Abstract
Background
Mean platelet volume (MPV) and plateletcrit (PCT) are indices used in evaluating immune‐mediated thrombocytopenia (IMT) in humans and in dogs with congenital macrothrombocytopenia. These indices may provide clinically valuable information in acquired thrombocytopenia.
Hypothesis/Objectives
Dogs with presumed primary IMT will have increased MPV, and therefore platelet mass (PCT) will increase faster than platelet count (PLT) during recovery.
Animals
Forty‐nine dogs with automated PLT < 30,000/μL because of presumed primary IMT and hematocrit (HCT), PCT, MPV, and platelet distribution width determined from the same complete blood count (CBC), and 46 healthy controls.
Methods
Case‐control retrospective study; PLT, PCT, MPV, and platelet distribution width (PDW) were recorded from CBCs from 49 dogs, with 45 having data collected on the day of presentation. Fifteen were confirmed to have attained a PLT ≥ 75,000/μL on at least 1 CBC within 15 days after admission. The PCT equivalent to a PLT of 75,000/μL (assuming an average MPV) was calculated for comparison with PLT in terms of time to achieve a threshold of platelet mass by the 2 measures.
Results
Mean platelet volume was higher in IMT dogs (17.3 fl) than the reference population (10.5 fl) (P < .0001). The PDW was not significantly different among the groups. The median time for PCT to reach threshold in confirmed responders was faster (3 days) compared with PLT (4 days).
Conclusions and Clinical Importance
Immune‐mediated thrombocytopenia is characterized by increased MPV. Time to achieve a threshold PCT tended to be shorter than PLT, suggesting that PCT may be a useful platelet parameter for monitoring dogs with IMT.
Keywords: Dog, Mean platelet volume, Platelet distribution width, Platelet indices, Plateletcrit
Abbreviations
- CBC
complete blood count
- HCT
hematocrit
- IMT
immune‐mediated thrombocytopenia
- MPV
mean platelet volume
- PCT
plateletcrit
- PDW
platelet distribution width
- PLT
platelet count
Immune‐mediated thrombocytopenia (IMT) is the most common cause of severe thrombocytopenia in dogs.1 The disease may be primary or can occur secondary to a variety of inflammatory or neoplastic conditions. A diagnosis of primary IMT is established by excluding other causes of thrombocytopenia.2 Because the pathogenesis involves the adherence of antiplatelet antibodies to antigens on the platelet surface, the detection of platelet‐bound antibodies may confirm an immune‐mediated pathogenesis for the disease.3 Unfortunately, this testing is not widely available, and although very sensitive, may lack specificity especially for primary IMT in dogs,2 as in human patients.4, 5 In addition to developing better diagnostic approaches to IMT, there is a clinical need for accurate monitoring of primary hemostatic competence.
New laser‐based hematology analyzers generate platelet data beyond a platelet count (PLT) that may be clinically useful in evaluating thrombocytopenia. Three parameters of interest are the mean platelet volume (MPV), platelet distribution width (PDW), and plateletcrit (PCT). Some automated analyzers use MPV and PLT to calculate PCT as an estimate of platelet mass analogous to the hematocrit (HCT) as a measure of red cell mass. Increased platelet size increases the PDW just as increased erythrocyte size increases red cell distribution width. Human IMT patients characteristically have increased MPV, reflecting release of large immature platelets from hyperplastic megakaryocytes in response to peripheral thrombocytopenia.5, 6, 7 In contrast, older or limited investigations suggest that MPV is normal or low in canine IMT.3, 8, 9 This discrepancy with the human literature warrants evaluation with data generated from current automated methods.3, 9, 10 Because the efficiency of platelet plug formation is likely to be more directly related to platelet mass than number, the PCT may provide additional information to better evaluate primary hemostasis, particularly in conditions associated with increased MPV.10 The value of the PCT in the assessment of platelet mass recently has been demonstrated in dogs with genetic causes of macrothrombocytopenia, such as that described in Cavaliar King Charles spaniels.11, 12 In dogs with IMT, low platelet counts are correlated with increased risk of bleeding, but PLT is not an absolute predictor of clinical hemorrhage,13 nor does PLT at presentation or lowest recorded platelet counts correlate with survival.2 These limitations demonstrate the need for exploring other options for platelet assessment beyond a simple PLT for comprehensive evaluation of dogs with IMT.
