Abstract
Background
Different aspiration techniques to retrieve bronchoalveolar lavage fluid (BALF) affect sample quality in healthy dogs. Studies evaluating these techniques in dogs with respiratory disease are lacking.
Objectives
To compare sample quality of BALF acquired by manual aspiration (MA) and suction pump aspiration (SPA).
Animals
Eighteen client‐owned dogs with respiratory disease.
Methods
Randomized, blinded prospective clinical trial. Manual aspiration was performed with a 35‐mL syringe attached directly to the bronchoscope biopsy channel and SPA was performed with a maximum of 50 mmHg negative pressure applied to the bronchoscope suction valve using the suction trap connection. Both aspiration techniques were performed in each dog on contralateral lung lobes, utilizing 2 mL/kg lavage volumes per site. Samples of BALF were analyzed by percentage of retrieved infusate, total nucleated cell count (TNCC), differential cell count, semiquantitative assessment of slide quality, and diagnosis score. Data were compared by paired Student's t‐test, Wilcoxon signed‐rank test, chi‐squared test, and ANOVA. Cohen's kappa coefficient was used to assess agreement.
Results
The percentage of retrieved BALF (P = .001) was significantly higher for SPA than MA. Substantial agreement was found between cytologic classification of BALF obtained with MA and SPA (kappa = 0.615). There was no significant difference in rate of definitive diagnosis achieved with cytologic assessment between techniques (P = .78).
Conclusions and Clinical Importance
Suction pump aspiration, compared to MA, improved BALF retrieval, but did not significantly affect the rate of diagnostic success of bronchoalveolar lavage (BAL) in dogs with pulmonary disease.
Keywords: Canine, Cytology, Lung
Abbreviations
- BAL
bronchoalveolar lavage
- BALF
bronchoalveolar lavage fluid
- MA
manual aspiration
- SPA
suction pump aspiration
- TNCC
total nucleated cell count
Bronchoalveolar lavage (BAL) is a minimally invasive technique that is widely used in veterinary medicine to investigate pulmonary disease.1 By retrieving infused saline from the airways, a sample representative of the lower generation bronchi, bronchioles, and alveolar spaces is collected for analysis.1 Studies in human and veterinary medicine, however, have shown that bronchoalveolar lavage fluid (BALF) sample quality, and therefore clinical utility, are affected by certain technical aspects of the BAL procedure.2, 3, 4, 5, 6 For example, a weight‐adjusted aliquot volume has been reported to provide more uniform epithelial lining fluid recovery than a fixed‐volume amount for BAL in healthy dogs.4 In addition, filtering retrieved BALF through gauze before analysis removes an unpredictable number of nucleated cells.6 As such, standardized BAL protocols have been implemented in human and equine medicine to decrease variability in BAL,2, 3, 7, 8 but such an approach is not used in small animal medicine. One technical aspect of BAL, which has not been objectively evaluated in human medicine, is the method used to retrieve BALF. Current recommendations for BALF retrieval in human medicine, based on clinician surveys and expert consensus, are to use suction pump aspiration (SPA) with <100 mmHg of negative pressure and to avoid visible airway collapse.2, 7 In small animal medicine, manual aspiration (MA) with a handheld syringe is the most commonly reported technique for BALF retrieval.1, 4 We have evaluated aspiration techniques for retrieval of BALF in healthy dogs and have reported that SPA yielded BALF samples of higher quality than MA through polyethylene tubing9 and that SPA retrieved a higher amount of BALF than did MA without polyethylene tubing.10 Because diseased lungs are more prone to collapse than are healthy lungs, the purpose of the study reported here was to compare MA and SPA using a suction trap connection for collection of BALF in dogs with respiratory tract disease and the effect of aspiration technique on BALF sample quality. We hypothesized that SPA would yield BALF of better sample quality than MA, and that samples collected with SPA would therefore be of greater diagnostic value.
Materials and Methods
Study Population
Client‐owned dogs that presented to the Ontario Veterinary College Health Sciences Centre, and for which bronchoscopy and BAL were recommended as part of their diagnostic evaluation were eligible for enrollment in this randomized, prospective, single‐blinded clinical trial. Written client consent was required for enrollment. Dogs were excluded if bronchoscopic‐guided BAL could not be performed or if the final diagnosis was unrelated to pulmonary disease. The study protocol was approved by the University of Guelph Animal Care Committee.
Information was collected from the medical record of each dog, including signalment, concurrent medications, and interpretation of orthogonal thoracic radiographs.
