Abstract
Background
Serum N‐terminal pro‐C‐natriuretic peptide (NT‐proCNP) concentration at hospital admission has sufficient sensitivity and specificity to differentiate naturally occurring sepsis from nonseptic systemic inflammatory response syndrome (SIRS). However, little is known about serum NT‐proCNP concentrations in dogs during the course of sepsis.
Objective
To determine serum NT‐proCNP and cytokine kinetics in dogs with endotoxemia, a model of canine sepsis.
Samples
Eighty canine serum samples.
Methods
Eight healthy adult Beagles were randomized to receive Escherichia coli lipopolysaccharide (LPS, 5 μg/kg) or placebo (0.9% NaCl) as a single IV dose in a randomized crossover study. Serum collected at 0, 1, 2, 4, and 24 hours was stored at −80°C for batch analysis. Serum NT‐proCNP was measured by ELISA and 13 cytokines and chemokines by multiplex magnetic bead‐based assay.
Results
Serum NT‐proCNP concentrations did not differ significantly between LPS‐ and placebo‐treated dogs at any time. When comparing serum cytokine concentrations, LPS‐treated dogs had higher interleukin‐6 (IL‐6), IL‐10, TNF‐α and KC‐like at 1, 2, and 4 hours; higher CCL2 at 1, 2, 4, and 24 hours; and higher IL‐8 and CXCL10 at 4 hours compared to placebo‐treated dogs. There were no differences in serum GM‐CSF, IFN‐γ, IL‐2, IL‐7, IL‐15 or IL‐18 between LPS‐ and placebo‐treated dogs.
Conclusions and Clinical Importance
Serum NT‐proCNP concentration does not change significantly in response to LPS administration in healthy dogs. Certain serum cytokine and chemokine concentrations are significantly increased within 1–4 hours after LPS administration and warrant further investigation as tools for the detection and management of sepsis in dogs.
Keywords: Biomarker, ELISA, Immunology, Inflammation, Multiplex, Sepsis
Abbreviations
- CCL2
C‐C motif chemokine ligand 2 (also referred to as monocyte chemotactic protein‐1, MCP‐1)
- CRP
C‐reactive protein
- CV
coefficient of variation
- CXCL10
C‐X‐C motif chemokine ligand 10 (also referred to as interferon gamma‐induced protein‐10, IP10)
- GM‐CSF
granulocyte macrophage‐colony stimulating factor
- IFN
interferon
- IL
interleukin
- KC‐like
keratinocyte‐derived chemokine (also referred to as CXCL1)
- LPS
lipopolysaccharide
- NK
natural killer
- NT‐proCNP
amino‐terminal pro‐C‐type natriuretic peptide
- OD
optical density
- SIRS
systemic inflammatory response syndrome
- TNF
tumor necrosis factor
A biological marker, or biomarker, refers to any objective measurement used to indicate a state of health or disease accurately and reproducibly. Biomarkers can be used to promptly identify sepsis, thereby enabling rapid initiation of antibiotic therapy.1 This feature is in contrast to bacterial culture and susceptibility testing, which require several days to complete. Cytokines and chemokines that have been investigated as biomarkers for sepsis in dogs include tumor necrosis factor‐alpha (TNF‐α), interleukin‐6 (IL‐6), interleukin‐10 (IL‐10), and interleukin‐8 (IL‐8, also known as C‐X‐C motif chemokine ligand‐8 or CXCL‐8).2, 3, 4 When measured using cell‐kill bioassays, increases in serum TNF‐α did not differentiate sepsis from nonseptic systemic inflammatory response syndrome (SIRS).2 In addition, although in 1 study IL‐6 plasma concentration was predictive of disease severity and mortality in dogs with SIRS and sepsis,3 in other studies, plasma IL‐6, IL‐8, and IL‐10 concentrations did not distinguish sepsis from nonseptic SIRS.2, 4
Amino‐terminal pro‐C‐type natriuretic peptide (NT‐proCNP) is a local regulator of vascular endothelial function that is expressed after stimulation by lipopolysaccharide (LPS) and TNF‐α. It is a stable propeptide that differentiates sepsis from other nonseptic causes of critical illness in people,5, 6, 7 and also is increased in dogs with naturally occurring sepsis.8 A study evaluating an enzyme‐linked immunosorbent assay (ELISA) for the quantification of NT‐proCNP indicated that a cut‐off concentration of 10.1 pmol/L had 65.5% sensitivity and 89.2% specificity for differentiating dogs with naturally occurring sepsis from dogs with nonseptic inflammation.8 A recent study also identified the induction of this biomarker from canine aortic endothelial cells in cell culture after IL‐1β, TNF‐α, and LPS stimulation.9 However, in clinical studies, NT‐proCNP has only been measured in dogs with naturally occurring sepsis at admission to the hospital, and to the authors’ knowledge, there are no published studies investigating blood concentrations of NT‐proCNP in dogs with endotoxemia.