The aims of this study were to evaluate platelet indices including MPV, PCT, and PDW in dogs with presumed primary IMT and to compare the time required for PLT and PCT to reach a threshold value presumed to be associated with decreased risk of bleeding. We hypothesized that dogs with presumed primary IMT would have increased MPV, and that platelet mass, as reflected by PCT, would increase faster than PLT after admission because of the presence of large platelets during the regenerative response.
Materials and Methods
The electronic medical record system of the University of Minnesota Veterinary Medical Center (UMN‐VMC) was searched from December 2007 to April 2012 to identify dogs with automated PLT1 < 30,000/μL and a HCT, PCT, MPV, and PDW available from the same complete blood count (CBC). We utilized a diagnostic cutoff of <30,000 platelets/μL for the diagnosis of IMT to maximize diagnostic specificity based on reports that 77% of dogs with IMT have PLT below this threshold and because of the increased risk of bleeding in this population.13 Medical records of dogs meeting the initial search criteria were reviewed, and dogs with evidence of secondary thrombocytopenia were excluded, as were cases for which follow‐up was inadequate to establish a diagnosis of presumptive primary IMT or when medical records were incomplete.
A diagnosis of primary IMT was made by excluding possible inciting causes using the following criteria:
Negative for exposure to Ehrlichia canis, Borrelia burgdorferi, Anaplasma phagocytophilum, and Dirofilaria immitis by Idexx 4DX ELISA antibody snap test, and specific serology in selected cases. Dogs with no identifiable etiology for IMT that did not have 4DX testing performed were retained in the group for analysis provided that no clinical signs of rickettsial disease other than thrombocytopenia were present.
Failure to document any other infectious disease, including sepsis, or sterile inflammatory disease (based on physical examination, history, diagnostic imaging, and laboratory testing performed at the discretion of the attending clinician).
No known drug or toxin exposure.
Negative for clinical evidence of neoplasia based on physical examination, medical imaging, cytology findings, biopsy results, or some combination of these.
Failure to document disseminated intravascular coagulation (DIC) based on concurrent prolongations in prothrombin time (PT) and partial thromboplastic time (PTT), increased fibrin degradation products, and identification of clinically relevant organ pathology.
Failure to document a primary bone marrow disorder if bone marrow aspiration cytology or core histopathology was performed.
Failure to document a disease process associated with consumption or sequestration of platelets such as hemorrhage secondary to trauma, liver lobe torsion, or congenital vascular anomaly.
Absence of a concurrent immune‐mediated disorder, unless a previously identified condition was in remission.
Dogs with renal azotemia also were excluded because of reported uremic thrombopathies described in humans and laboratory animal models14, 15, 16 PLT, PCT, MPV, and PDW were recorded for all study dogs from the first CBC performed at the VMC after presentation; dogs that did not have a CBC performed at the VMC on the day of presentation were excluded from analysis. PLT and PCT were recorded for all study dogs between presentation or admission to the UMN‐VMC and day 15. For dogs referred to the UMN‐VMC with IMT diagnosed using testing performed at an outside laboratory, platelet parameters (PLT and PCT) were recorded using the first CBC performed at the UMN‐VMC. Treatment history was evaluated. Dogs that attained a PLT ≥ 75,000/μL in response to treatment on at least 1 CBC within 15 days of presentation were evaluated for relative increases in PLT and PCT. A PLT threshold of 75,000/μL was selected as indicative of a clinical response because a higher PLT would not be expected to cause clinical bleeding, but it is low enough that CBCs would still be monitored to insure sufficient data for analysis. The selection of this platelet threshold was supported by previous studies.17, 18 To obtain the PCT threshold, we calculated the PCT that would correspond to a PLT of 75,000/μL in a healthy dog by multiplying that PLT threshold by the median MPV for the reference population, obtaining a corresponding PCT of 0.07575%. When evaluating time‐to‐threshold values for PLT and PCT, all available CBC data from the patient were used. A time frame of 15 days was chosen after evaluation of the retrospective dataset demonstrated relatively consistent clinical monitoring for most dogs over this time frame, which encompasses the usual response to treatment in most patients in our experience.