Anesthesia
Dogs were anesthetized with protocols tailored individually for each patient by the hospital's Anesthesia Service. Dogs were monitored by physical examination, blood pressure measurement (Doppler method), ECG, and pulse oximetry. Dogs of sufficient size to be intubated with a size 10 (or larger) cuffed endotracheal tube also were monitored with capnography and received supplemental oxygen via the anesthetic circuit. Smaller dogs received supplemental oxygen treatment via a sterile, semirigid urinary catheter1 placed transglotally into the trachea. The procedure was discontinued and appropriate treatment administered if the patient's clinical condition became unstable during general anesthesia or if unforeseen complications arose.
Bronchoscopy and BAL
Dogs were positioned in sternal recumbency. In intubated dogs, the procedures were performed through the endotracheal tube using a T‐port connection.2 In smaller dogs, the endoscope was passed transglotally into the trachea. The trachea, main stem bronchi, and second‐ and third‐generation bronchi of all lung lobes were examined visually. Four different video endoscopes (Olympus BF‐P40 video bronchoscope, outer diameter 5.0 mm, working length 55 cm, biopsy channel 2.0 mm3 ; Olympus GIF‐130 video endoscope, outer diameter 9.5 mm, working length 103 cm, biopsy channel 2.8 mm3; Olympus GIF‐140 video endoscope, outer diameter 8.9 mm, working length 140 cm, biopsy channel 2.8 mm3; Olympus GIF‐160 video endoscope, outer diameter 5.9 mm, working length 103 cm, biopsy channel 2.0 mm3) were used in this study, and were chosen by the supervising clinician based on patient size. One to 4 mL (based on patient size) of sterile 0.2% lidocaine solution was infused at the carina to decrease bronchospasm and cough. Bronchoscopy was performed by the primary clinician responsible for the case (an internist or a 1st, 2nd, or 3rd year internal medicine resident).
Two BAL aspiration techniques were performed in contralateral lung lobes of each dog. The order of and the side lavaged with each aspiration technique were randomized using a random number table. The sites for BAL were directed by pulmonary abnormalities visualized on thoracic radiography, bronchoscopy, or both.7 A weight‐adjusted BAL volume (2 mL/kg, divided into 2 aliquots) was used at each site.4 The second aliquot was infused immediately after retrieval of the first aliquot. To perform SPA, the tip of the bronchoscope was gently wedged in a distal bronchus, based on feeling resistance to advancement as well as visualization. Sterile 0.9% saline solution, warmed to 37°C, was rapidly infused through the biopsy channel of the endoscope, followed by 4 mL of air to clear the channel. Pulsatile aspiration with a maximum negative pressure of 50 mmHg was applied by the endoscopist immediately after infusion using a wall‐mounted suction unit with pressure regulator4 connected directly to the suction valve of the bronchoscope by a disposable suction trap.5 Disposable suction traps were replaced as needed if BALF recovery exceeded their maximum capacity (20 mL). To perform MA, the tip of the bronchoscope was similarly wedged into a distal bronchus of the contralateral lung lobe. A second clinician rapidly infused sterile 0.9% saline solution, warmed to 37°C, through the biopsy channel, followed by 4 mL of air to clear the channel. The second clinician inserted a 35‐mL syringe through the biopsy valve of the bronchoscope's biopsy channel and applied gentle pulsatile aspiration with the handheld syringe. For MA, the same syringe was emptied of air as necessary to continue aspiration. Aspiration by each technique was continued until fluid was no longer retrieved. The bronchoscope was cleaned and sterilized between patients by a cold sterilization method (Video bronchoscope cleaning and cold sterilization protocol).3 , 6 , 7 The time from infusion of saline to the first attempted aspiration was <20 seconds for each BAL aliquot.
Adverse Effects of Bronchoscopy and BAL
The lowest oxygen saturation during BAL was recorded for each aspiration technique. Patients that did not regain normal oxygen saturation (≥95%) on room air after recovery from general anesthesia were transferred to the intensive care unit for supplemental oxygen treatment. Whether or not supplemental oxygen treatment was required after recovery was recorded. Patient outcome, defined as survival to discharge from the hospital, also was recorded.