Although most biomarkers for sepsis, including chemokines and cytokines, are measured by individual ELISA, multiplex magnetic bead‐based assays offer the advantage of concurrently quantifying a large number of cytokines and chemokines.10, 11, 12, 13, 14, 15, 16, 17, 18 The simultaneous measurement of multiple biomarkers provides more information that might help to differentiate sepsis from nonseptic inflammation. Sepsis is a complex and heterogeneous disease during which the extent of proinflammatory cytokine production per patient depends on the severity of inflammation incited by the infection and varies with age, sex, genetics, and other concurrent diseases.19, 20, 21, 22 A multiplex assay enables the measurement of multiple cytokines and chemokines in a single blood sample.
The purpose of this study was to determine the kinetics of serum NT‐proCNP and multiplex‐based serum cytokine concentrations over a 24‐hour period in healthy dogs and dogs with endotoxemia after LPS administration. We hypothesized that dogs would have increased serum NT‐proCNP 1–4 hours after LPS administration as a consequence of the induction of systemic inflammation, and that NT‐proCNP concentration would decrease by 24 hours. We also hypothesized that proinflammatory cytokines such as TNF‐α and IL‐6 would increase within hours of LPS administration and would normalize within 24 hours coincident with resolution of systemic inflammation. Finally, we hypothesized that the multiplex assay would identify additional cytokines and chemokines that have not been investigated previously, but whose kinetics could aid in the diagnosis of sepsis in dogs.
Materials and Methods
Animals
Eight adult purpose‐bred Beagles deemed healthy based on normal physical examination, complete blood count, and serum biochemistry profile were included in this survival study. None of the dogs had received LPS as part of an experiment at any time before this study. Dogs were housed and handled according to the guidelines of the Canadian Council on Animal Care, the requirements of the Animals for Research Act Revised Statutes of Ontario, and institutional Animal Care Policy. This study was approved by the institution's Animal Care Committee.
Experimental Design
A randomized, placebo‐controlled, crossover study was performed. Dogs were randomized to receive a bolus of either 5 μg/kg LPS, IV (Escherichia coli serotype 0127:B8)1 or an equal volume of placebo (0.9% NaCl)2 IV through a cephalic catheter.3 Thirty minutes later, each dog was resuscitated with IV fluids (40 mL/kg 0.9% NaCl). After a minimum 14‐day washout period during which no treatments were administered beyond normal husbandry and care, the study was repeated with dogs receiving the alternate prefluid treatment (LPS or placebo).
Dogs were fasted for 12 hours before the study and allowed free access to water. At the initiation of the experiment, butorphanol4 (0.2 mg/kg IM) was given for sedation and analgesia and was repeated IV 2 and 4 hours after baseline. A 20‐gauge catheter was placed in the cephalic vein to enable IV administration of placebo, LPS, butorphanol, and resuscitation fluids. After 6 hours, the cephalic catheter was removed and the dogs were returned to their housing and offered food.
Sample Collection and Storage
Blood samples were collected by jugular venipuncture at baseline (0), 1, 2, 4, and 24 hours after LPS or placebo administration. Blood samples were transferred into plastic Vacutainer tubes with no additive5 and allowed to clot for 15 minutes. The samples then were centrifuged at 700 × g for 15 minutes, and serum was removed and stored at −80°C for batch analysis of NT‐proCNP, cytokine and chemokine concentrations. In addition, stored serum samples remaining after clinician‐ordered serum biochemistry profiles from a clinically healthy dog, a dog with sepsis secondary to bite wounds, and a nonseptic dog that was neutropenic 7 days after chemotherapy were used to confirm NT‐proCNP kit intra‐ and interassay coefficients of variation (CV).