The reference population consisted of 46 healthy dogs of various breeds (excluding Cavalier King Charles Spaniels), >6 months of age owned by students, staff, and faculty at UMN. An a priori direct selection process with defined selection criteria was used. Samples were collected and analyzed over a 3‐week period.
Statistical Analysis
To compare MPV, PLT, PCT, and PDW between IMT and reference dogs, we performed Welch's t‐tests on the day of presentation with differences considered to be significant at P < .05. To illustrate the rates of improvement in primary hemostasis according to the measures of PLT and PCT respectively, we performed survival analysis with the event being achieving a threshold of PLT 75,000/μL and PCT of 0.07575%. For each subject, we determined the interval over which the platelet parameters first reached each threshold. Using this interval to allow interval censoring, we created a Kaplan‐Meier plot to show the proportion of subjects each day that had not yet reached the thresholds. We compared the time‐to‐threshold curves using a Wilcoxon rank‐sum 2‐sample test for interval censoring. Differences were considered significant at P < .10. This less stringent P value was selected to avoid type II error because the purpose of this analysis was to determine if there was sufficient evidence to explore the effect in a better controlled prospective study.19 All analyses were performed in the statistical program R 3.1.0 (the free source code can be obtained at http://cran.r-project.org/src/base/R-3/R-3.1.0.tar.gz).
Results
Demographics
The initial medical record query yielded 217 dogs, of which 49 met the criteria for a diagnosis of presumptive primary IMT. Seventy‐nine dogs were excluded for neoplasia (of which 30 had lymphoma), 24 for infectious or inflammatory disease, 12 for DIC, 8 for hemorrhage unrelated to IMT, 7 for primary bone marrow disorders, 3 for renal azotemia, 4 for sepsis, and 1 dog for a 3‐year history of phenobarbital administration with suspected drug‐related pancytopenia. Thirteen dogs were excluded because of incomplete diagnostic testing, incomplete medical records, or inconclusive test results. The remaining 66 dogs were classified as having immune disorders. Fourteen dogs were removed from the cohort because of concurrent immune‐mediated hemolytic anemia, and 3 dogs because of concurrent pemphigus foliaceus, systemic lupus erythematosus, or prednisone‐responsive peripheral cytopenias and fever. In total, 49 dogs were included in the study, 45 of which had data generated on the day of presentation for comparison of platelet indices with the reference population.
Affected breeds were Labrador retriever (n = 6), 3 each of Cocker spaniel, miniature Schnauzer, and Yorkshire Terrier, 2 each of Australian shepherd, Bichon Frise, English springer spaniel, Malamute, Maltese, miniature Poodle, Rat Terrier, standard Poodle and 1 each of 18 other breeds. None were Cavaliar King Charles spaniels. The median age of the affected dogs was 7 years (range, 2–14). Thirty‐two dogs were spayed females, 1 was an intact female, 14 were castrated males, and 2 were intact males.
Treatment protocols were variable. All dogs received corticosteroids (prednisone, prednisolone, dexamethasone IV or some combination of these at various dosages), 28 received azathioprine, 7 received human immunoglobulin, 6 received cyclosporine, 3 received mycophenolate, and 2 received vincristine.
Platelet Indices
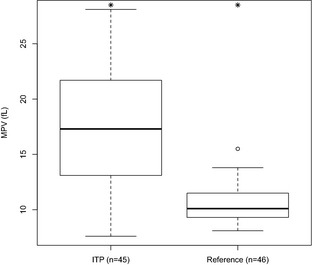
As expected according to the inclusion criteria, on admission all study dogs had severe thrombocytopenia (mean PLT 9,977/μL) compared with the reference population (mean PLT 260,934/μL, P < .0001). Similarly, the mean PCT for IMT dogs was 0.018% compared with 0.267% for the reference population (P < .0001). Figure 1 shows that MPV values were significantly higher in IMT cases compared with the reference population (17.3 fl versus 10.5 fl, P < .0001). No significant differences in the variation in platelet size as measured by the PDW were noted between IMT and reference dogs (61.8% versus 59.9%, P = .58).
Figure 1.
Mean platelet volume (MPV) for a reference population of healthy dogs and canine immune‐mediated thrombocytopenia (IMT) patients treatment. *Groups were significantly different from each other (P < .05), Welch's t‐test.