Evaluation of BALF
The amount of fluid retrieved was recorded for each aspiration technique. For this study, low retrieval of BALF was defined as recovery of <40% of the original aliquot volume.4 Each BALF sample was identified by the patient's hospital number, and processed for analysis within 40 minutes of collection.7 Samples were not filtered during processing. The total nucleated cell counts (TNCCs) were determined by electrical impedance.8 A 200‐μL aliquot of each BALF sample was cytocentrifuged (180 × g for 6 minutes),9 and direct smear slides were prepared from fluid concentrated by centrifugation for 5 minutes at 500 × g and decanting of supernatant. Slides were stained with Wright stain.10 The cytologic preparations were assessed by the board‐certified veterinary clinical pathologist on duty and results were reported to supervising clinicians. Slide preparations were stored for up to 12 months until analysis by a board‐certified veterinary clinical pathologist (DB) who was blinded to the patient history, original interpretations, and aspiration technique used to collect each BALF sample. Differential cell counts of a minimum of 400 leukocytes7, 11 were performed at 400× magnification (Olympus BX53 system microscope)3 on cytocentrifuge preparations. Cells were evaluated for erythrophagocytosis and presence of intracellular bacteria and pigment during differential counting. The presence of erythrophagocytosis or hemosiderin was considered to be consistent with clinically relevant intrapulmonary hemorrhage. Samples also were assessed microscopically for 5 variables reflective of sample quality.9, 10 Semiquantitative scores were applied for cellularity, cell preservation, and the presence of RBCs (without erythrophagocytosis), extracellular bacteria, and epithelial cells. Scores ≥2 for cellularity and cell preservation were considered adequate for diagnostic utility.9, 10 Predetermined cutoffs for RBC and epithelial cell scores were not defined because increased bronchial epithelial cell sloughing and intrapulmonary hemorrhage can occur in dogs with pulmonary disease.12 However, an overall trend for increased RBC scores (without erythrophagocytosis) was considered to indicate increased trauma to the bronchial mucosa during BAL.
Diagnosis
The final diagnosis for each patient was based on a combination of history, and findings from physical examination, thoracic radiographs, bronchoscopic examination, BALF cytology, BALF microbial cultures, and response to treatment (when available). A diagnosis of bacterial pneumonia was made on the basis of semiquantitative microbial cultures of pooled BALF samples, the presence of intracellular bacterial on BALF cytology, or both.13 The veterinary laboratory reported semiquantitative culture results as follows: no growth, R+ for bacteria isolated on replating, 1+ for occasional organisms isolated, 2+ for few organisms isolated, 3+ for moderate number of organisms isolated, and 4+ for large number of organisms isolated. For aerobic bacterial cultures, 3+ to 4+ growth was considered clinically relevant, as was 2+ growth in samples from patients receiving concurrent antibiotic treatment.
Inflammation identified in BALF was defined as TNCC >500 cells/μL.13 Inflammatory BALF was further classified as suppurative (>12% neutrophils), eosinophilic (>14% eosinophils), lymphocytic (>16% lymphocytes), or mixed (increased proportions of ≥2 types of leukocyte).13, 14 The information obtained from cytologic analysis of BALF was compared with the final patient diagnosis and coded into 3 categories, based on a previous retrospective assessment of diagnostic yield of BAL in dogs (cytologic diagnosis score).14 A cytologic diagnosis score of 0 indicated a nonhelpful sample.14 A score of 1 indicated that the cytologic analysis was supportive of the overall diagnosis.14 A score of 2 indicated that a definitive diagnosis was achieved from cytology alone.14 For example, a score of 2 was allocated for the presence of intracellular bacteria, neoplastic cells, or fungal organisms.
Statistical Analysis
Descriptive statistics for patient and BAL parameters were determined by spreadsheet software.11 Remaining statistical analyses were performed by statistical software programs.12 , 13 All data were assessed for normality by the Shapiro‐Wilk test, with P > .05 considered to have a normal distribution. Normally distributed data (percent of retrieved BALF) were assessed using a paired Student's t‐test. Nonparametric data (TNCC, proportional differential cell counts, semiquantitative cytology scale, and cytologic diagnosis score) were compared using Wilcoxon Signed‐Rank test for matched pairs. ANOVA was used to assess influence of endoscope on BALF retrieval. Chi‐square testing was used to assess the effect of thoracic radiographs on cytologic diagnosis score. Agreement between classification of BALF for MA and SPA samples and between cytologic diagnosis scores for MA and SPA were assessed using Cohen's unweighted kappa coefficient. For all analyses, values of P < .05 were considered significant.
Results
Study Population
Twenty‐five dogs were evaluated for inclusion in this study. Two dogs were excluded, 1 because of deteriorating condition that precluded BAL and the other because of a hypoplastic trachea that precluded bronchoscopic BAL. Four dogs that had final diagnoses unrelated to pulmonary disease also were excluded. One dog was excluded from analysis because laboratory error resulted in acellular slides from the MA BALF sample. The final study group consisted of 18 dogs, with mean (±SD) age of 6.7 ± 4.3 years and weight of 27.5 ± 18.2 kg. Ten dogs were male (7 castrated, 3 intact) and 8 were female (8 spayed). Breeds represented in the study population included mixed breed (3), Labrador Retriever (3), German Shepherd Dog (2), and 1 of each of the following: Beagle, Boston Terrier, Cane Corso, Coonhound, Havanese Terrier, Portuguese Water Dog, Rhodesian Ridgeback, Toy Poodle, Whippet, and Yorkshire Terrier. Eleven (61.1%) of dogs were receiving medications at the time of BAL. Antibiotics, corticosteroids, and bronchodilators were the most common classes of concurrent medications in these patients. Thoracic radiographs acquired before bronchoscopy were assessed by the board‐certified veterinary radiologist on duty, and identified diffuse bronchial pulmonary pattern in 4 dogs, interstitial pulmonary pattern in 4 dogs (1 focal, 3 diffuse), diffuse mixed broncho‐interstitial pattern in 2 dogs, and alveolar pulmonary pattern in 4 dogs (3 focal, 1 diffuse). The remaining 4 dogs had thoracic radiographs without radiographic abnormalities.
