeo, Bernex, Switzerland
SAS Institute Inc, Cary, NC
References
- 1. Cagle L. Concurrent occipital hypoplasia, occipital dysplasia, syringohydromyelia, and hydrocephalus in a Yorkshire Terrier. Can Vet J 2010;51:904–908. [PMC free article] [PubMed] [Google Scholar]
- 2. Cross HR, Cappello R, Rusbridge C. Comparison of cerebral cranium volumes between cavalier King Charles Spaniels with Chiari‐like malformation, small breed dogs and Labradors. J Small Anim Pract 2009;50:399–405. [DOI] [PubMed] [Google Scholar]
- 3. Marino DJ, Loughin CA, Dewey CW, et al. Morphometric features of the craniocervical junction region in dogs with suspected Chiari‐like malformation determined by combined use of magnetic resonance imaging and computed tomography. Am J Vet Res 2012;73:105–111. [DOI] [PubMed] [Google Scholar]
- 4. Parker JE, Knowler SP, Rusbridge C, et al. Prevalence of asymptomatic syringomyelia in Cavalier King Charles spaniels. Vet Rec 2011;168:667. [DOI] [PubMed] [Google Scholar]
- 5. Rusbridge C, Knowler SP, Pieterse L, et al. Chiari‐like malformation in the Griffon Bruxellois. J Small Anim Pract 2009;50:386–393. [DOI] [PubMed] [Google Scholar]
- 6. Cappello R, Rusbridge C. Report from the Chiari‐like Malformation and Syringomyelia Working Group Round Table. Vet Surg 2007;36:509–512. [DOI] [PubMed] [Google Scholar]
- 7. Cerda‐Gonzalez S, Olby NJ, McCullough S, et al. Morphology of the caudal fossa in Cavalier King Charles Spaniels. Vet Radiol Ultrasound 2009;50:37–46. [DOI] [PubMed] [Google Scholar]
- 8. Cerda‐Gonzalez S, Olby NJ, Broadstone R, et al. Characteristics of cerebrospinal fluid flow in Cavalier King Charles Spaniels analyzed using phase velocity cine magnetic resonance imaging. Vet Radiol Ultrasound 2009;50:467–476. [DOI] [PubMed] [Google Scholar]
- 9. Driver CJ, Volk HA, Rusbridge C, et al. An update on the pathogenesis of syringomyelia secondary to Chiari‐like malformations in dogs. Vet J 2013;198:551–559. [DOI] [PubMed] [Google Scholar]
- 10. Shaw TA, McGonnell IM, Driver CJ, et al. Increase in cerebellar volume in Cavalier King Charles Spaniels with Chiari‐like malformation and its role in the development of syringomyelia. PLoS One 2012;7:e33660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Driver CJ, Rusbridge C, Cross HR, et al. Relationship of brain parenchyma within the caudal cranial fossa and ventricle size to syringomyelia in Cavalier King Charles Spaniels. J Small Anim Pract 2010;51:382–386. [DOI] [PubMed] [Google Scholar]
- 12. Schmidt MJ, Biel M, Klumpp S, et al. Evaluation of the volumes of cranial cavities in Cavalier King Charles Spaniels with Chiari‐like malformation and other brachycephalic dogs as measured via computed tomography. Am J Vet Res 2009;70:508–512. [DOI] [PubMed] [Google Scholar]
- 13. Couturier J, Rault D, Cauzinille L. Chiari‐like malformation and syringomyelia in normal Cavalier King Charles spaniels: A multiple diagnostic imaging approach. J Small Anim Pract 2008;49:438–443. [DOI] [PubMed] [Google Scholar]
- 14. Carrera IDR, Mellor D, Penderis J, Sullivan M. Use of magnetic resonance imaging for morphometric analysis of caudal cranial fossa in Cavalier King Charles Spaniels. Am J Vet Res 2009;70:340–345. [DOI] [PubMed] [Google Scholar]
- 15. Schmidt MJ, Volk H, Klingler M, et al. Comparison of closure times for cranial base synchondroses in mesaticephalic, brachycephalic, and Cavalier King Charles Spaniel dogs. Vet Radiol Ultrasound 2013;54:497–503. [DOI] [PubMed] [Google Scholar]
- 16. Shaw TA, McGonnell IM, Driver CJ, et al. Increase in cerebellar volume in Cavalier King Charles Spaniels with Chiari‐like malformation and its role in the development of syringomyelia. PLoS One 2012;7:e33660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Hu HZ, Rusbridge C, Constantino‐Casas F, et al. Histopathological investigation of syringomyelia in the Cavalier King Charles spaniel. J Comp Pathol 2012;146:192–201. [DOI] [PubMed] [Google Scholar]