PLT Versus PCT Analysis
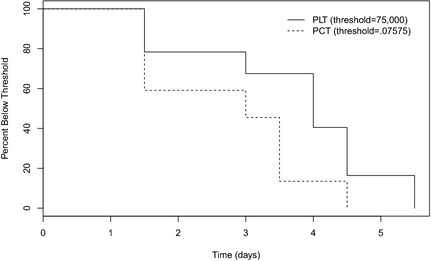
The Wilcoxon rank‐sum 2‐sample test identified a significant difference between the PLT and PCT time‐to‐threshold curves (P = .0772) for the 15 dogs meeting the criteria for this analysis, designated as “responders.” The median time to threshold for the PLT was 4 days, whereas it was 3 days for PCT (Fig 2).
Figure 2.
Survival analysis of time for a subset of 15 dogs demonstrated to have reached thresholds for platelet count (PLT) and plateletcrit (PCT). The median time for PCT to reach the threshold was 3 days, shorter than the median time for PLT to reach the threshold of 4 days (P = .0772).
Discussion
Our data suggest that presumptive primary IMT is characterized by increased MPV, and increases in PCT preceded increases in PLT during the early recovery phase in treatment responders. This finding is likely because of the presence of large young platelets that would be expected to accompany a regenerative process. No significant differences were detected in the variation in platelet size.
In our study, only 1 dog had a decreased MPV, 10 were within the reference interval, and the remainder had MPV values above the reference interval. These results are consistent with expected production of large immature platelets during a regenerative response. Information in the literature about platelet indices in dogs with IMT is limited. Two early studies reported increased MPV values with regenerative thrombocytopenias such as IMT,8, 9 although a subpopulation of dogs with IMT had low MPV in 1 of the studies.8 A third small study using a hematology analyzer similar to the 1 utilized for our study reported that 0/13 dogs suspected to have primary IMT had increased MPV.3 The reason for the discrepancy with our larger study remains unclear, but this data also are inconsistent with studies of humans with IMT. There is a complex relationship between platelet size indices and immune‐mediated destruction, which may lead to variability in results among studies and partially explain discrepant findings in the veterinary literature regarding MPV in IMT. Platelet size can be decreased by division in circulation and by platelet apoptosis, which has been observed as a mechanism for thrombocytopenia in murine models of IMT.20, 21, 22 These mechanisms could decrease MPV in IMT before a regenerative response and could lead to increased variability in platelet size in this disease, although a decrease was not observed in the dogs in our study population.
In human patients, MPV and PDW are considered the best validated of the platelet volume indices.23, 24 Studies demonstrating a high MPV in patients with IMT led to inclusion of thrombocytopenia with a population of large platelets as an initial diagnostic criterion for IMT.21 A predominance of large platelets in a thrombocytopenic patient also could be consistent with inherited macrothrombocytopenia such as Bernard‐Soulier syndrome.4 However, a combination of MPV, mean platelet diameter, and blood film evaluation can distinguish macrothrombocytopenia caused by IMT from inherited causes with good sensitivity and specificity (0.83 and 0.88, respectively).5 Another study found that MPV was significantly lower in patients with primary bone marrow disease as a cause for thrombocytopenia, but receive operator curve analysis could not establish a cutoff value that was sufficiently sensitive and specific for routine diagnostic application.6
Presumably as a result of increased MPV, dogs in our study with sufficient serial data for analysis achieved a threshold PCT earlier than the corresponding PLT. This finding suggests there may be value in further evaluating the potential of PCT as a more sensitive indicator of recovery of platelet mass than PLT. Replacement of PLT with PCT as a standard for transfusions in a human neonatal intensive care unit resulted in fewer transfusions with no increased incidence of bleeding, indicating the potential value of PCT to guide clinical decision making, assuming the risk of bleeding would be decreased as functional platelet mass increases.10 Interestingly, the development of hemorrhage in dogs with IMT is unpredictable, and survival does not correlate directly with PLT.2 Immune‐mediated vascular damage may be a mechanism for coagulopathy in IMT less directly dependent on platelet mass or function, and may contribute to poor correlation between platelet mass and clinical evidence of hemorrhage. In human IMT patients, a spectrum of autoantibodies with specificities to many epitopes has been identified, including those with cross‐reactivity to endothelial membrane proteins.21 In dogs with IMT, antibodies that cause platelet destruction may also impair function.25
Our study dogs were derived from a referral population, which could be a source of bias if cases were particularly severe or refractory to treatment. This study was retrospective, which precluded standardization of treatments, diagnostic evaluation, and follow‐up. Hematologic data were collected at the discretion of the clinician rather than at regular intervals or clinical decision points. This variability impacted options for statistical analysis and contributed to exclusion of animals from the study. We studied platelet parameters as surrogate endpoints and did not directly evaluate survival. Group size in serial assessment of PLT and PCT limited statistical power and analysis, and thus the ability to draw definitive conclusions. These results are consistent with data from human ITP patients, and should be more fully explored in prospective studies of dogs.