Bronchoscopy and BAL
A pediatric gastroscope3 developed for use in humans (10 dogs) and video bronchoscope3 (5 dogs) were used for the majority of the procedures (15/18, 83.3%). Two larger diameter gastroscopes3 were used for procedures in the remaining dogs. In general, the video bronchoscope was used in patients weighing <30 kg and the pediatric gastroscope was used in patients weighing >30 kg for its increased working length. However, the pediatric gastroscope was used in 2 dogs weighing <10 kg as further endoscopic evaluation was performed after BAL, and the pediatric gastroscope provided an improved image. Bronchoscopic abnormalities were identified grossly in 15 of the 18 dogs, with increased airway secretions and erythema being the most common abnormalities, each identified in 6 dogs. Other abnormalities included tracheal or bronchial collapse (5), nodular bronchial mucosa (2), bronchiectasis (2), and hemorrhage (1). Multiple suction trap connections were required for SPA in 10 dogs (>24 kg) when BALF exceeded 20 mL.
Adverse Effects of Bronchoscopy and BAL
Twelve dogs maintained normal oxygen saturation during the procedure. Three dogs (16.7%) had transient decreases in oxygen saturation (<2 minutes), which resolved without intervention. The remaining 3 dogs (16.6%) required oxygen supplementation after recovery from anesthesia. One dog did not survive to discharge from hospital, resulting in a mortality or euthanasia rate of 5.5%.
Examination of BALF
The percentage of infusate retrieved by MA ranged from 6.6 to 61.4% (mean ± SD, 26.8 ± 15.2%; Fig 1). The percentage of infusate retrieved with SPA ranged from 17.0 to 66.7% (mean ± SD, 44.3± 16.3%). A significantly higher percentage of BALF was retrieved using the SPA technique, when compared with MA (mean ± SD difference, 17.5 ± 17.4%; P = .001; Table 1). The type of endoscope used for the procedure had no significant effect on the amount of BALF retrieved (MA, P = .99; SPA, P = .26).
Figure 1.
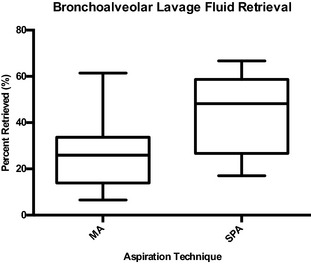
Box and whisker plot showing the percentage of canine bronchoalveolar lavage fluid (BALF) retrieved with manual aspiration (MA) and suction pump aspiration (SPA) from 18 dogs with respiratory tract disease. The top end of the box represents the 75th percentile and the bottom end of the box indicates the 25th percentile. The line within the box represents the median. The whiskers on the top and the bottom of the boxes indicate the 95th and 5th percentiles, respectively. A significantly higher percentage of BALF was retrieved using the SPA technique when compared with MA (mean difference 17.5 ± SD 17.4%, P = .001).
Table 1.
Comparison of results for BALF samples obtained via MA and SPA from 18 client‐owned dogs with naturally occurring respiratory tract disease.
| Variable | Mean by Technique | Median by Technique | Mean Difference | SD of Difference | Median Distribution of Differencesa | IQR for Distribution of Differences | P Valueb | ||
|---|---|---|---|---|---|---|---|---|---|
| MA | SPA | MA | SPA | ||||||
| Percentage (%) of retrieved infusate | 26.8 | 44.3 | — | — | 17.5 | 17.4 | — | — | .001 |
| Quality score | |||||||||
| Cellularityc | — | — | 4 | 4 | — | — | 0 | 0.5 | .10 |
| Cell preservationd | — | — | 4 | 4 | — | — | 0 | 0 | .41 |
| RBCse | — | — | 0 | 0 | — | — | 0 | 0 | .32 |
| Epithelial cellsf | — | — | 0 | 0 | — | — | 0 | 0 | .56 |
| Extracellular bacteriag | — | — | 0 | 0 | — | — | 0 | 1 | 1.0 |
| TNCC (No. of cells/μL) | — | — | 575 | 695 | — | — | 90 | 492 | .87 |
| Differential cell count | |||||||||
| Macrophage (%) | — | — | 29 | 54 | — | — | 2 | 14.5 | .40 |
| Neutrophil (%) | — | — | 10 | 7 | — | — | −2 | 7 | .43 |
| Lymphocyte (%) | — | — | 5 | 7 | — | — | 0 | 3 | .96 |
| Eosinophils (%) | — | — | 2 | 3 | — | — | 0 | 4 | .41 |
| Mast cell (%) | — | — | 0 | 0 | — | — | 0 | 0 | .71 |
| Cytologic diagnosis score | — | — | 1 | 1 | — | — | 0 | 0 | .78 |
BALF, bronchoalveolar lavage fluid; MA, manual aspiration; SPA, suction pump aspiration.