NT‐proCNP ELISA
An NT‐proCNP ELISA kit6 previously validated in dogs was obtained, and the assay was performed according to the manufacturer's instructions.9 This kit was reported to have a lower limit of detection of 0.55 pmol/L, and inter‐ and intra‐assay CV of 7–9 and 4–5%, respectively.9 The kit control, as well as serum samples from the above‐mentioned individual dogs, each were assayed 5 times in 1 assay run to further evaluate intra‐assay CV, and on 5 different dates to assess interassay variability. All study samples were assayed in duplicate, and 1 well was left empty as a blank reference. The absorbance was measured at 450 nm with a 620 nm reference; the measured optical density (OD) was (OD at 450 nm) − (OD at 620 nm). The OD of the blank well was subtracted from all sample measurements. Log transformation was performed to obtain a standard curve that was used to determine the NT‐proCNP concentration of the samples using the formula 10([logOD – intercept] / slope).
Multiplex Cytokine Immunoassay Kit
An antibody‐coated magnetic microsphere‐based multiplex cytokine immunoassay kit7 designed for the simultaneous quantification of 13 cytokines, including several not previously investigated in dogs with sepsis, was used to determine cytokine kinetics. Those included in the kit were IL‐2, IL‐6, IL‐7, IL‐8, IL‐10, IL‐15, IL‐18, TNF‐α, interferon‐γ (IFN‐γ), granulocyte macrophage‐colony stimulating factor (GM‐CSF), keratinocyte‐derived chemokine (KC)‐like, C‐C motif chemokine ligand 2 (CCL2), and CXCL10. This multiplex kit gives an accurate measurement of serum cytokines when compared with individual ELISAs and has been used previously to measure serum cytokine concentrations in dogs.23, 24, 25 All 80 samples from the LPS‐ or placebo‐treated dogs were assayed once, and the assay standards and quality control sample each were assayed twice, for a total of 116 samples. The assay was performed according to the manufacturer's instructions. Overnight incubation at 4°C was performed and a magnetic plate washer was utilized. The plates then were read using a multiplex plate reader8 with the companion software.9
Statistical Analyses
Intra‐ and interassay CVs for the NT‐proCNP ELISA kit were calculated by dividing the standard deviation by the mean of the 5 results obtained for each sample. Duplicate NT‐proCNP results were averaged and the mean value was used for statistical analysis. Single values were obtained for the quantification of cytokines. The NT‐proCNP and cytokine concentrations over time were analyzed by a generalized linear mixed‐model using the mixed procedure. Different error structures were tried, with the final model for auto‐regression chosen based on the Akaike information criterion. The assumptions of the ANOVA were assessed by comprehensive residual analyses and the Shapiro–Wilk test was conducted to analyze for normal distribution. The residuals were plotted against the predicted values and explanatory variables (treatment, time, dogs) to identify outliers or unequal variance. If residual analyses suggested a need for data transformation, logarithmic transformation was done before data analysis. The level of significance was corrected using a Dunnett's or Tukey's test for multiple comparisons where appropriate. Significance was set at P < .05 for all variables. Data are presented as mean ± SD. All analyses were performed using standard statistical software.10 Graphs were generated using commercially available software.11
Results
All dogs were female (3 intact, 5 spayed) ranging in age from 16 to 44 months (median, 18 months). The median body weight was 8.85 kg (range, 7.8–11 kg). Within 30 minutes of LPS administration, all LPS‐treated dogs demonstrated lethargy and gastrointestinal upset characterized by diarrhea and hypersalivation. Rectal temperature was significantly increased after 3 (P < .001) and 4 (P < .001) hours in LPS‐ versus placebo‐treated dogs.
The intra‐assay CV for the NT‐proCNP kit ranged from 9.2 to 17.9% and the interassay CV ranged from 6.3 to 18.0%. There was no significant difference in NT‐proCNP concentration at any time point when samples from placebo‐treated dogs were compared to LPS‐treated dogs (Fig 1).
Figure 1.