Despite design advances, there are technical limitations to generation of platelet volume indices and PCT by automated hematology analyzers that could influence PLT, PCT, and platelet size indices. These include imprecision in the identification of very small and very large platelets based on internal thresholds in the instrument software, variation in results among analyzers even within the reference range, and lack of universal calibration material that would permit comparison of MPV among different analyzers.4, 21, 22 Artifactual increases in MPV can occur with storage in EDTA.24 Despite these potential impediments, MPV and PDW are of clinical utility in the evaluation of thrombocytopenia in human patients.
In conclusion, we found that IMT in dogs is associated with increased MPV, consistent with IMT being a regenerative thrombocytopenia. PCT tended to increase to a threshold value earlier than PLT and use of this platelet index warrants further study of its ability to improve platelet evaluation. Evidence is accumulating that platelet indices may contribute to the evaluation of primary hemostasis in thrombocytopenia in dogs, especially considering the availability of this data at no additional cost to the client when reference analyzers are used. Confirmation of these preliminary studies with larger prospective studies is required to more fully explore the diagnostic potential of these laboratory parameters.
Acknowledgment
Conflict of Interest Declaration: The authors disclose no conflict of interest.
All work was performed at the University of Minnesota College of Veterinary Medicine and School of Statistics.
Footnotes
ADVIA 120; Siemens Medical Solutions USA Inc, Malvern, PA
References
- 1. Botsch V, Küchenhoff H, Hartmann K, Hirschberger J. Retrospective study of 871 dogs with thrombocytopenia. Vet Rec 2009;164:647–651. [DOI] [PubMed] [Google Scholar]
- 2. O'Marra SK, Delaforcase AM, Shaw SP. Treatment and predictors of outcome in dogs with immune‐mediated thrombocytopenia. J Am Vet Med Assoc 2011;238:346–352. [DOI] [PubMed] [Google Scholar]
- 3. Dircks BH, Schuberth HJ, Mischke R. Underlying diseases and clinicopathologic variables of thrombocytopenic dogs with and without platelet‐bound antibodies detected by use of a flow cytometric assay: 83 cases (2004–2006). J Am Vet Med Assoc 2009;235:960–966. [DOI] [PubMed] [Google Scholar]
- 4. Geddis AE, Balduini CL. Diagnosis of immune thrombocytopenic purpura in children. Curr Opin Hematol 2007;14:520–525. [DOI] [PubMed] [Google Scholar]
- 5. Noris P, Klersy C, Zecca M, et al. Platelet size distinguishes between inherited macrothrombocytopenias and immune thrombocytopenia. J Thromb Haemost 2009;7:2131–2136. [DOI] [PubMed] [Google Scholar]
- 6. Chandra H, Chandra S, Rawat A, Verma SK. Role of mean platelet volume as discriminating guide for bone marrow disease in patients with thrombocytopenia. Int J Lab Hematol 2010;32:498–505. [DOI] [PubMed] [Google Scholar]
- 7. DiQuattro M, Gagliano F, Calabro GM, et al. Relationships between platelet counts, platelet volumes and reticulated platelets in patients with ITP: Evidence for significant platelet count inaccuracies with conventional instrument methods. Int J Hematol 2009;31:199–206. [DOI] [PubMed] [Google Scholar]
- 8. Northern J, Tvedten HW. Diagnosis of microthrombocytopenia and immune‐mediated thrombocytopenia in dogs with thrombocytopenia: 68 cases (1987–1989). J Am Vet Med Assoc 1992;200:368–372. [PubMed] [Google Scholar]