A positive value indicates that SPA was higher than MA, whereas a negative value indicates that MA was higher than SPA.
Values <0.05 indicate that results were significantly different between the MA and SPA techniques.
Scored on the basis of cytologic evaluation using a scale of 0–4 (0 = <10 cells/slide; 1 = 10–100 cells/slide; 2 = 101–200 cells/slide; 3 = 201–500 cells/slide; 4 = >500 cells/slide).
Scored on the basis of cytologic evaluation using a scale of 0–4 (0 = <10% well‐preserved cells/slide; 1 = 10–25% well‐preserved cells/slide; 2 = 26–50% well‐preserved cells/slide; 3 = 51–80% well‐preserved cells/slide; 4 = >80% well‐preserved cells/slide).
Scored on the basis of cytologic evaluation using a scale of 0–3 (0 = ≤1% cells/slide; 1 = 2–3% cells/slide; 2 = 4–5% cells/slide; 3 = ≥6% cells/slide).
Scored on the basis of cytologic evaluation on a scale of 0–4 (0 = absent; 1 = <50 cells/slide; 2 = 51–100 cells/slide; 3 = 101–200 cells/slide; 4 = >201 cells/slide).
Scored on the basis of cytologic evaluation on a scale of 0–4 (0 = absent; 1 = <5 cells/slide; 2 = 6–10 cells/slide; 3 = 11–20 cells/slide; 4 = >21 cells/slide).
Median TNCC for samples obtained by MA was 575 cells/μL (range, 55–14,900 cells/μL; IQR, 800 cells/μL) and median TNCC for samples obtained by SPA was 695 cells/μL (range, 200–3,900 cells/μL; IQR, 800 cells/μL); these values did not differ significantly (P = .87; Fig 2). Differential cell counts (neutrophil, P = .43; macrophage, P = .40; lymphocyte, P = .96; eosinophil, P = .41; mast cell, P = .71) were not significantly different between aspiration techniques. Five of 18 cytocentrifuge preparations from BALF acquired by MA were acellular. These 5 MA BALF samples also had low TNCC, lacked bronchoalveolar cells, or both on the direct smear of concentrated fluid, and were considered nondiagnostic. In contrast, all 18 BALF samples acquired by SPA had adequate cell numbers for analysis. There were no significant differences between scores for cellularity (P = .10), cell preservation (P = .41), epithelial cells (P = .56), extracellular bacteria (P = 1.0), or RBCs (P = .32) between MA and SPA.
Figure 2.
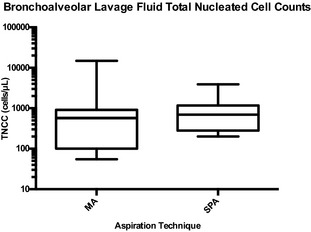
Box and whisker plot showing the total nucleated cell count (TNCC) (cells/μL) from canine bronchoalveolar lavage fluid retrieved with manual aspiration (MA) and suction pump aspiration (SPA) from 18 dogs with respiratory tract disease. The top end of the box represents the 75th percentile and the bottom end of the box indicates the 25th percentile. The line within the box represents the median. The whiskers on the top and the bottom of the box indicate the 95th and 5th percentiles, respectively. The y‐axis is on a logarithmic (log10) scale. The TNCCs were not significantly different between aspiration techniques (median distribution of differences 90 ± IQR 492 cells/μL, P = .87).
Diagnosis
When history, physical examination findings, bronchoscopic findings, and microbial analysis of BALF were assessed in conjunction with BALF cytology, a diagnosis was achieved in 16/18 (88.9%) dogs. Definitive diagnoses (cytologic diagnosis score of 2) were achieved from cytologic analysis of 3/18 (16.7%) and 4/18 (22.2%) of the BALF samples acquired by MA and SPA techniques, respectively. Definitive diagnoses included bacterial pneumonia (4) and blastomycosis (1). Cytology was considered supportive of the diagnosis (cytologic diagnosis score of 1) in 7/18 (38.9%) and 6/18 (33.3%) of samples acquired by MA and SPA, respectively. These diagnoses included bacterial pneumonia (5), eosinophilic bronchopneumopathy (3), and chronic bronchitis (1). There was no significant difference between cytologic diagnosis score achieved using either technique (P = .78). Excluding the 5 dogs with nondiagnostic MA BALF samples, substantial agreement was found between the classification of inflammatory BALF between MA and SPA (kappa = 0.615). Moderate agreement was found between the cytologic diagnostic score achieved with SPA and MA (kappa = 0.563).