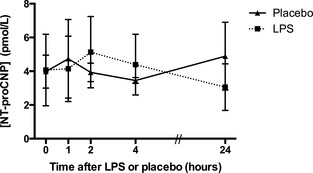
Serum NT‐proCNP concentrations in dogs after lipopolysaccharide (LPS) or placebo administration. Values are presented as group mean ± SD.
When comparing serum cytokine concentrations in LPS‐treated dogs to placebo‐treated dogs, IL‐6, IL‐10, TNF‐α, and KC‐like were significantly higher at 1, 2, and 4 hours (P < .05; Fig 2). In addition, CCL2 was higher at 1, 4, and 24 hours, whereas IL‐8 and CXCL10 were higher at 4 hours (P < .05; Fig 2). There were no significant differences in serum GM‐CSF, IFN‐γ, IL‐2, IL‐7, IL‐15 or IL‐18 between LPS‐ and placebo‐treated dogs (data not shown).
Figure 2.
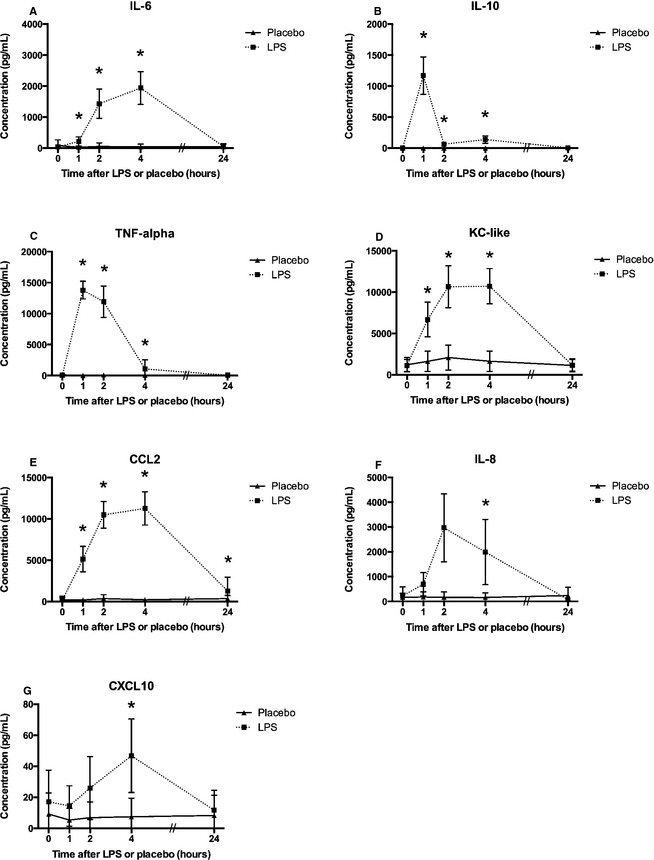
Serum cytokine concentrations in dogs after lipopolysaccharide (LPS) or placebo administration: interleukin‐6 (IL‐6) (A), IL‐10 (B), tumor necrosis factor (TNF‐α) (C), keratinocyte‐derived chemokine (KC‐like) (D), C‐C motif chemokine ligand 2 (CCL2) (E), IL‐8 (F), and C‐X‐C motif chemokine ligand 10 (CXCL10) (G). Significant differences between LPS and placebo dogs are indicated with *(P < .05). Values are presented as group mean ± SD.
Discussion
Using an assay with inter‐ and intra‐assay CVs ≤ 18%, this study indicated that NT‐proCNP was not increased in dogs given a single IV injection of a low dose of LPS, but increases in several other cytokines and chemokines were observed in the same dogs. Potent simulators of NT‐proCNP include LPS, TNF‐α and transforming growth factor‐β.5, 26 In addition, inflammation can create an environment in which NT‐proCNP is produced by the monocyte/macrophage system, resulting in vasodilatation and the inhibition of microbial growth and pathogenicity.5, 27, 28
Although LPS induced NT‐proCNP in a dose‐dependent manner in a canine cell culture system,8 LPS at the dosage administered to dogs in this study (5 μg/kg, IV) did not produce an increase in serum NT‐proCNP. This LPS dosage was chosen because it produces clinical illness in healthy dogs without being fatal.29, 30, 31 Nevertheless, the absence of an increase in the concentration of NT‐proCNP over time in the dogs with endotoxemia could have been because the dosage of LPS was too low, or because the duration of endotoxemia was too short. However, the measured increases in IL‐6, IL‐8, IL‐10, and TNF‐α confirmed induction of inflammation in the dogs given LPS. These proinflammatory cytokines are similarly increased in dogs with naturally occurring sepsis.2 Therefore, it is likely that the endotoxemia model used in this study, as in previous studies in other species, cannot fully reproduce the complexity of the in vivo interactions resulting in NT‐proCNP expression during naturally occurring sepsis.