- 18. Rusbridge C, Jeffery ND. Pathophysiology and treatment of neuropathic pain associated with syringomyelia. Vet J 2008;175:164–172. [DOI] [PubMed] [Google Scholar]
- 19. Schmidt MJ, Roth J, Ondreka N, et al. A potential role for substance P and interleukin‐6 in the cerebrospinal fluid of Cavalier King Charles Spaniels with neuropathic pain. J Vet Intern Med 2013;27:530–535. [DOI] [PubMed] [Google Scholar]
- 20. Rusbridge C, Carruthers H, Dube MP, et al. Syringomyelia in cavalier King Charles Spaniels: The relationship between syrinx dimensions and pain. J Small Anim Pract 2007;48:432–436. [DOI] [PubMed] [Google Scholar]
- 21. Levine DN. The pathogenesis of syringomyelia associated with lesions at the foramen magnum: A critical review of existing theories and proposal of a new hypothesis. J Neurol Sci 2004;220:3–21. [DOI] [PubMed] [Google Scholar]
- 22. Speer MC, Enterline DS, Mehltretter L, et al. Chiari type I malformation with or without syringomyelia: Prevalence and genetics. J Genet Couns 2003;12:297–311. [DOI] [PubMed] [Google Scholar]
- 23. Rusbridge C, MacSweeny JE, Davies JV, et al. Syringohydromyelia in Cavalier King Charles Spaniels. J Am Anim Hosp Assoc 2000;36:34–41. [DOI] [PubMed] [Google Scholar]
- 24. Oldfield EH, Muraszko K, Shawker TH, et al. Pathophysiology of syringomyelia associated with Chiari I malformation of the cerebellar tonsils. Implications for diagnosis and treatment. J Neurosurg 1994;80:3–15. [DOI] [PubMed] [Google Scholar]
- 25. Chandler K, Volk H, Rusbridge C, et al. Syringomyelia in Cavalier King Charles Spaniels. Vet Rec 2008;162:324. [DOI] [PubMed] [Google Scholar]
- 26. Rusbridge C, Knowler P, Rouleau GA, et al. Inherited occipital hypoplasia/syringomyelia in the Cavalier King Charles Spaniel: Experiences in setting up a worldwide DNA collection. J Hered 2005;96:745–749. [DOI] [PubMed] [Google Scholar]
- 27. Rusbridge C, Knowler SP. Inheritance of occipital bone hypoplasia (Chiari type I malformation) in Cavalier King Charles Spaniels. J Vet Intern Med 2004;18:673–678. [DOI] [PubMed] [Google Scholar]
- 28. Upchurch JJ, McGonnell IM, Driver CJ, et al. Influence of head positioning on the assessment of Chiari‐like malformation in Cavalier King Charles Spaniels. Vet Rec 2011;169:277. [DOI] [PubMed] [Google Scholar]
- 29. Banerji NK, Millar JH. Chiari malformation presenting in adult life. Its relationship to syringomyelia. Brain 1974;97:157–168. [DOI] [PubMed] [Google Scholar]
- 30. Fernandez AA, Guerrero AI, Martinez MI, et al. Malformations of the craniocervical junction (Chiari type I and syringomyelia: Classification, diagnosis and treatment). BMC Musculoskelet Disord 2009;10(Suppl 1):S1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Mohr PD, Strang FA, Sambrook MA, et al. The clinical and surgical feature in 40 patients with primary cerebellar ectopia (adult Chiari malformation). Q J Med 1977;46:85–96. [PubMed] [Google Scholar]
- 32. Schady W, Metcalfe RA, Butler P. The incidence of craniocervical bony anomalies in the adult Chiari malformation. J Neurol Sci 1987;82:193–203. [DOI] [PubMed] [Google Scholar]
- 33. Heiss JD, Patronas N, DeVroom HL, et al. Elucidating the pathophysiology of syringomyelia. J Neurosurg 1999;91:553–562. [DOI] [PubMed] [Google Scholar]
- 34. Bilston LE, Fletcher DF, Brodbelt AR, et al. Arterial pulsation‐driven cerebrospinal fluid flow in the perivascular space: A computational model. Comput Methods Biomech Biomed Engin 2003;6:235–241. [DOI] [PubMed] [Google Scholar]