No significant associations were found between patterns (MA, P = .20; SPA, P = .22) or distribution (MA, P = .78; SPA, P = .64) of changes identified on thoracic radiographs and acquiring a definitive diagnosis from BALF cytology.
Discussion
Although SPA previously had been found to result in increased BALF retrieval from healthy dogs when compared with MA, this finding had not yet been confirmed in dogs with respiratory tract disease.9, 10 In this group of dogs with pulmonary disease, an increased frequency of airway collapse and an overall decreased BALF retrieval was observed with both aspiration techniques, when compared to previous groups of healthy dogs.9, 10 However, SPA retrieved a significantly higher amount of BALF than did MA. Dwell time and duration of BAL procedure are suspected to increase BALF retrieval volume because a substantial amount of additional water passes from the intravascular space into the alveolar space during BAL.15 It is possible that BAL using the SPA technique required more time in larger dogs, when multiple suction traps were required. Alternatively, if the bronchoscope suction valve did not seal properly, MA may have retrieved air (via the suction channel) preferentially over fluid from the airways. Another explanation for this finding could be that SPA creates more consistent negative pressure, without increased airway collapse, than does MA. It is also possible that SPA creates a better wedge between the bronchoscope and bronchial mucosa by collapsing the bronchi aboral to the bronchoscope's tip.
Recovery of 40–90% of BAL infusate has been reported in canine medicine using various BAL techniques, and currently a minimum 40% BALF retrieval has been recommended in dogs to maximize chances of a diagnostic sample.4 In this study, 61.1% of BALF samples (15 MA and 7 SPA) comprised <40% of the original aliquot volume. All 9 BALF samples with cellularity scores <4 (8 MA and 1 SPA) were associated with <40% infusate retrieval. In as much as the majority of cells in BALF originate from the alveoli, this finding was in agreement with previous reports in human medicine that indicated that decreased fluid retrieval and low TNCC in retrieved infusate are consistent with bronchial, and not bronchoalveolar, washes.16 However, in the present study of dogs with pulmonary disease, only 5 of the 22 low‐retrieval BALF samples had inadequate cell numbers for analysis and half (11/22) still yielded a definitive or supportive cytologic diagnosis. This finding agrees with a report in humans that BALF retrieval of at least 10% of the original aliquot still can reflect alveolar sampling.17 In general, maximal infusate retrieval should be attempted for any BAL to maximize the likelihood of retrieving alveolar cells. If patient condition allows, additional lavages may be performed because higher fluid retrieval has been reported with subsequent lavages.18 In cases where low BALF retrieval persists, however, the samples still should be assessed because they still may contain adequate cell numbers for analysis.
The variables of the semiquantitative quality score did not differ significantly between techniques. Similar cellularity, cell preservation, RBC, and epithelial cell scores indicated that SPA at a maximum negative pressure of 50 mmHg did not result in increased cell lysis or bronchial mucosa trauma when compared with MA. Although only substantial agreement was found between the cytologic classification of BALF from the aspiration techniques, a higher level of agreement between BALF cytology from different lung segments was not necessarily expected because some dogs in this study had focal pulmonary disease on preprocedure thoracic radiographs. In addition, it was reported that BALF cytologic assessment from cats with lower respiratory disease often differed among different pulmonary segments, even with diffuse disease.19 Similarly, a retrospective review of canine BALF cytology determined that 37% of samples had different types of inflammation among different lung lobes in the same dog.14 These findings indicated that cytology results obtained by both MA and SPA were comparable.
A relationship between increased BALF retrieval and improved rate of diagnosis from cytologic assessment has been identified inconsistently in human medicine.20, 21 Although SPA yielded higher BALF retrieval, there was no significant difference between the cytologic diagnosis score for the 2 techniques and thus this study failed to demonstrate a relationship between increased BALF and improved diagnostic yield in dogs.