Previous studies using cell‐kill bioassay methods to measure cytokine concentrations in dogs after LPS administration have identified rapid changes in TNF‐α concentration. TNF‐α increased within 30 minutes after IV LPS administration, and a large overlap in sustained peak concentrations was observed after approximately 2 hours in response to dosages ranging from 0.1 to 40 μg/kg, suggesting that its activity was not dose‐dependent.30, 32 In contrast, the increase in IL‐6 after the IV administration of LPS over the same dosage range had a slower onset, peak activity that persisted >2 hours longer than TNF‐α activity, and greater dose‐dependency.32 TNF‐α and IL‐6 kinetics seen in this study are consistent with these previous results, which reflect the proinflammatory response to LPS administration.
Other chemokines, including CCL2, KC‐like, and CXCL 10, were increased at ≥1 time point after LPS administration in this study, suggesting their possible utility as biomarkers of systemic inflammation or sepsis. The multiplex assay has been previously used to measure IL‐18, CCL2, IL‐6, IL‐15, IL‐8, and TNF‐α in dogs with immune‐mediated hemolytic anemia,25 immune‐mediated thrombocytopenia,12 and in endurance racing sled dogs that experience a sustained inflammatory response.23 To the authors’ knowledge, CCL2 has not yet been evaluated as a potential biomarker for sepsis in dogs. In murine models of pancreatitis and sepsis, CCL2 mediates the immediate proinflammatory response by recruiting circulating monocytes, T lymphocytes, natural killer (NK) cells and neutrophils to the site of infection or inflammation33; this cytokine could play a similar role in dogs. KC‐like is expressed by macrophages, neutrophils, and epithelial cells, resulting in neutrophil chemoattractant activity and increased bacterial phagocytosis, thereby playing a key role in sepsis.33, 34 Similarly, CXCL10 is secreted by cells such as monocytes, endothelial cells, and fibroblasts in response to IFN‐γ.34 This cytokine plays a role in the chemoattraction of monocytes and macrophages, T‐cells, NK cells, and dendritic cells, and promotes T‐cell adhesion to endothelial cells in order to resist bacterial and viral infections.34 As in mouse models of sepsis,34 CXCL10 also could prove to be a useful biomarker of sepsis in dogs.
Cytokines such as IL‐6, IL‐10, and TNF‐α are associated with the innate immune response, which is the nonspecific first line of host defense during infection, responsible for triggering a proinflammatory response after LPS administration. The cytokines that did not increase after LPS administration (GM‐CSF, IFN‐γ, IL‐2, IL‐7, IL‐15, or IL‐18) in this study typically play a role in the adaptive immune response, which is responsible for the elimination of pathogens in the late phase of infection. Consequently, the timing of sampling could have precluded these cytokines from becoming increased. For example, the lack of a significant increase in IL‐15 is consistent with studies demonstrating an increase in this cytokine only when measured beyond 24 hours.35 Similarly IL‐2 and IL‐7 are expected to be increased 24–72 hours or 48 hours, respectively, after bacterial infection.36, 37
Limitations of this study include sample collection at a limited number of time points after LPS or placebo administration. Additional time points might have yielded more information detailing the kinetics of NT‐proCNP and cytokines; specifically, concentrations of IL‐2, IL‐7, and IL‐15 might have increased after 24 hours. Blood was sampled at only a small number of time points because of funding limitations. Similar constraints limited cytokine multiplex analysis to single replicates, although duplication of samples would have been preferred. Likewise, validation of the multiplex analysis using cytokine controls or mass spectrometry was not within the scope of this study. In addition, only female Beagle dogs were available through the Central Animal Facility at the university from which the dogs were acquired. Consequently, any effect of sex on NT‐proCNP or cytokine kinetics could not be assessed. Finally, the average NT‐proCNP inter‐ and intra‐assay CVs in this study (13.5% and 13.2%, respectively) were higher than anticipated based on those reported previously.8 The lowest CVs (9.2% for intra‐assay CV and 6.3% for interassay CV) were obtained for the control provided in the kit. The higher CVs were obtained from the samples with the lowest NT‐proCNP concentrations. Although these concentrations were not below the detection limit of the assay, they were toward the lower end of the standard curve and therefore more variable.