- 9. Sullivan PS, Manning KL, McDonald TP. Association of mean platelet volume and bone marrow megakaryocytopoiesis in thrombocytopenic dogs: 60 cases (1984–1993). J Am Vet Med Assoc 1995;206:332–334. [PubMed] [Google Scholar]
- 10. Gerday E, Baer VL, Lambert DK, et al. Testing platelet mass versus platelet count to guide platelet transfusions in the neonatal intensive care unit. Transfusion 2009;49:2034–2039. [DOI] [PubMed] [Google Scholar]
- 11. Kelley J, Sharkey LC, Christopherson P, Rendahl A. Platelet count and plateletcrit in Cavalier King Charles Spaniels and Greyhounds using the Advia 120/2120. Vet Clin Pathol 2014;43:43–49. [DOI] [PubMed] [Google Scholar]
- 12. Tvedten HW, Lilleihöök IE, Oberg J, et al. Validation of Advia plateletcrit for assessing platelet mass in dogs, including Cavalier King Charles spaniels. Vet Clin Pathol 2012;41:336–343. [DOI] [PubMed] [Google Scholar]
- 13. Putsche JC, Kohn B. Primary immune‐mediated thrombocytopenia in 30 dogs (1997–2003). J Am Anim Hosp Assoc 2008;44:250–257. [DOI] [PubMed] [Google Scholar]
- 14. Kaw D, Malhotra D. Platelet dysfunction and end‐stage renal disease. Semin Dial 2006;19:317–322. [DOI] [PubMed] [Google Scholar]
- 15. Lindsay RM, Clark WF. Platelet destruction in renal disease. Semin Thromb Hemost 1982;8:138–155. [DOI] [PubMed] [Google Scholar]
- 16. Schetz MR. Coagulation disorders in acute renal failure. Kidney Int Suppl 1998;66:S96–S101. [PubMed] [Google Scholar]
- 17. Bigge LA, Brown DJ, Penninck DG. Correlation between coagulation profile findings and bleeding complications after ultrasound‐guided biopsies: 434 cases (1993–1996). J Am Anim Hosp Assoc 2001;37:228–233. [DOI] [PubMed] [Google Scholar]
- 18. Davidow EB, Brainard B, Martin LG, et al. Use of fresh platelet concentrate or lyophilized platelets in thrombocytopenic dogs with clinical signs of hemorrhage: A preliminary trial in 37 dogs. J Vet Emerg Crit Care 2012;22:116–125. [DOI] [PubMed] [Google Scholar]
- 19. Curran‐Everett D, Benos DJ. Guidelines for reporting statistics in journals published by the American Physiological Society. Physiol Genomics 2004;18:249–251. [DOI] [PubMed] [Google Scholar]
- 20. Leytin V. Apoptosis in the anucleate platelet. Blood Rev 2012;26:51–63. [DOI] [PubMed] [Google Scholar]
- 21. Gyulkhandanyan AV, Mutlu A, Freedman J, Leytin V. Markers of platelet apoptosis: Methodology and applications. J Thromb Thrombolysis 2012;33:397–411. [DOI] [PubMed] [Google Scholar]
- 22. Leytin V, Mykhaylov S, Starkey AF, et al. Intravenous immunoglobulin inhibits anti‐glycoprotein IIb‐induced platelet apopotosis in a murine model of immune thrombocytopenia. Br J Haematol 2006;133:78–82. [DOI] [PubMed] [Google Scholar]
- 23. Leader A, Pereg D, Lishner M. Are platelet volume indices of clinical use? A multidisciplinary review. Ann Med 2012;44:805–816. [DOI] [PubMed] [Google Scholar]
- 24. Latger‐Cannard V, Hoarau M, Salignac S, et al. Mean platelet volume: Comparison of three analysers towards standardization of platelet morphological phenoypte. Int J Lab Hematol 2012;34:300–310. [DOI] [PubMed] [Google Scholar]
- 25. Kristensen AT, Weiss DJ, Klausner JS. Platelet dysfunction associated with immune‐mediated thrombocytopenia in dogs. J Vet Intern Med 1994;8:323–327. [DOI] [PubMed] [Google Scholar]
- 26. Handagama P, Feldman B, Kono C, Farver T. Mean platelet volume artifacts: The effect of anticoagulants and temperature on canine platelets. Vet Clin Pathol 1986;15:13–17. [DOI] [PubMed] [Google Scholar]