In this study, a weight‐adjusted aliquot volume was used based on a report that they provided more uniform epithelial lining fluid recovery in healthy Beagles.4 Compared with previous reports, we did not experience an increased mortality rate associated with the weight‐adjusted aliquot volume,14 but we did experience technical challenges associated with 1 mL/kg aliquots in larger dogs. Multiple suction traps were used during the BAL procedure with SPA, which required equipment changes during the procedure. Suction trap connections with larger capacities14 are available and their use should be considered for BAL with SPA. In addition, the use of weight‐adjusted BAL aliquots in small dogs (<9 kg),4 large dogs (>20 kg)4, or in dogs with respiratory tract disease has not been critically evaluated. This situation warrants further investigation because 1 mL/kg BAL aliquots may be unnecessary in all dogs.
A limitation of this study was that bronchoscopy and BAL were performed by various clinicians, with different levels of experience in the technique. Operator experience may have affected BAL retrieval and BALF sample quality. However, because the same clinician performed both aspiration techniques on an individual dog, the variable expertise should have affected both MA and SPA results. Another limitation was that the majority of dogs had received concurrent medications at the time of BAL, which may have affected the composition of BALF. Furthermore, none of our diagnoses were confirmed by pulmonary histopathology. Other limitations included the lack of long‐term patient follow‐up and absence of cases of pulmonary neoplasia. A high diagnostic yield had been reported previously from BALF cytologic assessment for dogs with lymphoma and carcinoma.14, 22 Inclusion of patients with pulmonary neoplasia may have altered the rate of definitive diagnoses achieved from BALF cytology.
The results identified that SPA with a maximum negative pressure of 50 mmHg resulted in higher BALF retrieval in dogs with respiratory tract disease when compared to MA. These findings, however, did not correspond to a higher diagnostic yield from BALF cytology. There was substantial agreement between cytology of BALF acquired by both techniques and moderate agreement between the cytologic diagnosis scores. Both the MA and SPA techniques were suitable for BAL in dogs with respiratory tract disease. Further evaluation of weight‐adjusted and fixed‐volume BAL aliquot volumes may be required in dogs with respiratory tract disease.
Acknowledgment
Supported financially by the Ontario Veterinary College Pet Trust. The authors thank Alison Downie for technical assistance and Gabrielle Monteith for assistance with statistical analysis.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
Research was performed at the Ontario Veterinary College, University of Guelph in Guelph, ON N1G 2W1.
This manuscript represents a portion of a thesis submitted by Dr Woods to the Ontario Veterinary College Department of Clinical Studies as partial fulfillment of the requirements for a DVSc degree.
Footnotes
Arnolds dog catheter with female Luer mount, 8F × 50 cm; Smith Medical International, Kent, UK
Right‐angled swivel connector; Vygon Corp., PA
Olympus Canada Inc, Richmond Hill, ON
Vacuum tracheal suction regulator with Ohmeda adapter; Western Medica, Westlake, OH
Kendall Luki 20‐mL (6.25‐inch) disposable aspirating tube; Tyco Healthcare Group, Mansfield, MA
Endozime, dual enzymatic cleaning; Ruhof Corp., Mineola, NY
Glutacide; Pharmax Limited, Etobicoke, ON
Z2 Coulter counter; Beckman Coulter, Mississauga, ON
Shandon Cytospin 4; Thermo‐Fisher Scientific Inc, Waltham, MA
Hematek Slide Stainer 4488C; Siemens Healthcare Diagnostics Inc, Tarrytown, NY
Microsoft Excel for Mac 2011, version 14.3.1.
SAS Institute Inc. 2007. SAS OnlineDoc® 9.2. Cary, NC
IBM SPSS Statistics, version 21; IBM Corporation, Armonk, NY
40 and 80 mL sterile mucus specimen trap, Stevens Company Ltd, Brampton, ON
References
- 1. Hawkins EC, DeNicola DB, Kuehn NF. Bronchoalveolar lavage in the evaluation of pulmonary disease in the dog and cat: State of the art. J Vet Intern Med 1990;4:267–274. [DOI] [PubMed] [Google Scholar]
- 2. Baughman RP. Technical aspects of bronchoalveolar lavage: Recommendations for a standard procedure. Semin Respir Crit Care Med 2007;28:475–485. [DOI] [PubMed] [Google Scholar]