In conclusion, serum NT‐proCNP concentrations did not differ significantly between healthy dogs and dogs with endotoxemia after LPS administration. Future studies using either higher dosages of LPS, or a low dose endotoxin infusion, or an alternative model that might better mimic naturally occurring sepsis, could be useful for determining the kinetics of this protein biomarker during sepsis. Serum cytokines and chemokines including IL‐6, IL‐8, IL‐10, TNF‐α, KC‐like, CCL2, and CXCL10 were significantly increased in dogs within 1–4 hours of LPS administration, and warrant further investigation as tools for the detection and management of sepsis in dogs. Considering the complexity and redundancy of the inflammatory response to sepsis, it is unlikely that a single biomarker will have adequate sensitivity and specificity for diagnosis of sepsis, but a multiplex assay could yield valuable diagnostic information if available to the clinician.
Acknowledgments
The authors thank Jonathan Liu for technical assistance as well as Gabrielle Monteith and William Sears for their assistance with statistical analyses. Funding for this project was generously provided by the Ontario Veterinary College Pet Trust Fund.
Conflict of Interest Declaration: The authors disclose no conflict of interest.
All of the ELISAs and cytokine assays were performed at Ontario Veterinary College.
This manuscript represents a portion of a thesis to be submitted by Dr Floras to the Department of Clinical Studies, Ontario Veterinary College, as partial fulfillment of the requirements for a Doctor of Veterinary Science degree. The abstract of this manuscript was presented as a poster at the International Veterinary Emergency and Critical Care Symposium, September 2013, San Diego, CA.
Footnotes
Sigma‐Aldrich, St. Louis, MO
0.9% NaCl; Baxter, Mississauga, ON
BD Insyte Autoguard catheter; BD Medical, Sandy, UT
Torbugesic; Fort Dodge Animal Health, Overland Park, KS
BD Vacutainer plus plastic plasma tubes; Becton Dickinson and Company, Franklin Lakes, NJ
Biomedica Gruppe, Vienna, Austria
MILLIPLEX MAP for Luminex® xMAP® technology canine cytokine/chemokine magnetic bead panel immunoassay, EMD Millipore Corporation, Billerica, MA
Bio‐Plex® 200; Bio‐Rad Laboratories, Mississauga, ON
Bio‐Plex® Data‐Pro software; Bio‐Rad Laboratories
SAS v.9.2; SAS Institute Inc., Cary, NC
Prism 5; GraphPad Software, La Jolla, CA
LeVine DN, Birkenheuer AJ, Brooks MB, et al. A novel canine model of immune thrombocytopenia. 2012;http://www.repository.lib.ncsu.edu
References
- 1. Wacker C, Prkno A, Brunkhorst FM, et al. Procalcitonin as a diagnostic marker for sepsis: A systematic review and meta‐analysis. Lancet Infect Dis 2013;13:426–435. [DOI] [PubMed] [Google Scholar]
- 2. DeClue AE, Sharp CR, Harmon M. Plasma Inflammatory mediator concentrations at ICU admission in dogs with naturally developing sepsis. J Vet Intern Med 2012;26:624–630. [DOI] [PubMed] [Google Scholar]
- 3. Rau S, Kohn B, Richter C, et al. Plasma interleukin‐6 response is predictive for severity and mortality in canine systemic inflammatory response syndrome and sepsis. Vet Clin Pathol 2007;36:253–260. [DOI] [PubMed] [Google Scholar]
- 4. Fransson BA, Lagerstedt AS, Bergstrom A, et al. C‐reactive protein, tumor necrosis factor α, and interleukin‐6 in dogs with pyometra and SIRS. J Vet Emerg Crit Care 2007;17:373–381. [Google Scholar]
- 5. Hama N, Itoh H, Shirakami G, et al. Detection of C‐type natriuretic peptide in human circulation and marked increase of plasma CNP level in septic shock patients. Biochem Biophys Res Commun 1994;198:1177–1182. [DOI] [PubMed] [Google Scholar]