- 35. Rusbridge C, Knowler SP. Coexistence of occipital dysplasia and occipital hypoplasia/syringomyelia in the Cavalier King Charles Spaniel. J Small Anim Pract 2006;47:603–606. [DOI] [PubMed] [Google Scholar]
- 36. Driver CJ, De Risio L, Hamilton S, et al. Changes over time in craniocerebral morphology and syringomyelia in Cavalier King Charles Spaniels with Chiari‐like malformation. BMC Vet Res 2012;8:215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Klekamp J, Iaconetta G, Batzdorf U, et al. Syringomyelia associated with foramen magnum arachnoiditis. J Neurosurg 2002;97:317–322. [DOI] [PubMed] [Google Scholar]
- 38. Levine DN. Intracranial pressure and ventricular expansion in hydrocephalus: Have we been asking the wrong question? J Neurol Sci 2008;269:1–11. [DOI] [PubMed] [Google Scholar]
- 39. Portnoy HD, Branch C, Castro ME. The relationship of intracranial venous pressure to hydrocephalus. Childs Nerv Syst 1994;10:29–35. [DOI] [PubMed] [Google Scholar]
- 40. Schmidt MJ, Ondreka N, Sauerbrey M, et al. Volume reduction of the jugular foramina in Cavalier King Charles Spaniels with syringomyelia. BMC Vet Res 2012;8:158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Rusbridge C, Greitz D, Iskandar BJ. Syringomyelia: Current concepts in pathogenesis, diagnosis, and treatment. J Vet Intern Med 2006;20:469–479. [DOI] [PubMed] [Google Scholar]
- 42. Bhadelia RA, Frederick E, Patz S, et al. Cough‐associated headache in patients with Chiari I malformation: CSF Flow analysis by means of cine phase‐contrast MR imaging. AJNR Am J Neuroradiol 2011;32:739–742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: Clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999;44:1005–1017. [DOI] [PubMed] [Google Scholar]
- 44. Taylor FR, Larkins MV. Headache and Chiari I malformation: Clinical presentation, diagnosis, and controversies in management. Curr Pain Headache Rep 2002;6:331–337. [DOI] [PubMed] [Google Scholar]
- 45. Todor DR, Mu HT, Milhorat TH. Pain and syringomyelia: A review. Neurosurg Focus 2000;8:E11. [DOI] [PubMed] [Google Scholar]
- 46. Yeom JS, Lee CK, Park KW, et al. Scoliosis associated with syringomyelia: Analysis of MRI and curve progression. Eur Spine J 2007;16:1629–1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Child G, Higgins RJ, Cuddon PA. Acquired scoliosis associated with hydromyelia and syringomyelia in two dogs. J Am Vet Med Assoc 1986;189:909–912. [PubMed] [Google Scholar]
- 48. Dewey CW, Berg JM, Barone G, et al. Foramen magnum decompression for treatment of caudal occipital malformation syndrome in dogs. J Am Vet Med Assoc 2005;227:1270–1275, 1250–1271. [DOI] [PubMed] [Google Scholar]
- 49. Dewey CW, Marino DJ, Bailey KS, et al. Foramen magnum decompression with cranioplasty for treatment of caudal occipital malformation syndrome in dogs. Vet Surg 2007;36:406–415. [DOI] [PubMed] [Google Scholar]
- 50. Kirberger RM, Jacobson LS, Davies JV, et al. Hydromyelia in the dog. Vet Radiol Ultrasound 1997;38:30–38. [DOI] [PubMed] [Google Scholar]
- 51. McGrath JT. Spinal dysraphism in the dog. With comments on syringomyelia. Pathol Vet 1965;2(Suppl):1–36. [PubMed] [Google Scholar]
- 52. Schmahl W, Kaiser E. Hydrocephalus, syringomyelia, and spinal cord angiodysgenesis in a Lhasa‐apso dog. Vet Pathol 1984;21:252–254. [DOI] [PubMed] [Google Scholar]
- 53. Whittaker DE, English K, McGonnell IM, et al. Evaluation of cerebrospinal fluid in Cavalier King Charles Spaniel dogs diagnosed with Chiari‐like malformation with or without concurrent syringomyelia. J Vet Diagn Invest 2011;23:302–307. [DOI] [PubMed] [Google Scholar]
- 54. Parker JE, Knowler SP, Rusbridge C, et al. Prevalence of asymptomatic syringomyelia in Cavalier King Charles Spaniels. Vet Rec 2011;168:667. [DOI] [PubMed] [Google Scholar]