- 3. Klech H, Pohl W. Technical recommendations and guidelines for bronchoalveolar lavage (BAL). Eur Respir J 1989;2:561–585. [PubMed] [Google Scholar]
- 4. Melamies MA, Jarvinen AK, Seppala KM, et al. Comparison of results for weight‐adjusted and fixed‐amount bronchoalveolar lavage techniques in healthy Beagles. Am J Vet Res 2011;72:694–698. [DOI] [PubMed] [Google Scholar]
- 5. Mordelet‐Dambrine MM, Arnoux AA, Stanislas‐Leguern GG, et al. Processing of lung lavage fluid causes variability in bronchoalveolar cell count. Am Rev Respir Dis 1984;130:305–306. [DOI] [PubMed] [Google Scholar]
- 6. Kelly C, Ward C, Bird G, et al. The effect of filtration on absolute and differential cell counts in fluid obtained at bronhcolaveolar lavage. Respir Med 1989;83:107–110. [DOI] [PubMed] [Google Scholar]
- 7. Meyer KC, Raghu G, Baughman RP, et al. An official American Thoracic Society clinical practice guideline: The clinical utility of bronchoalveolar lavage cellular analysis in interstitial lung disease. Am J Respir Crit Care Med 2012;185:1004–1014. [DOI] [PubMed] [Google Scholar]
- 8. Hewson J, Viel L. Sampling, microbiology and cytology of the respiratory tract In: Lekeux P, ed. Equine Respiratory Diseases. Ithaca, NY: IVIS; 2002. http://www.ivis.org/special_books/lekeux/viel/ivis.pdf. Accessed October 4, 2012. [Google Scholar]
- 9. Woods KS, Defarges AMN, Abrams‐Ogg ACG, et al. Comparison between manual aspiration via polyethylene tubing and aspiration via a suction pump with a suction trap connection for performing bronchoalveolar lavage in healthy dogs. Am J Vet Res 2013;74:523–529. [DOI] [PubMed] [Google Scholar]
- 10. Woods KS, Defarges AMN, Abrams‐Ogg AC, et al. Comparison of bronchoalveolar lavage fluid obtained by manual aspiration with a handheld syringe with that obtained by automated suction pump aspiration from healthy dogs. Am J Vet Res 2014;75:85–90. [DOI] [PubMed] [Google Scholar]
- 11. De Lorenzi D, Masserdotti C, Bertoncello D, et al. Differential cell counts in canine cytocentrifuge bronchoalveolar lavage fluid: A study on reliable enumeration of each cell type. Vet Clin Pathol 2009;38:532–536. [DOI] [PubMed] [Google Scholar]
- 12. Silverstein DC, Drobratz KJ. Clinical evaluation of the respiratory tract In: Ettinger SJ, Feldman EC, eds. Textbook of Veterinary Internal Medicine, 7th ed St Louis, MO: Saunders Elsevier; 2010:1055–1066. [Google Scholar]
- 13. Johnson LR, Queen EV, Vernau W, et al. Microbiologic and cytologic assessment of bronchoalveolar lavage fluid from dogs with lower respiratory tract infection: 105 cases (2001–2011). J Vet Intern Med 2013;27:259–267. [DOI] [PubMed] [Google Scholar]
- 14. Hawkins EC, DeNicola DB, Plier ML. Cytological analysis of bronchoalveolar lavage fluid in the diagnosis of spontaneous respiratory tract disease in dogs: A retrospective study. J Vet Intern Med 1995;9:386–392. [DOI] [PubMed] [Google Scholar]
- 15. Kelly CA, Fenwick JD, Corris PA, et al. Fluid dynamics during bronchoalveolar lavage. Am Rev Respir Dis 1988;138:81–84. [DOI] [PubMed] [Google Scholar]
- 16. Lam S, Leriche JC, Kijek K, Phillips D. Effect of bronchial lavage volume on cellular and protein recovery. Chest 1985;88:856–859. [DOI] [PubMed] [Google Scholar]
- 17. Baughman RP, Spencer RE, Kleykamp BO, et al. Ventilator associated pneumonia: Quality of nonbronchoscopic bronchoalveolar lavage sample affects diagnostic yield. Eur Respir J 2000;16:1152–1157. [DOI] [PubMed] [Google Scholar]
- 18. Hawkins EC, Kennedystoskopf S, Levy J, et al. Cytologic characterization of bronchoalveolar lavage fluid collected through an endotracheal tube in cats. Am J Vet Res 1994;55:795–802. [PubMed] [Google Scholar]
- 19. Ybarra WL, Johnson LR, Drazenovich TL, et al. Interpretation of multisegment bronchoalveolar lavage in cats. J Vet Intern Med 2012;26:1281–1287. [DOI] [PubMed] [Google Scholar]
- 20. Rosell A, Xaubet A, Agusti C, et al. A new BAL fluid instillation and aspiration technique: A multicenter randomized study. Respir Med 2006;100:529–535. [DOI] [PubMed] [Google Scholar]
- 21. Sampsonas F, Kontoyiannis DP, Dickey BF, et al. Performance of a standardized bronchoalveolar lavage protocol in a comprehensive cancer center. Cancer 2011;117:3424–3433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Hawkins EC, Morrison WB, DeNicola DB, et al. Cytologic analysis of bronchoalveolar lavage fluid from 47 dogs with multicentric malignant lymphoma. J Am Vet Med Assoc 1993;203:1418–1425. [PubMed] [Google Scholar]