- 6. Bahrami S, Pelinka L, Khadem A, et al. Circulating NT‐proCNP predicts sepsis in multiple‐traumatized patients without traumatic brain injury. Crit Care Med 2010;38:161–166. [DOI] [PubMed] [Google Scholar]
- 7. Koch A, Voigt S, Sanson E, et al. Prognostic value of circulating amino‐terminal pro‐C‐type natriuretic peptide in critically ill patients. Crit Care 2011;15:R45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. DeClue AE, Osterbur K, Bigio A, et al. Evaluation of serum NT‐pCNP as a diagnostic and prognostic biomarker for sepsis in dogs. J Vet Intern Med 2011;25:453–459. [DOI] [PubMed] [Google Scholar]
- 9. Osterbur K, Yu D‐H, DeClue AE. Interleukin‐1b, tumour necrosis factor‐a and lipopolysaccharide induce C‐type natriuretic peptide from canine aortic endothelial cells. Res Vet Sci 2013;94:478–483. [DOI] [PubMed] [Google Scholar]
- 10. Ebong S, Call D, Nemzek JA, et al. Immunopathologic alterations in murine sepsis models of increasing lethality. Infect Immunol 1999;67:6603–6610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Remick D, Bolgos GL, Siddiqui J, et al. Six at six: Interleukin‐6 measured 6 hours after the initiation of sepsis predicts mortality over 3 days. Shock 2002;17:463–467. [DOI] [PubMed] [Google Scholar]
- 12. Oberhoffer M, Vogelsang H, Russwurm S, et al. Outcome prediction by traditional and new markers of inflammation in patients with sepsis. Clin Chem Lab Med 1999;37:363–368. [DOI] [PubMed] [Google Scholar]
- 13. Dienstknecht T, Schwacha MG, Kang S, et al. Sex steroid‐mediated regulation of macrophage/monocyte function in a two‐hit model of trauma‐hemorrhage and sepsis. Cytokine 2004;25:110–118. [DOI] [PubMed] [Google Scholar]
- 14. Jarrar D, Wang P, Cioffi WG, et al. The female reproductive cycle is an important variable in the response to trauma‐hemorrhage. Am J Physiol Heart Circ Physiol 2000;279:H1015–H1021. [DOI] [PubMed] [Google Scholar]
- 15. Kang S, Matsutani T, Choudhry MA, et al. Are the immune responses different in middle‐aged and young mice following bone fracture, tissue trauma and hemorrhage? Cytokine 2004;26:223–230. [DOI] [PubMed] [Google Scholar]
- 16. Tateda K, Matsumoto T, Miyazaki S, et al. Lipopolysaccharide‐induced lethality and cytokine production in aged mice. Infect Immunol 1996;64:769–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Faulker JW, Simeeka MK, Davidson JK, et al. Gene expression and production of tumor necrosis factor alpha, interleukin 1, interleukin 6, and gamma interferon in C3H/HeN and C57BL/6N mice in acute Mycoplasma pulmonis disease. Infect Immunol 1995;63:4084–4090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Matsutani T, Samy TS, Kang S, et al. Mouse genetic background influences severity of immune responses following trauma‐hemorrhage. Cytokine 2005;30:168–176. [DOI] [PubMed] [Google Scholar]
- 19. Otto CM, Drobatz KJ, Soter C. Endotoxemia and tumor necrosis factor activity in dogs with naturally occurring parvoviral enteritis. J Vet Intern Med 1997;11:65–70. [DOI] [PubMed] [Google Scholar]
- 20. Ruaux CG, Pennington HL, Worrall S, et al. Tumor necrosis factor‐α at presentation in 60 cases of spontaneous canine acute pancreatitis. Vet Immunol Immunopathol 1999;72:369–376. [DOI] [PubMed] [Google Scholar]
- 21. Nemzek JA, Agrodnia MD, Hauptman JG. Breed‐specific pro‐inflammatory cytokine production as a predisposing factor for susceptibility to sepsis in the dog. J Vet Emerg Crit Care 2007;17:368–372. [Google Scholar]
- 22. Gebhardt C, Hirschberger J, Rau S, et al. Use of C‐reactive protein to predict outcome in dogs with systemic inflammatory response syndrome or sepsis. J Vet Emerg Crit Care 2009;19:450–458. [DOI] [PubMed] [Google Scholar]
- 23. Yazwinski M, Milizio JG, Wakshlag JJ. Assessment of serum myokines and markers of inflammation associated with exercise in endurance racing sled dogs. J Vet Intern Med 2013;27:371–376. [DOI] [PubMed] [Google Scholar]
- 24. Dossus L, Becker S, Achaintre D, et al. Validity of multiplex‐based assays for cytokine measurements in serum and plasma from “non‐diseases” subjects: Comparison with ELISA. J Immunol Methods 2009;350:125–132. [DOI] [PubMed] [Google Scholar]
- 25. Kjelgaard‐Hansen M, Goggs R, Wiinberg B, et al. Use of serum concentration of interleukin‐18 and monocyte chemoattractant protein‐1 as prognostic indicators in primary immune‐mediated haemolytic anemia in dogs. J Vet Intern Med 2011;25:76–82. [DOI] [PubMed] [Google Scholar]
- 26. Suga S, Itoh H, Komatsu Y, et al. Cytokine‐induced C‐type natriuretic peptide (CNP) secretion from vascular endothelial cells—Evidence for CNP as a novel autocrine/paracrine regulator from endothelial cells. Endocrinology 1993;133:3038–3041. [DOI] [PubMed] [Google Scholar]
- 27. Kubo A, Isumi Y, Ishizaka Y, et al. C‐type natriuretic peptide is synthesized and secreted from leukemia cell lines, peripheral blood cells, and peritoneal macrophages. Exp Hematol 2001;29:609–615. [DOI] [PubMed] [Google Scholar]
- 28. Veron W, Orange N, Feuilloley MG, et al. Natriuretic peptides modify Pseudomonas fluorescens cytotoxicity by regulating cyclic nucleotides and modifying LPS structure. BMC Microbiol 2008;8:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Schultz MJ, van der Poll T. Animal and human models for sepsis. Ann Med 2002;34:73–581. [DOI] [PubMed] [Google Scholar]
- 30. DeClue AE, Cohn LA, Lechner ES, et al. Effects of subanesthetic doses of ketamine on hemodynamic and immunologic variables in dogs with experimentally induced endotoxemia. Am J Vet Res 2008;69:228–232. [DOI] [PubMed] [Google Scholar]
- 31. Holowaychuk MK, Birkenheuer AJ, Li J, et al. Alterations in calcium homeostasis in dogs with induced endotoxemia. J Vet Emerg Crit Care 2010;20:A5. [Google Scholar]
- 32. LeMay DR, LeMay LG, Kluger MJ, et al. Plasma profiles of IL‐6 and TNF with fever‐inducing doses of lipopolysaccharide in dogs. Am J Physiol 1990;259:R126. [DOI] [PubMed] [Google Scholar]
- 33. Shanmugam MK, Bhatia M. The role of pro‐inflammatory molecules and pharmacological agents in acute pancreatitis and sepsis. Inflamm Allergy Drug Targets 2010;9:20–31. [DOI] [PubMed] [Google Scholar]
- 34. Kelly‐Scumpia KM, Scumpia PO, Delano MJ, et al. Type I interferon signalling in hematopoietic cells is required for survival in mouse polymicrobial sepsis by regulating CXCL10. J Exp Med 2010;207:319–326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Inoue S, Unsinger J, Davis CG, et al. IL‐15 prevents apoptosis, reverses innate and adaptive immune dysfunction, and improves survival in sepsis. J Immunol 2010;184:1401–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. O'Sullivan ST, Lederer JA, Horgan AF, et al. Major injury leads to predominance of the T helper‐2 lymphocyte phenotype and diminished interleukin‐12 production associated with decreased resistance to infection. Ann Surg 1995;222:482–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Unsinger J, Burnham CD, McDonough J, et al. Interleukin‐7 ameliorates immune dysfunction and improves survival in a 2‐hit model of fungal sepsis. J Infect Dis 2012;206:606–616. [DOI] [PMC free article] [PubMed] [Google Scholar]


